Application of High-Pressure Processing to Assure the Storage Stability of Unfiltered Lager Beer
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of High-Pressure Processing on the Basic Analytical Parameters of Unfiltered Beer
2.2. Impact of High-Pressure Processing on the Foam Stability of Unfiltered Beer
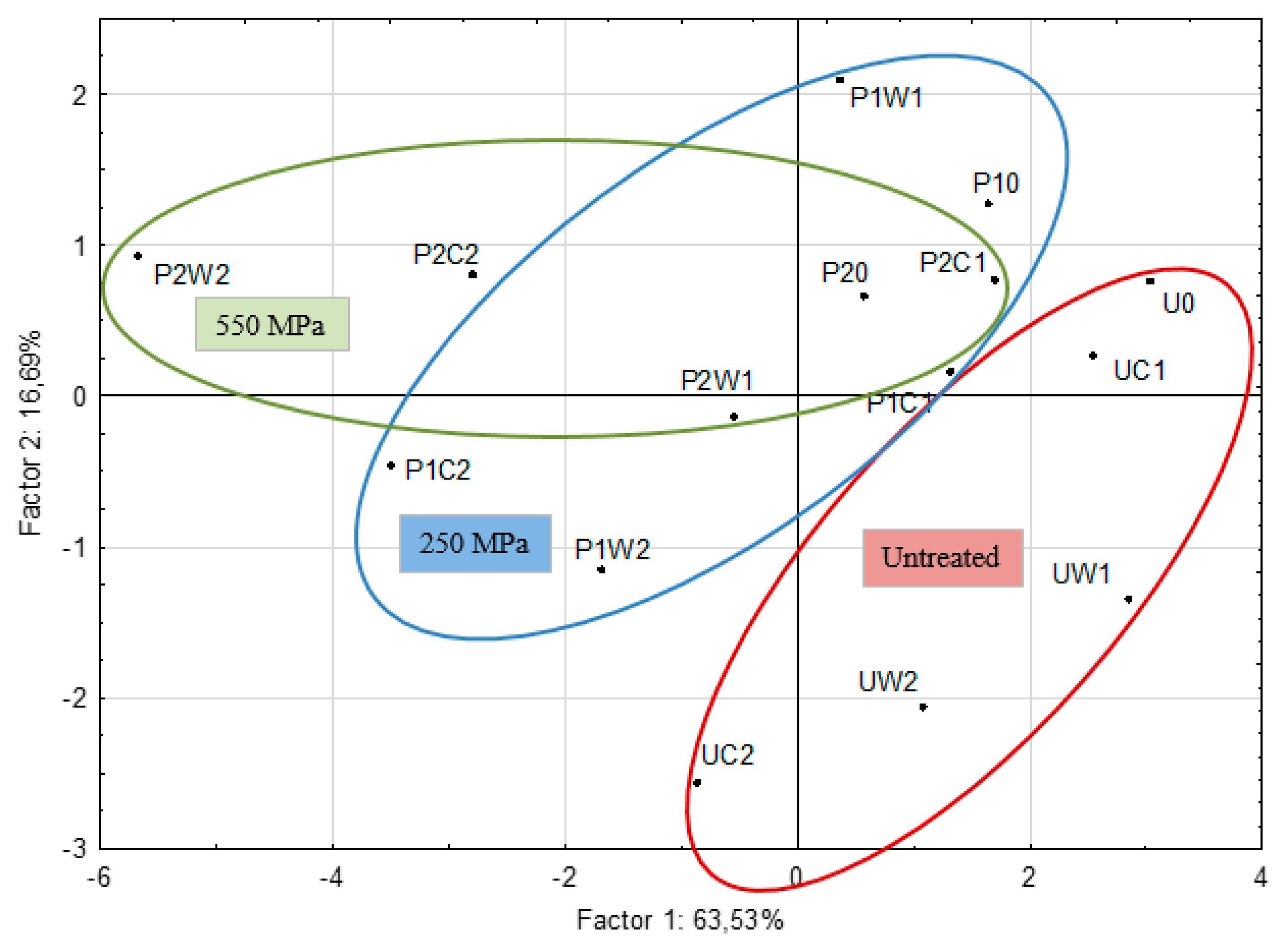
2.3. Changes in Concentrations of Carbonyl Compounds after Processing and during Storage
2.4. Effect of HPP on the Sensory Properties
3. Materials and Methods
3.1. Beer Samples and High-Pressure Treatment
3.2. Measurement of Basic Analytical Parameters
3.3. Foam Stability and the Activity of Proteinase A
3.4. Assessment of Selected Volatile Carbonyl Compounds
3.5. Sensory Evaluation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Corona, C.; Escalona-Buendía, H.B.; García, M.; Chollet, S.; Valentin, D. Craft vs. industrial: Habits, attitudes and motivations towards beer consumption in Mexico. Appetite 2016, 96, 358–367. [Google Scholar]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Lučan, M.; Mastanjević, K. Craft brewing–is it really about the sensory revolution? Kvas. Prum. 2019, 65, 13–16. [Google Scholar] [CrossRef]
- Bamforth, C.W. Nutritional aspects of beer—A review. Nutr. Res. 2002, 22, 227–237. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1, 3/1, 6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Torrelli, P.; Marini, F. Flavour component analysis by HS-SPME/GC–MS and chemometric modeling to characterize Pilsner-style Lager craft beers. Microchem. J. 2019, 149, 103991. [Google Scholar] [CrossRef]
- Donadini, G.; Porretta, S. Uncovering patterns of consumers’ interest for beer: A case study with craft beers. Food Res. Int. 2017, 91, 183–198. [Google Scholar] [CrossRef]
- Choi, D.Y.; Stack, M.H. The all-American beer: A case of inferior standard (taste) prevailing? Bus. Horiz. 2005, 48, 79–86. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging–a critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Wray, E. Reducing microbial spoilage of beer using pasteurization. In Brewing Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 253–269. [Google Scholar]
- Cao, L.; Zhou, G.; Guo, P.; Li, Y. Influence of pasteurising intensity on beer flavour stability. J. Inst. Brew. 2011, 117, 587–592. [Google Scholar] [CrossRef]
- Hoff, S.; Lund, M.N.; Petersen, M.A.; Frank, W.; Andersen, M.L. Storage stability of pasteurized non-filtered beer. J. Inst. Brew. 2013, 119, 172–181. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Aquilanti, L.; Clementi, F. The occurrence of beer spoilage lactic acid bacteria in craft beer production. J. Food Sci. 2015, 80, M2845–M2852. [Google Scholar] [CrossRef] [PubMed]
- Maifreni, M.; Frigo, F.; Bartolomeoli, I.; Buiatti, S.; Picon, S.; Marino, M. Bacterial biofilm as a possible source of contamination in the microbrewery environment. Food Control. 2015, 50, 809–814. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control. 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High pressure processing in food industry–characteristics and applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Kurowska, A.; Szajkowska, A.; van der Meulen, B. EU regulatory approach to high-pressure processing. In High Pressure Processing of Food; Springer: Berlin, Germany, 2016; pp. 717–732. [Google Scholar]
- Santos, L.M.; Oliveira, F.A.; Ferreira, E.H.; Rosenthal, A. Application and possible benefits of high hydrostatic pressure or high-pressure homogenization on beer processing: A review. Food Sci. Technol. Int. 2017, 23, 561–581. [Google Scholar] [CrossRef]
- Buzrul, S.; Alpas, H.; Bozoglu, F. Effect of high hydrostatic pressure on quality parameters of lager beer. J. Sci. Food Agric. 2005, 85, 1672–1676. [Google Scholar] [CrossRef]
- Castellari, M.; Arfelli, G.; Riponi, C.; Carpi, G.; Amati, A. High hydrostatic pressure treatments for beer stabilization. J. Food Sci. 2000, 65, 974–977. [Google Scholar] [CrossRef]
- Yin, H.; Dong, J.; Yu, J.; Chang, Z.; Qian, Z.; Liu, M.; Huang, S.; Hu, X.; Liu, X.; Deng, Y. A preliminary study about the influence of high hydrostatic pressure processing on the physicochemical and sensorial properties of a cloudy wheat beer. J. Inst. Brew. 2016, 122, 462–467. [Google Scholar] [CrossRef]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of high-pressure processing on the microbial inactivation in fruit preparations and other vegetable based beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]
- Hartmann, C.; Mathmann, K.; Delgado, A. Mechanical stresses in cellular structures under high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2006, 7, 1–12. [Google Scholar] [CrossRef]
- Perrier-Cornet, J.M.; Hayert, M.; Gervais, P. Yeast cell mortality related to a high-pressure shift: Occurrence of cell membrane permeabilization. J. Appl. Microbiol. 1999, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E.; Bamforth, C.W. Beer foam: Achieving a suitable head. In Beer: A Quality Perspective; Master Brewers Association of Americas: Madison, WI, USA, 2009; pp. 1–60. [Google Scholar]
- Kosin, P.; Branyik, T.; Savel, J.; Ulmann, F.; Vlcek, J. Use of Sorbents to Increase Beer Foam Stability. J. Am. Soc. Brew. Chem. 2018, 76, 58–61. [Google Scholar] [CrossRef]
- He, G.-Q.; Wang, Z.-Y.; Liu, Z.-S.; Chen, Q.-H.; Ruan, H.; Schwarz, P.B. Relationship of proteinase activity, foam proteins, and head retention in unpasteurized beer. J. Am. Soc. Brew. Chem. 2006, 64, 33–38. [Google Scholar] [CrossRef]
- Kanauchi, M.; Bamforth, C. A Challenge in the study of flavour instability. Mon. Brauwiss. 2018, 71, 82–84. [Google Scholar]
- Stewart, G.G. Beer Shelf Life and Stability. In The Stability and Shelf Life of Food; Elsevier: London, UK, 2016; pp. 293–309. [Google Scholar]
- Jaskula-Goiris, B.; De Causmaecker, B.; De Rouck, G.; Aerts, G.; Paternoster, A.; Braet, J.; De Cooman, L. Influence of transport and storage conditions on beer quality and flavour stability. J. Inst. Brew. 2019, 125, 60–68. [Google Scholar] [CrossRef]
- Begrow, W. Fighting quality threats: Notable microbiological contaminations of craft beer in the United States. Brew. Beverage Ind. Int. 2017, 5, 10–13. [Google Scholar]
- Franchi, M.A.; Tribst, A.A.L.; Cristianini, M. Effects of high pressure homogenization on beer quality attributes. J. Inst. Brew. 2011, 117, 195–198. [Google Scholar] [CrossRef]
- Perez-Lamela, C.; Reed, R.; Simal-Gándara, J. High pressure application to wort and beer. Deut. Lebensm-Rundsch. 2004, 100, 52–56. [Google Scholar]
- Moreno, F.J.; Molina, E.; Olano, A.; López-Fandiño, R. High-pressure effects on Maillard reaction between glucose and lysine. J. Agric. Food Chem. 2003, 51, 394–400. [Google Scholar] [CrossRef]
- Kuchel, L.; Brody, A.L.; Wicker, L. Oxygen and its reactions in beer. Packag. Technol. Sci. 2006, 19, 25–32. [Google Scholar] [CrossRef]
- Driscoll, M.; Ramsay, C.; Hulse, G.; Simpson, W. A method of detecting autolysis of brewers’ yeast by measurement of extracellular adenylate kinase activity. J. Am. Soc. Brew. Chem. 2002, 60, 176–180. [Google Scholar] [CrossRef]
- Parr, C.L.; Keates, R.A.; Bryksa, B.C.; Ogawa, M.; Yada, R.Y. The structure and function of Saccharomyces cerevisiae proteinase A. Yeast 2007, 24, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Iglesias, C.; Nešpor, J.; Karabín, M.; Montero, O.; Blanco, C.A.; Dostálek, P. Comparison of carbonyl profiles from Czech and Spanish lagers: Traditional and modern technology. Lwt-Food Sci. Technol. 2016, 66, 390–397. [Google Scholar]
- Baert, J.J.; De Clippeleer, J.; Hughes, P.S.; De Cooman, L.; Aerts, G. On the origin of free and bound staling aldehydes in beer. J. Agric. Food Chem. 2012, 60, 11449–11472. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Improved flavor stability by aging beer in the presence of yeast. J. Am. Soc. Brew. Chem. 2011, 69, 50–56. [Google Scholar] [CrossRef]
- Chen, E.C.-H.; Jamieson, A.; Van Gheluwe, G. The release of fatty acids as a consequence of yeast autolysis. J. Am. Soc. Brew. Chem. 1980, 38, 13–18. [Google Scholar] [CrossRef]
- Molina-García, A.D. The effect of hydrostatic pressure on biological systems. Biotechnol. Genet. Eng. Rev. 2002, 19, 3–54. [Google Scholar] [CrossRef]
- Olšovská, J.; Čejka, P.; Sigler, K.; Hönigová, V. The phenomenon of Czech beer: A review. Czech. J. Food Sci. 2014, 32, 309–319. [Google Scholar] [CrossRef]
- Chen, Y.; Song, L.; Han, Y.; Liu, M.; Gong, R.; Luo, W.; Guo, X.; Xiao, D. Decreased proteinase a excretion by strengthening its vacuolar sorting and weakening its constitutive secretion in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 149–159. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds are not available from authors. |


| Sample | Untreated | 250 MPa | 550 MPa |
|---|---|---|---|
| Original gravity (% w/w) | 12.26 ± 0.01 | 12.18 ± 0.01 | 12.22 ± 0.01 |
| Alcohol (% vol.) | 4.84 | 4.80 | 4.81 |
| Apparent extract (% w/w) | 3.19 ± 0.01 | 3.20 ± 0.01 | 3.21 ± 0.01 |
| pH | 4.73 | 4.73 ± 0.01 | 4.73 ± 0.01 |
| Color (EBC) | 12.5 ± 0.2 | 12.3 ± 0.4 | 14.1 ± 0.1 * |
| Density (g/cm3) | 1.0106 | 1.0107 | 1.0107 |
| Sample Code | Processing Pressure (MPa) a | Storage Temperature (°C) | Length of Storage (Months) | 2-Methyl Propanal | 2-Methyl Butanal | 3-Methyl Butanal | Benzaldehyde | Heptanal | Hexanal | Octanal | (2E)-Non-2-enal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| U0 | - | 0 | 14 ± 2 | 8 | 15 | 2 ± 1 | 1 | 2 | 3 | 2 | |
| UC1 | - | 8 | 1 | 14 ± 2 | 8 ± 1 | 17 ± 1 | 3 ± 1 | 1 | 3 | 5 ± 2 | 2 ± 1 |
| UW1 | - | 22 | 1 | 12 | 6 | 10 | 4 | 1 | 4 | 8 ± 1 | 1 ± 1 |
| UC2 | - | 8 | 2 | 17 ± 2 | 9 ± 1 | 25 ± 1 | 16 ± 6 | 7 | 5 | 6 ± 2 | 4 ± 1 |
| UW2 | - | 22 | 2 | 11 ± 2 | 6 ± 1 | 14 ± 1 | 13 ± 2 | 5 ± 1 | 4 ± 1 | 5 | 2 |
| P10 | 250 | - | 0 | 26 ± 2 | 12 ± 1 | 23 ± 1 | 3 ± 1 | 1 | 3 | 4 | 2 |
| P1C1 | 250 | 8 | 1 | 23 ± 4 | 12 ± 1 | 23 ± 1 | 8 ± 3 | 2 | 3 | 6 ± 2 | 2 ± 1 |
| P1W1 | 250 | 22 | 1 | 43 ± 2 | 17 ± 2 | 31 ± 1 | 5 ± 1 | 1 | 5 ± 1 | 4 ± 1 | 3 ± 1 |
| P1C2 | 250 | 8 | 2 | 41 ± 4 | 19 ± 3 | 39 ± 2 | 17 | 6 ± 1 | 8 ± 1 | 5 ± 1 | 7 ± 2 |
| P1W2 | 250 | 22 | 2 | 26 ± 11 | 11 ± 3 | 23 ± 2 | 12 ± 3 | 5 ± 2 | 7 ± 1 | 3 ± 1 | 4 ± 1 |
| P20 | 550 | - | 0 | 30 ± 1 | 13 ± 1 | 24 ± 1 | 6 | 2 | 4 | 5 ± 1 | 3 |
| P2C1 | 550 | 8 | 1 | 24 ± 1 | 10 ± 1 | 20 | 6 | 1 | 4 | 4 ± 1 | 2 ± 1 |
| P2W1 | 550 | 22 | 1 | 37 ± 1 | 15 | 29 | 15 ± 1 | 2 | 4 | 5 ± 1 | 3 |
| P2C2 | 550 | 8 | 2 | 46 ± 4 | 19 ± 1 | 39 ± 2 | 14 ± 1 | 4 ± 2 | 7 | 4 | 6 ± 1 |
| P2W2 | 550 | 22 | 2 | 80 ± 10 | 25 ± 2 | 48 ± 2 | 24 ± 7 | 5 ± 2 | 8 ± 1 | 5 | 8 ± 1 |
| Factor 1 | Factor 2 | |
|---|---|---|
| 2-Methylpropanal | −0.835952 | 0.475608 |
| 2-Methylbutanal | −0.850818 | 0.491356 |
| 3-Methylbutanal | −0.914484 | 0.352284 |
| Hexanal | −0.943668 | −0.124385 |
| Heptanal | −0.714630 | −0.600826 |
| Octanal | 0.125436 | −0.503644 |
| Benzaldehyde | −0.904390 | −0.315189 |
| Nonenal | −0.960025 | 0.095859 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štulíková, K.; Bulíř, T.; Nešpor, J.; Jelínek, L.; Karabín, M.; Dostálek, P. Application of High-Pressure Processing to Assure the Storage Stability of Unfiltered Lager Beer. Molecules 2020, 25, 2414. https://doi.org/10.3390/molecules25102414
Štulíková K, Bulíř T, Nešpor J, Jelínek L, Karabín M, Dostálek P. Application of High-Pressure Processing to Assure the Storage Stability of Unfiltered Lager Beer. Molecules. 2020; 25(10):2414. https://doi.org/10.3390/molecules25102414
Chicago/Turabian StyleŠtulíková, Kateřina, Tomáš Bulíř, Jakub Nešpor, Lukáš Jelínek, Marcel Karabín, and Pavel Dostálek. 2020. "Application of High-Pressure Processing to Assure the Storage Stability of Unfiltered Lager Beer" Molecules 25, no. 10: 2414. https://doi.org/10.3390/molecules25102414
APA StyleŠtulíková, K., Bulíř, T., Nešpor, J., Jelínek, L., Karabín, M., & Dostálek, P. (2020). Application of High-Pressure Processing to Assure the Storage Stability of Unfiltered Lager Beer. Molecules, 25(10), 2414. https://doi.org/10.3390/molecules25102414







