Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive
Abstract
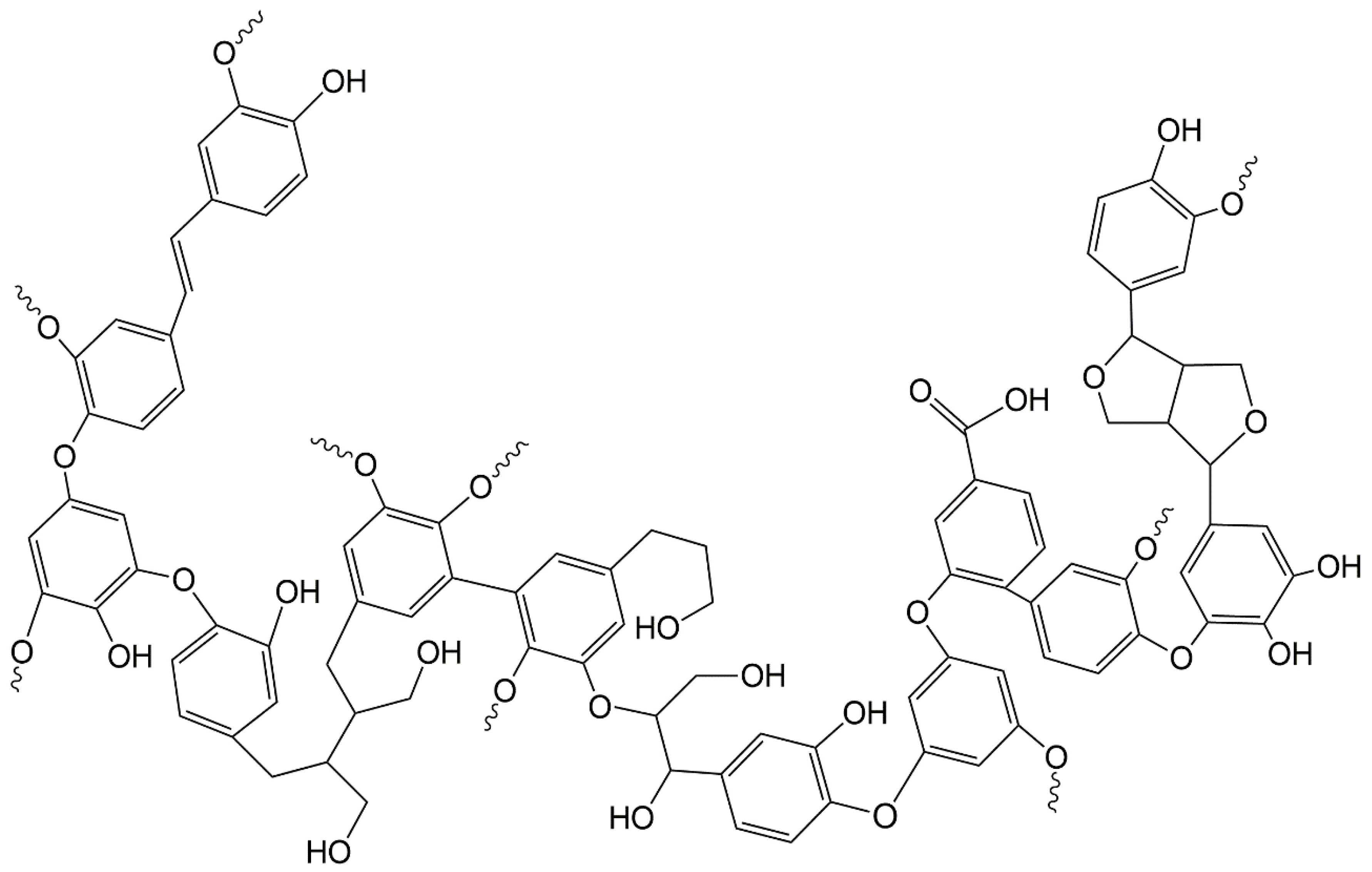
:1. Introduction
2. Experimental Procedures
2.1. Materials
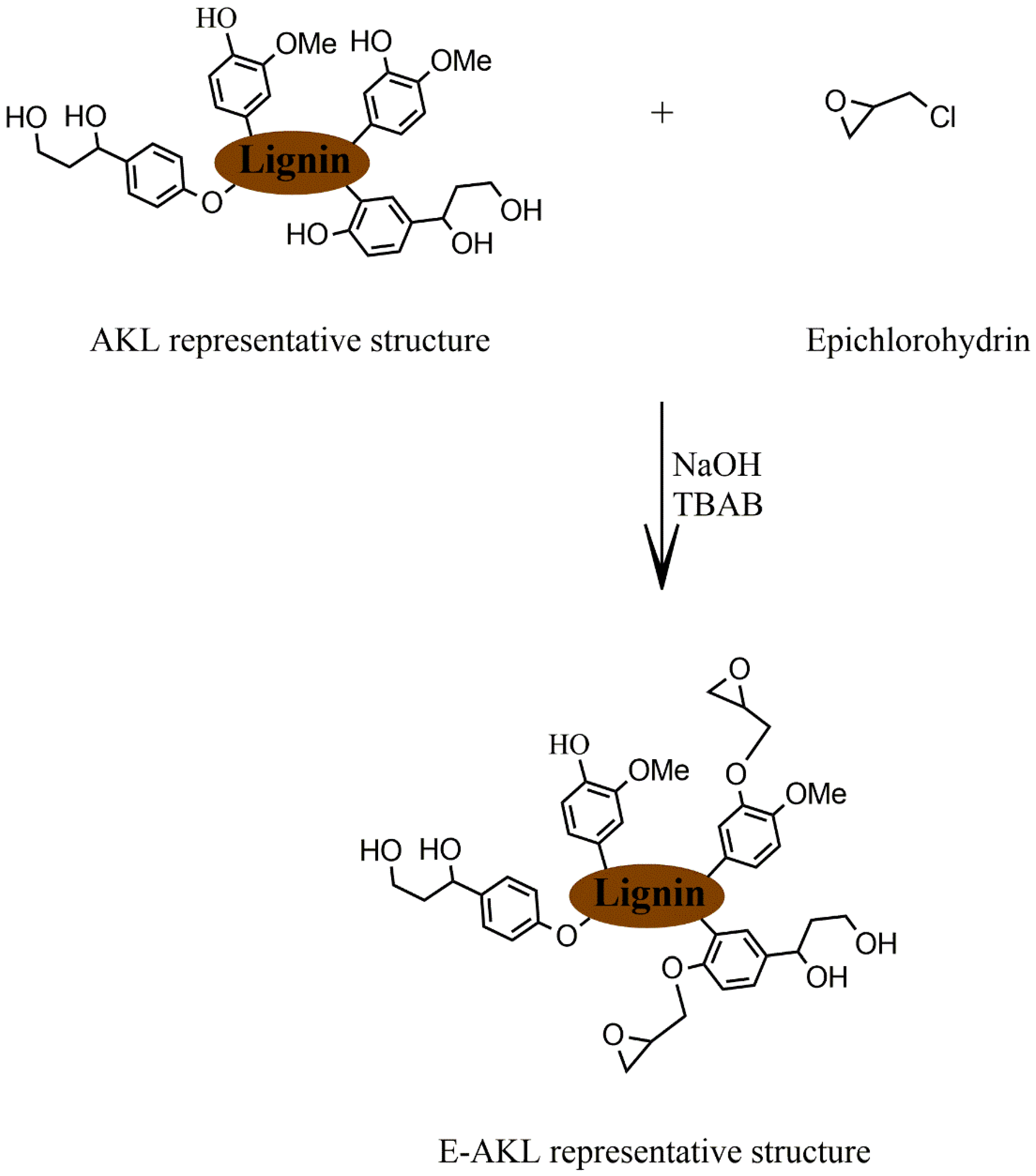
2.2. Lignin Epoxidation
2.3. Epoxy Synthesis
2.4. Infrared Spectroscopy (FTIR)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Temperature Modulated Optical Refractometry (TMOR)
2.7. Izod Impact Test
2.8. Single-Lap Shear Test
3. Results and Discussion
3.1. Characterization of Modified Lignin
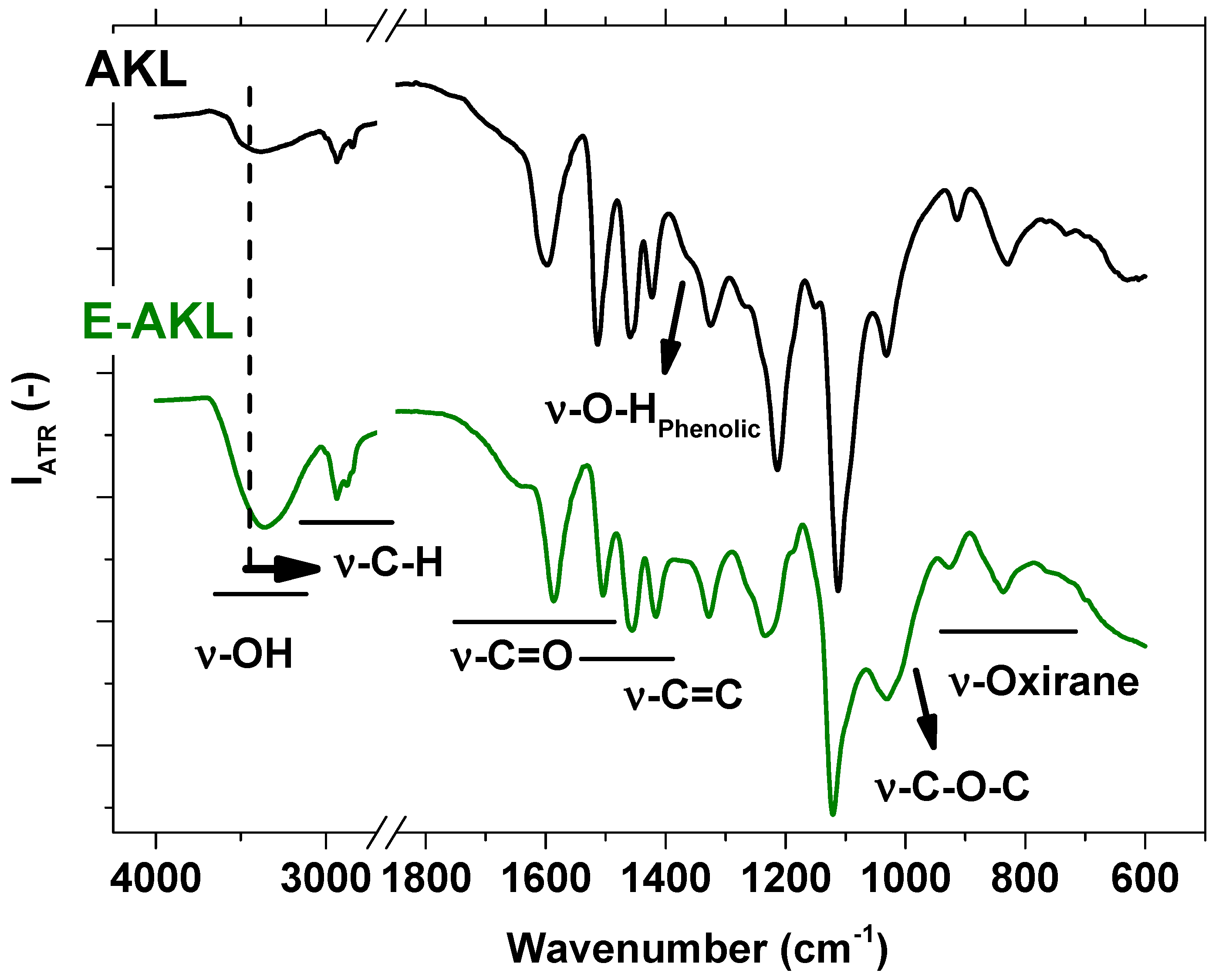
3.1.1. FTIR-ATR
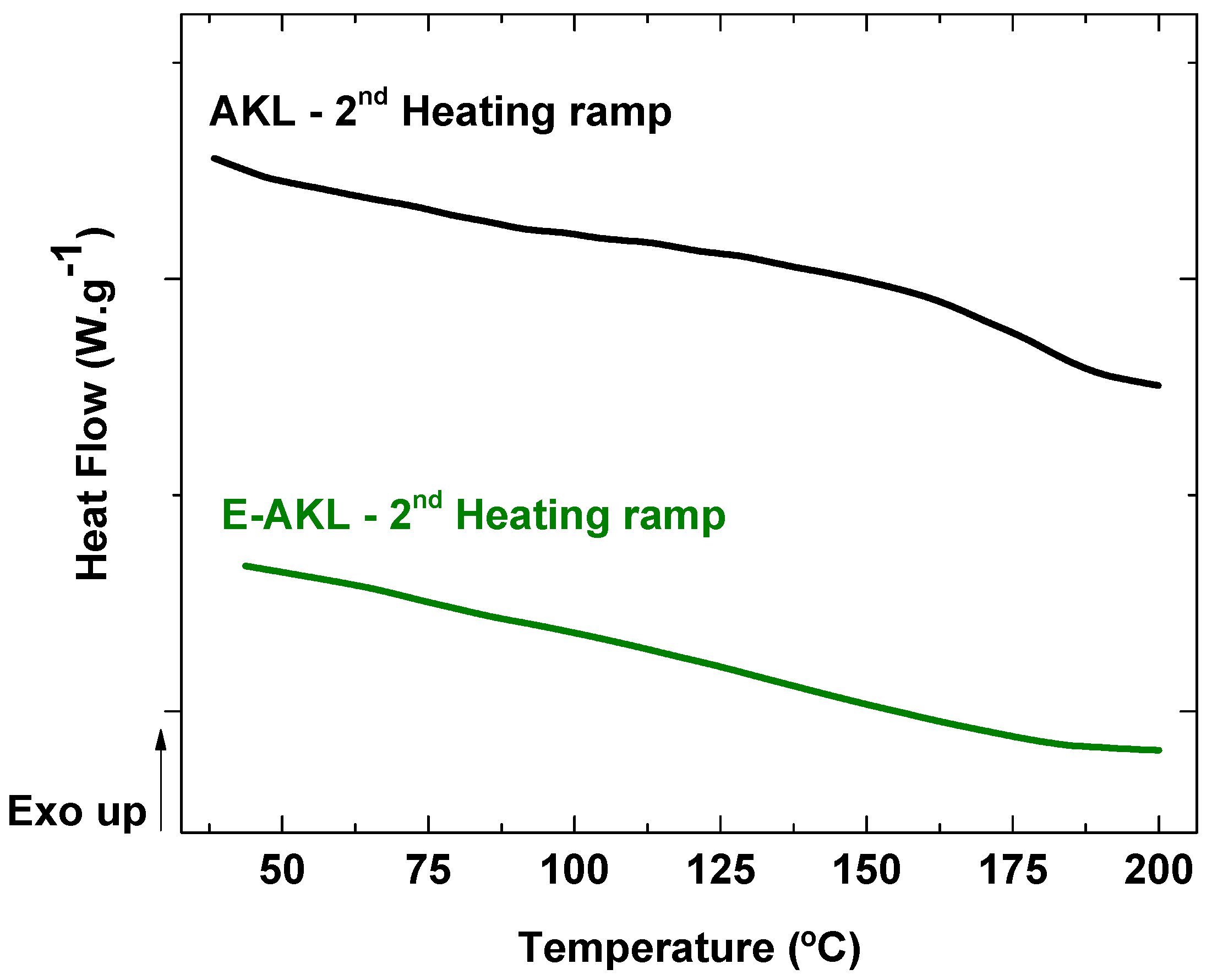
3.1.2. Differential Scanning Calorimetry
3.2. Lignin-Based Epoxy Adhesives Characterization
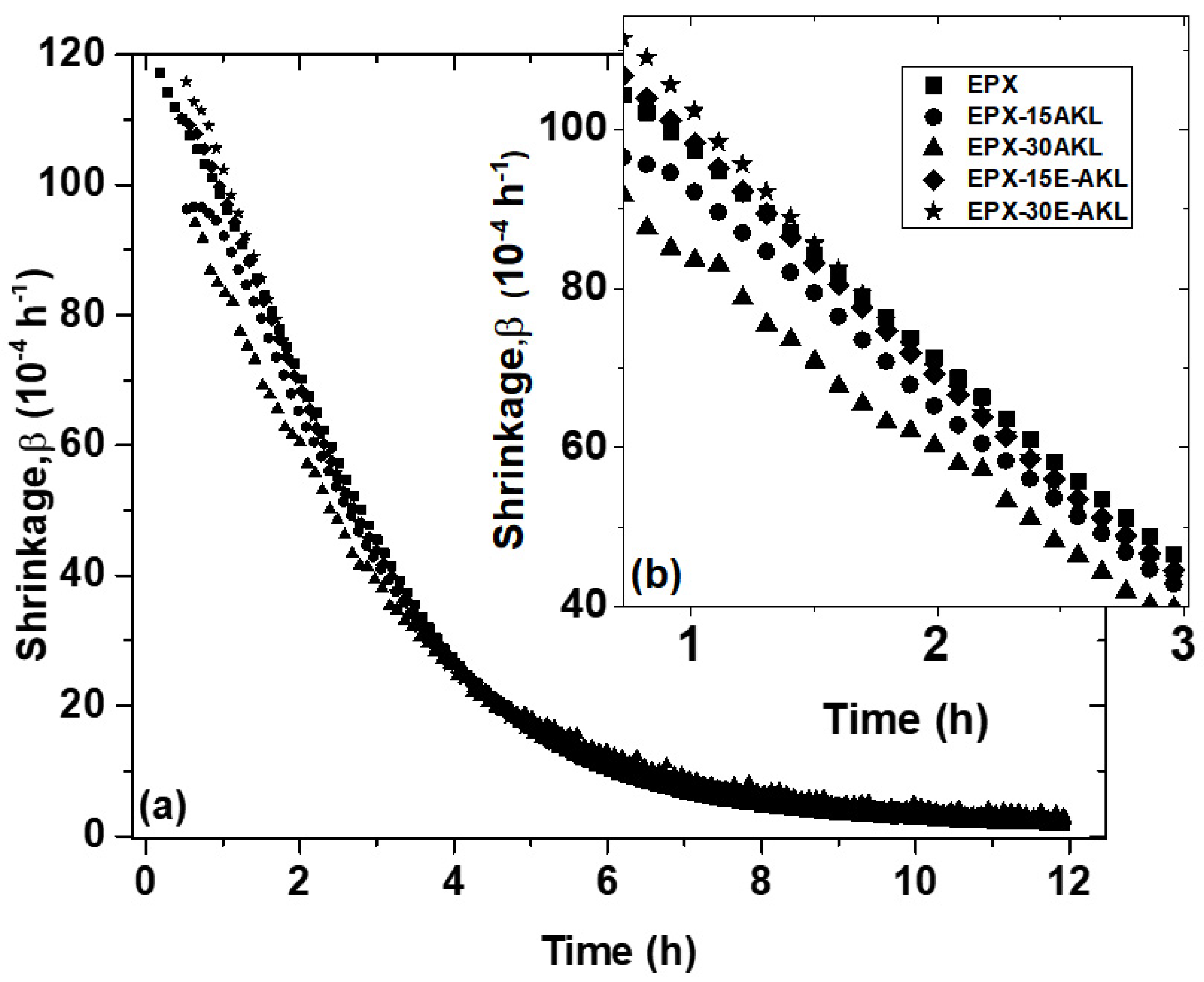
3.2.1. Temperature Modulated Optical Refractometry (TMOR)
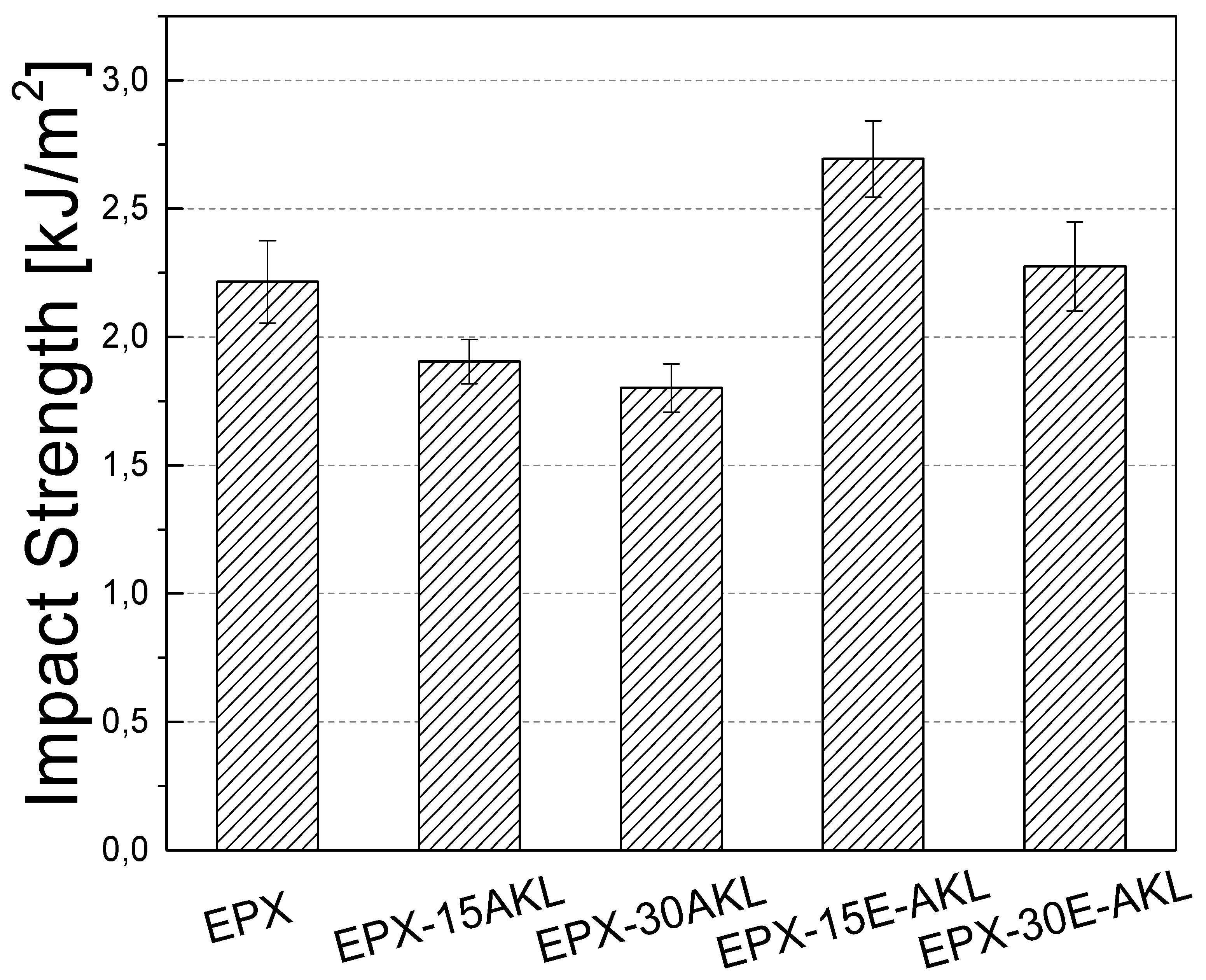
3.2.2. IZOD Impact Test
3.2.3. Lap Shear Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy Resin Synthesis Using Low Molecular Weight Lignin Separated from Various Lignocellulosic Materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Moradi, Y.; Ahmadi, M.; Amiri, S.; Naebe, M. Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin. Polymers (Basel) 2017, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and Characterization of Petroleum and Biobased Epoxy Resins: A Review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Jia, Z.; Hui, D.; Yuan, G.; Lair, J.; Lau, K.T.; Xu, F. Mechanical Properties of an Epoxy-Based Adhesive under High Strain Rate Loadings at Low Temperature Environment. Compos. Part B Eng. 2016, 105, 132–137. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Oh, S.; Lee, J.; Roh, H.G.; Park, J. Changes of Lignin Molecular Structures in a Modification of Kraft Lignin Using Acid Catalyst. Materials (Basel) 2016, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J. Bisphenol A–Sources, Toxicity and Biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Fan, L.; Jiang, Y.; Cao, L.; Tang, Z.; Zhu, J. Synthesis and Properties of a Bio-Based Epoxy Resin with High Epoxy Value and Low Viscosity. ChemSusChem 2014, 7, 555–562. [Google Scholar] [CrossRef]
- Fernandes, F.C.; Kirwan, K.; Lehane, D.; Coles, S.R. Epoxy Resin Blends and Composites from Waste Vegetable Oil. Eur. Polym. J. 2017, 89, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Stemmelen, M.; Lapinte, V.; Habas, J.-P.; Robin, J.-J. Plant Oil-Based Epoxy Resins from Fatty Diamines and Epoxidized Vegetable Oil. Eur. Polym. J. 2015, 68, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Karak, N. Vegetable Oil-Based Flame Retardant Epoxy/Clay Nanocomposites. Polym. Degrad. Stab. 2009, 94, 1948–1954. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Shakeri, A. Analysis and Testing of Bisphenol A—Free Bio-Based Tannin Epoxy-Acrylic Adhesives. Polymers 2016, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, N.; Salimi, A.; Zohuriaan-Mehr, M.J.; Vafayan, M.; Meyer, W. Bio-Based Thermosetting Epoxy Foam: Tannic Acid Valorization toward Dye-Decontaminating and Thermo-Protecting Applications. J. Hazard. Mater. 2018, 357, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized Green Tea Tannins as Phenolic Prepolymers for Bio-Based Epoxy Resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.-R.M.; El-Sockary, M.A. Rosin Based Epoxy Coating: Synthesis, Identification and Characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Huo, L.; Wang, D.; Liu, H.; Jia, P.; Gao, J. Cytoxicity, Dynamic and Thermal Properties of Bio-Based Rosin-Epoxy Resin/ Castor Oil Polyurethane/ Carbon Nanotubes Bio-Nanocomposites. J. Biomater. Sci. Polym. Ed. 2016, 27, 1100–1114. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin, GmbH Germany, 2017. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully Biobased Epoxy Resins from Fatty Acids and Lignin. Molecules 2020, 25, 1158. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset Materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [Green Version]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the Structure of Softwood Kraft Lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Delmas, G.H.; Benjelloun-Mlayah, B.; Bigot, Y.L.; Delmas, M. Biolignin Based Epoxy Resins. J. Appl. Polym. Sci. 2013, 127, 1863–1872. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Rusan, V.; Macoveanu, M.M.; Cazacu, G.; Lipsa, R.; Vasile, C.; Stoleriu, A.; Ioanid, A. Lignin/epoxy composites. Compos. Sci. Technol. 1993, 48, 317–323. [Google Scholar] [CrossRef]
- Feng, P.; Chen, F. Preparation and Characterization of Acetic Acid Lignin-Based Epoxy Blends. BioResources 2012, 7, 2860–2870. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W.G. Engineering Plastics from Lignin. 22. Cure of Lignin Based Epoxy Resins. J. Adhes. 1993, 40, 229–241. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W. Engineering Plastics from Lignin, 23. Network Formation of Lignin-Based Epoxy Resins. Macromol. Chem. Phys. 2018, 195, 65–80. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Sustainable Lignin-Based Epoxy Resins Cured with Aromatic and Aliphatic Amine Curing Agents: Curing Kinetics and Thermal Properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Quantitative Phosphorus-31 NMR Analysis of Lignins, a New Tool for the Lignin Chemist. J. Wood Chem. Technol. 1994, 14, 45–63. [Google Scholar] [CrossRef]
- Faix, O.; Robert, D.; Neirinck, V.; Argyropoulos, D.S. Determination of Hydroxyl Groups in Lignins. Evaluation of 1H-, 13C-, 31P-NMR, FTIR and Wet Chemical Methods. Holzforschung 1994, 48, 387–394. [Google Scholar] [CrossRef]
- Malutan, T.; Nicu, R.; Popa, V.I. Lignin Modification by Epoxidation. BioResources 2008, 3, 1371–1376. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward Thermoplastic Lignin Polymers. Part 1. Selective Masking of Phenolic Hydroxyl Groups in Kraft Lignins via Methylation and Oxypropylation Chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Müller, U.; Philipp, M.; Thomassey, M.; Sanctuary, R.; Krüger, J.K. Temperature Modulated Optical Refractometry: A Quasi-Isothermal Method to Determine the Dynamic Volume Expansion Coefficient. Thermochim. Acta 2013, 555, 17–22. [Google Scholar] [CrossRef]
- Aleksandrova, R.; Philipp, M.; Müller, U.; Rioboo, R.J.; Ostermeyer, M.; Sanctuary, R.; Müller-Buschbaum, P.; Krüger, J.K. Phase Instability and Molecular Kinetics Provoked by Repeated Crossing of the Demixing Transition of PNIPAM Solutions. Langmuir 2014, 30, 11792–11801. [Google Scholar] [CrossRef] [PubMed]
- Philipp, M.; Nies, C.; Ostermeyer, M.; Possart, W.; Krüger, J.K. Thermal Glass Transition beyond Kinetics of a Non-Crystallizable Glass-Former. Soft Matter 2018, 14, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.R.; Cristina, K.; Lixandr, D.L.; Bas, L.; Henrique, P.; Elias, G.; Garcia, S.; Jackson, D. Thermal Transitions of Cocoa Butter: A Novel Characterization Method by Temperature Modulation. Foods 2019, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Philipp, M.; Zimmer, B.; Ostermeyer, M.; Krüger, J.K. Polymerization-Induced Shrinkage and Dynamic Thermal Expansion Behavior during Network Formation of Polyurethanes. Thermochim. Acta 2019. [Google Scholar] [CrossRef]
- Jackson dos Santos, D.; Gouveia, J.R.; Philipp, M.; Augusto, A.C.; Ito, N.M.; Krüger, J.K. Temperature Modulated Optical Refractometry: A Novel and Practical Approach on Curing and Thermal Transitions Characterizations of Epoxy Resins. Polym. Test. 2019, 77, 105915. [Google Scholar] [CrossRef]
- Bauer, C.; Bohmer, R.; Moreno-Flores, S.; Richer, R.; Sillescu, H. Capacitive Scanning Dilatometry and Frequency-Dependent Thermal Expansion of Polymer Films. Phys. Rev. E 2000, 61, 1755–1764. [Google Scholar] [CrossRef]
- Ding, J.; Gu, L.; Dong, W.; Yu, H. Epoxidation Modification of Renewable Lignin to Improve the Corrosion Performance of Epoxy Coating. Int. J. Electrochem. Sci. 2016, 11, 6256–6265. [Google Scholar] [CrossRef]
- Mendis, G.P.; Hua, I.; Youngblood, J.P.; Howarter, J.A. Enhanced Dispersion of Lignin in Epoxy Composites through Hydration and Mannich Functionalization. J. Appl. Polym. Sci. 2014, 132, 1–8. [Google Scholar] [CrossRef]
- Jablonskis, A.; Arshanitsa, A.; Arnautov, A.; Telysheva, G.; Evtuguin, D. Evaluation of Ligno BoostTM Softwood Kraft Lignin Epoxidation as an Approach for Its Application in Cured Epoxy Resins. Ind. Crops Prod. 2018, 112, 225–235. [Google Scholar] [CrossRef]
- Podkościelna, B.; Sobiesiak, M.; Yadong, Z.; Gawdzik, B.; Sevastyanova, O. Preparation of lignin-containing porous microspheres through the copolymerization of lignin acrylate derivatives withstyrene and divinylbenzene. Holzforschung 2015, 69, 769–776. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis and Characterization of Hydrolysis Lignin-Based Epoxy Resins. Ind. Crops Prod. 2016, 91, 295–301. [Google Scholar] [CrossRef]
- Cui, C.; Sadeghifar, H.; Sen, S.; Argyropoulos, D.S. Toward Thermoplastic Lignin Polymers; Part II: Thermal & Polymer Characteristics of Kraft Lignin & Derivatives. BioResources 2013, 8, 864–886. [Google Scholar] [CrossRef] [Green Version]
- Muzammil, E.M.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef] [Green Version]
- De, B.; Gupta, K.; Mandal, M.; Karak, N. Biodegradable Hyperbranched Epoxy from Castor Oil-BasedHyperbranched Polyester Polyol. ACS Sustain. Chem. Eng. 2014, 2, 445–453. [Google Scholar] [CrossRef]
- Harani, H.; Fellahi, S.; Bakar, M. Toughening of Epoxy Resin Using Synthesized Polyurethane Prepolymer Based on Hydroxyl-Terminated Polyesters. J. Appl. Polym. Sci. 1998, 70, 2603–2618. [Google Scholar] [CrossRef]
- Galpaya, D.G.D.; Fernando, J.F.S.; Rintoul, L.; Motta, N.; Waclawik, E.R.; Yan, C.; George, G.A. The Effect of Graphene Oxide and Its Oxidized Debris on the Cure Chemistry and Interphase Structure of Epoxy Nanocomposites. Polymer (Guildf) 2015, 71, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of Completely Bio-Based Epoxy Networks Derived from Epoxidized Linseed and Castor Oil Cured with Citric Acid. Polym. Adv. Technol. 2018, 29, 2080–2090. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Novel High Performance Tough Hyperbranched Epoxy by an A2 + B3 Polycondensation Reaction. J. Mater. Chem. A 2013, 1, 348–353. [Google Scholar] [CrossRef]
- Ito, N.M.; Gouveia, J.R.; Vidotti, S.E.; Julienne, M.; Ferreira, G.C.; Jackson, D.; Minako, N.; Gouveia, J.R.; Vidotti, S.E.; Julienne, M. Interplay of Polyurethane Mechanical Properties and Practical Adhesion of Flexible Multi-Layer Laminates. J. Adhes. 2019, 1–14. [Google Scholar] [CrossRef]
- Brandrup, E.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Abdul Khalil, H.P.S.; Marliana, M.M.; Alshammari, T. Material Properties of Epoxy-Reinforced Biocomposites with Lignin from Empty Fruit Bunch as Curing Agent. BioResources 2011, 6, 5206–5223. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Introduction and Adhesion Theories. In Handbook of Adhesives and Surface Preparation; Elsevier: Oxford, UK, 2011; pp. 3–13. [Google Scholar] [CrossRef]
- De Junior, R.R.S.; Gouveia, J.R.; Ito, N.M.; dos Santos, D.J. Failure Prediction of Hybrid Composite Using Arcan’s Device and Drucker-Prager Model. Polym. Test. 2017. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Ramos, R.; Júnior, D.S.; Orzari, A.; Adriano, S.; Jackson, D. Effect of Soft Segment Molecular Weight and NCO: OH Ratio on Thermomechanical Properties of Lignin-Based Thermoplastic Polyurethane Adhesive. Eur. Polym. J. 2020, 131. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Natansohn, A.; Wang, J. Structure–Properties Relations of Thermally Cured Epoxy–Lignin Polyblends. J. Appl. Polym. Sci. 1991, 42, 1537–1550. [Google Scholar] [CrossRef]
- Feldman, D.; Khoury, M. Epoxy-Lignin Polyblends. Part II. Adhesive Behavior and Weathering. J. Adhes. Sci. Technol. 1988, 2, 107–116. [Google Scholar] [CrossRef]
- Wang, J.; Banu, D.; Feldman, D. Epoxy-Lignin Polyblends: Effects of Various Components on Adhesive Properties. J. Adhes. Sci. Technol. 1992, 6, 58–598. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A Lignin-Epoxy Resin Derived from Biomass as an Alternative to Formaldehyde-Based Wood Adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Kong, X.; Xu, Z.; Guan, L.; Di, M. Study on Polyblending Epoxy Resin Adhesive with Lignin I-Curing Temperature. Int. J. Adhes. Adhes. 2014, 48, 75–79. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and Characterization of Lignin-Polyurethane Based Wood Adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Lima García, J.; Pans, G.; Phanopoulos, C. Use of Lignin in Polyurethane-Based Structural Wood Adhesives. J. Adhes. 2018, 94, 814–828. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C. Curing Kinetics and Mechanical Properties of Bio-Based Epoxy Composites Comprising Lignin-Based Epoxy Resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, J.R.; Garcia, G.E.S.; Antonino, L.D.; Tavares, L.B.; dos Santos, D.J. Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive. Molecules 2020, 25, 2513. https://doi.org/10.3390/molecules25112513
Gouveia JR, Garcia GES, Antonino LD, Tavares LB, dos Santos DJ. Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive. Molecules. 2020; 25(11):2513. https://doi.org/10.3390/molecules25112513
Chicago/Turabian StyleGouveia, Julia R., Guilherme E. S. Garcia, Leonardo Dalseno Antonino, Lara B. Tavares, and Demetrio J. dos Santos. 2020. "Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive" Molecules 25, no. 11: 2513. https://doi.org/10.3390/molecules25112513
APA StyleGouveia, J. R., Garcia, G. E. S., Antonino, L. D., Tavares, L. B., & dos Santos, D. J. (2020). Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive. Molecules, 25(11), 2513. https://doi.org/10.3390/molecules25112513





