Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Sewage Sludge Ash
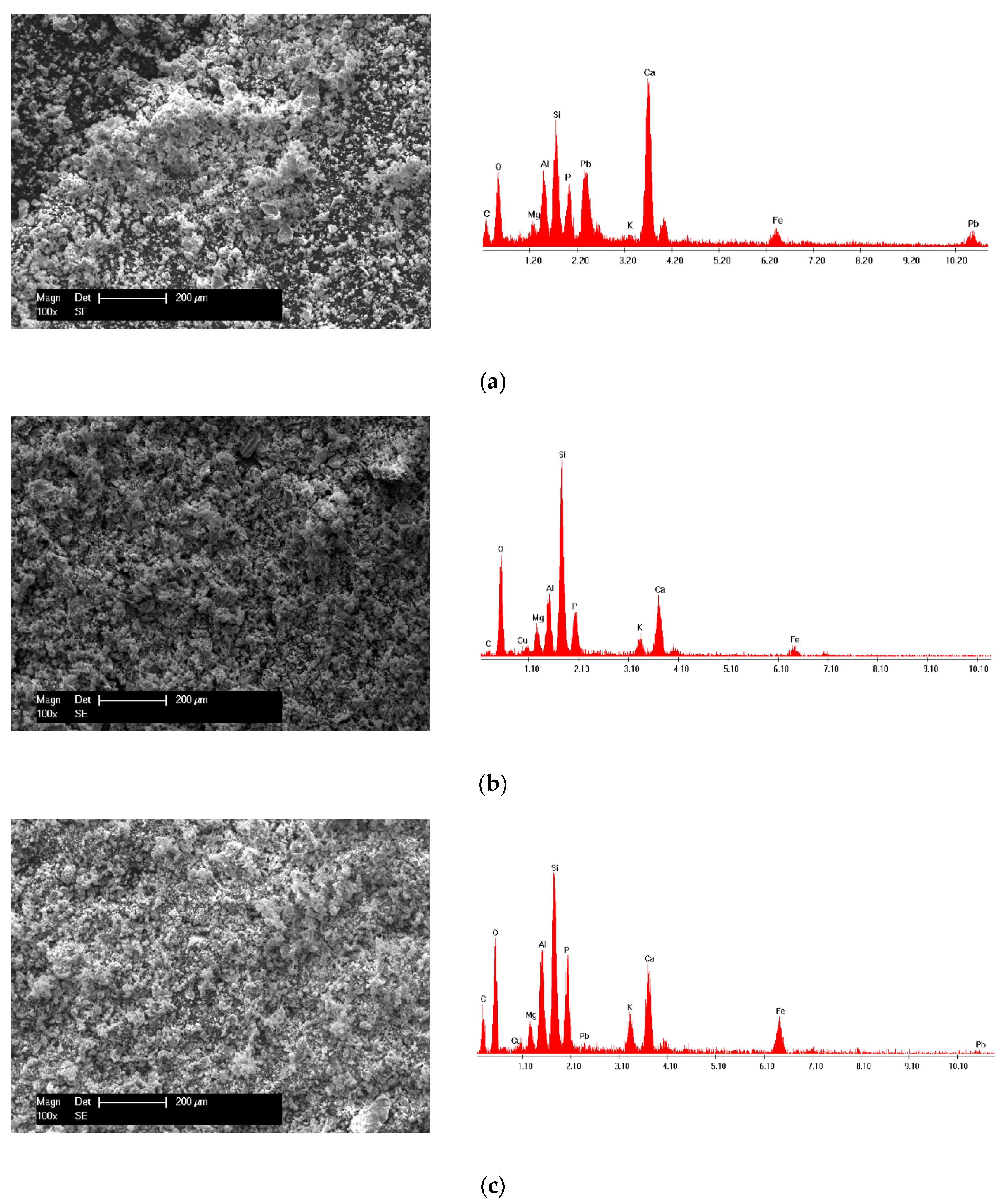
XRD and SEM–EDX Analysis
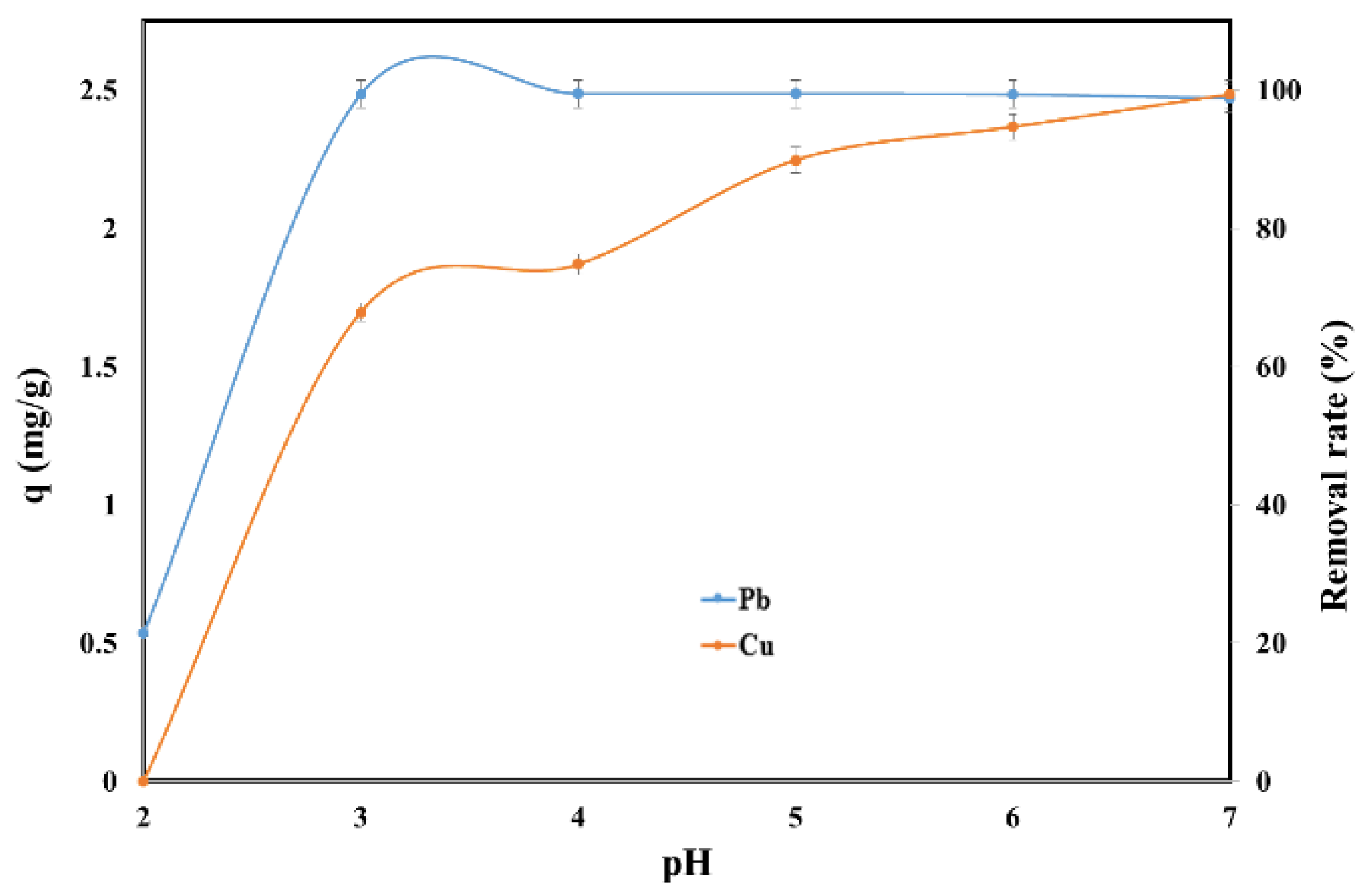
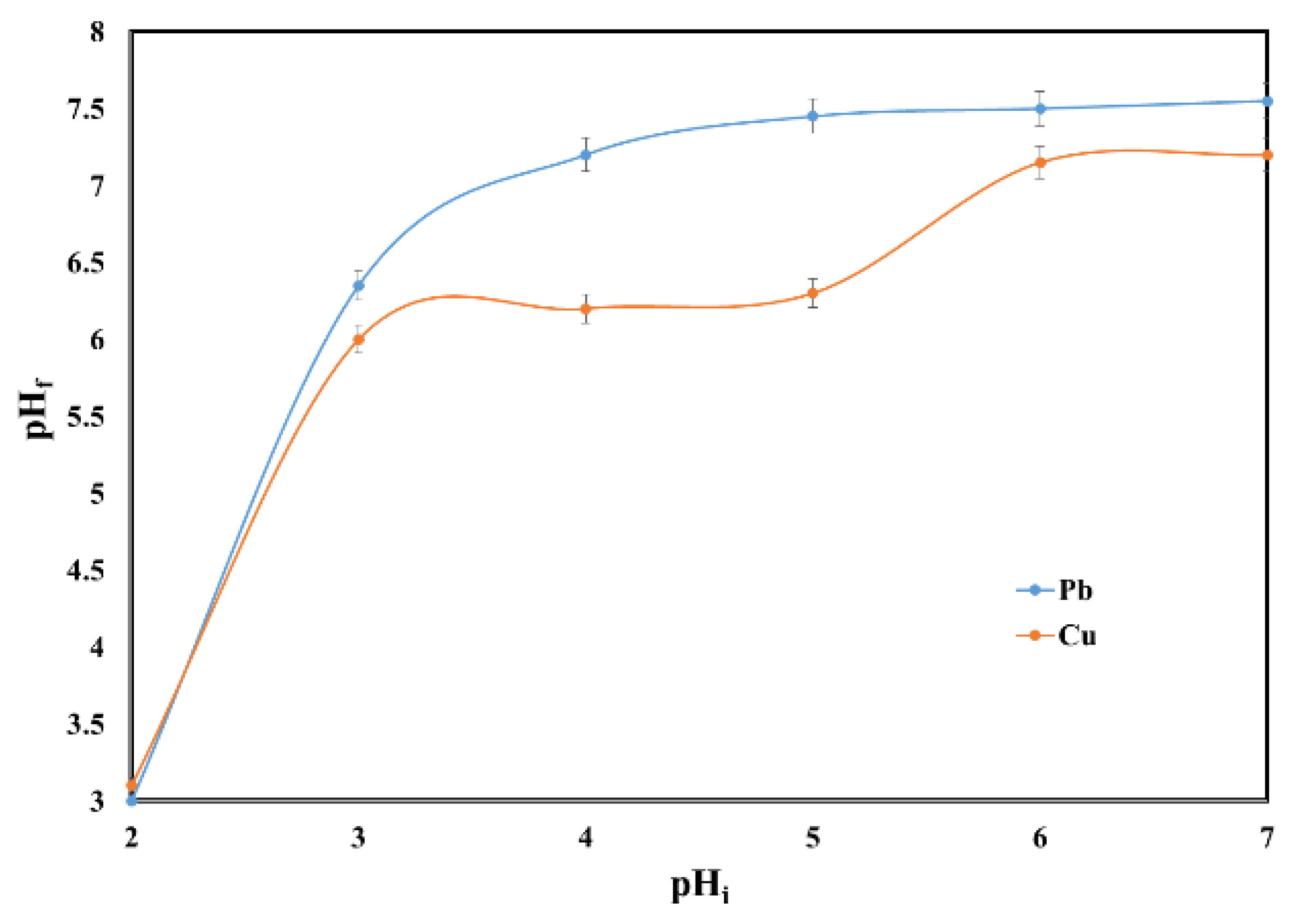
2.2. Effect of pH
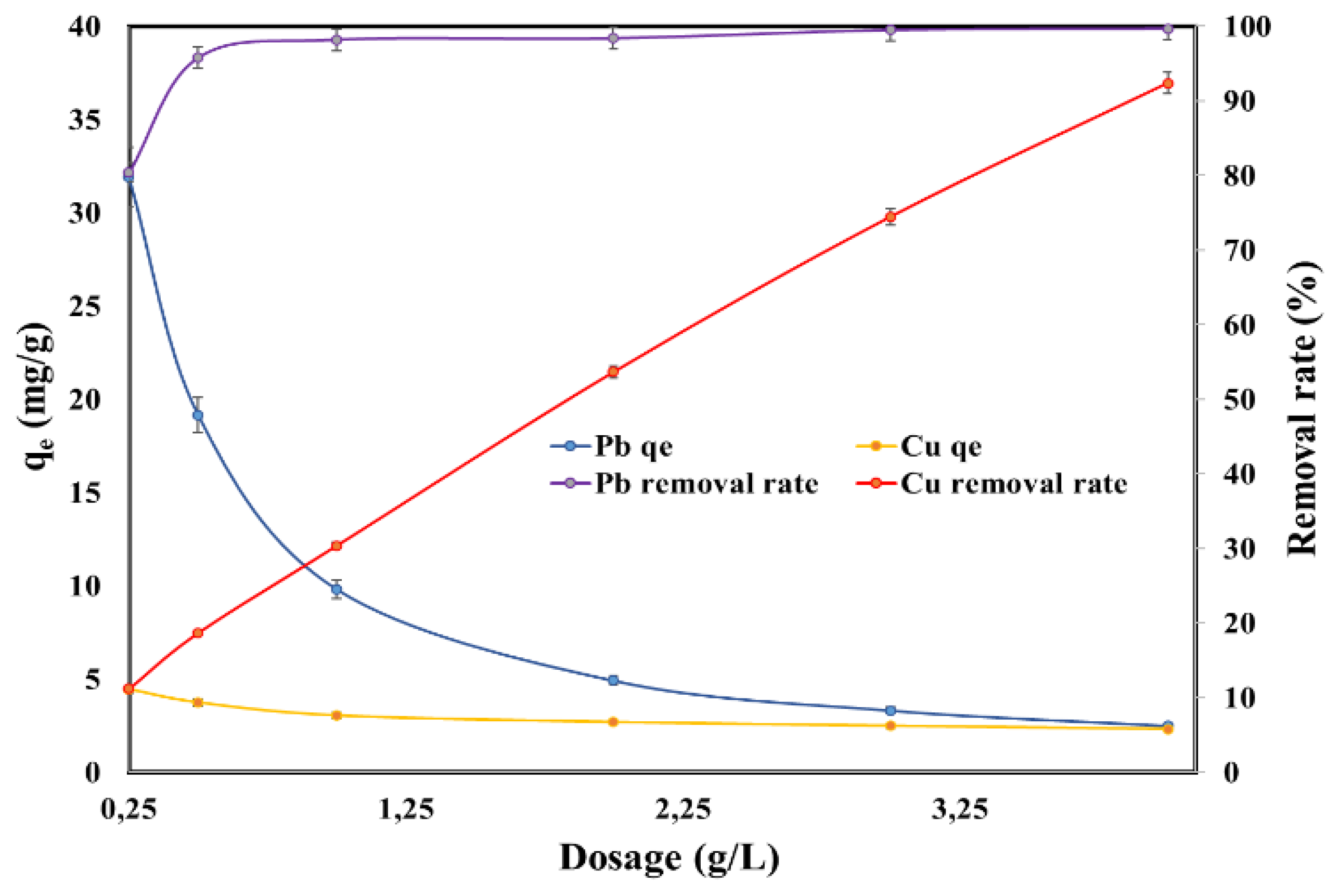
2.3. Effect of Adsorbent Dosage
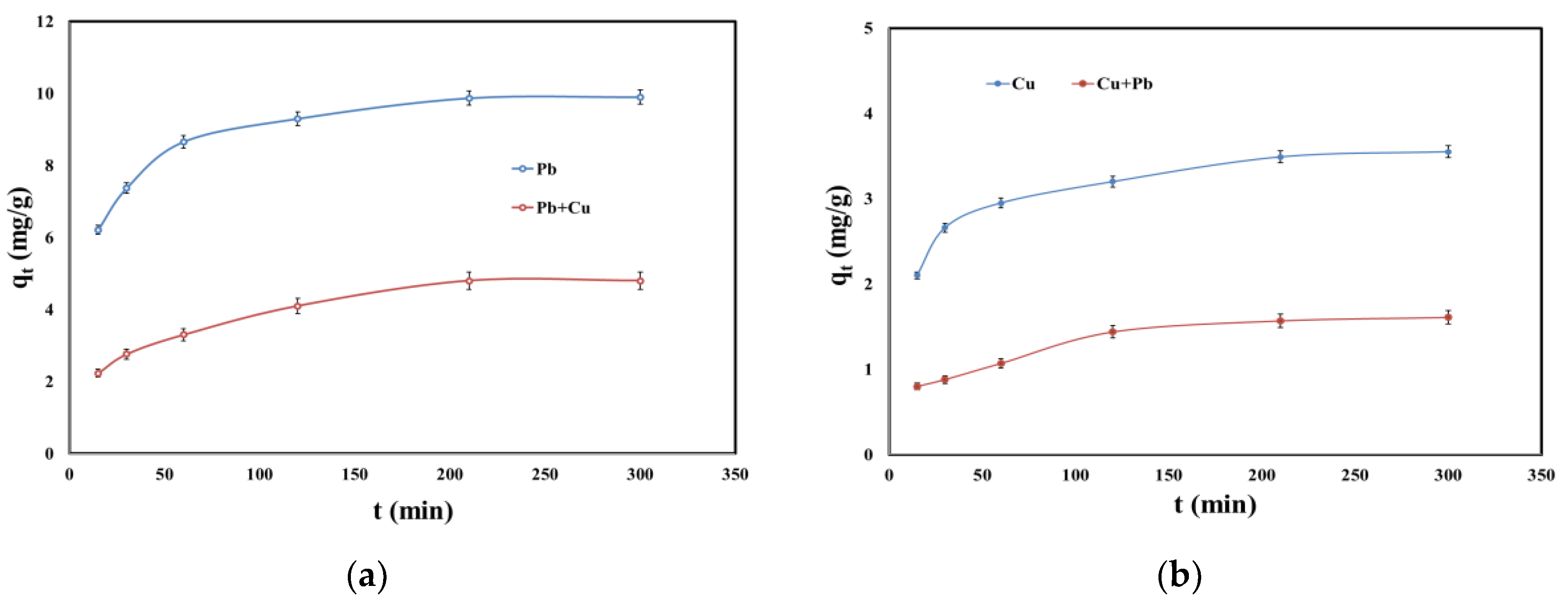
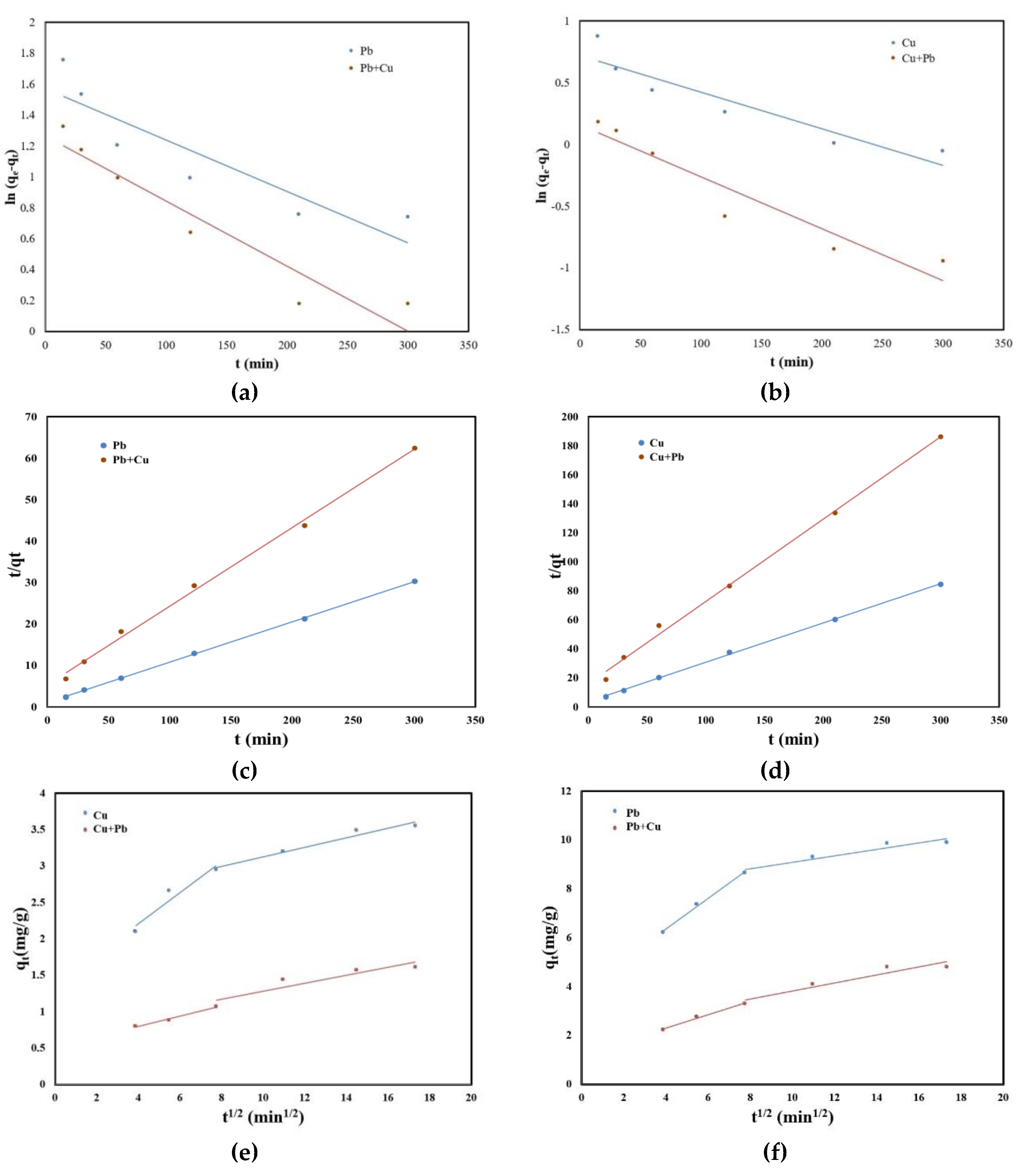
2.4. Kinetic Studies
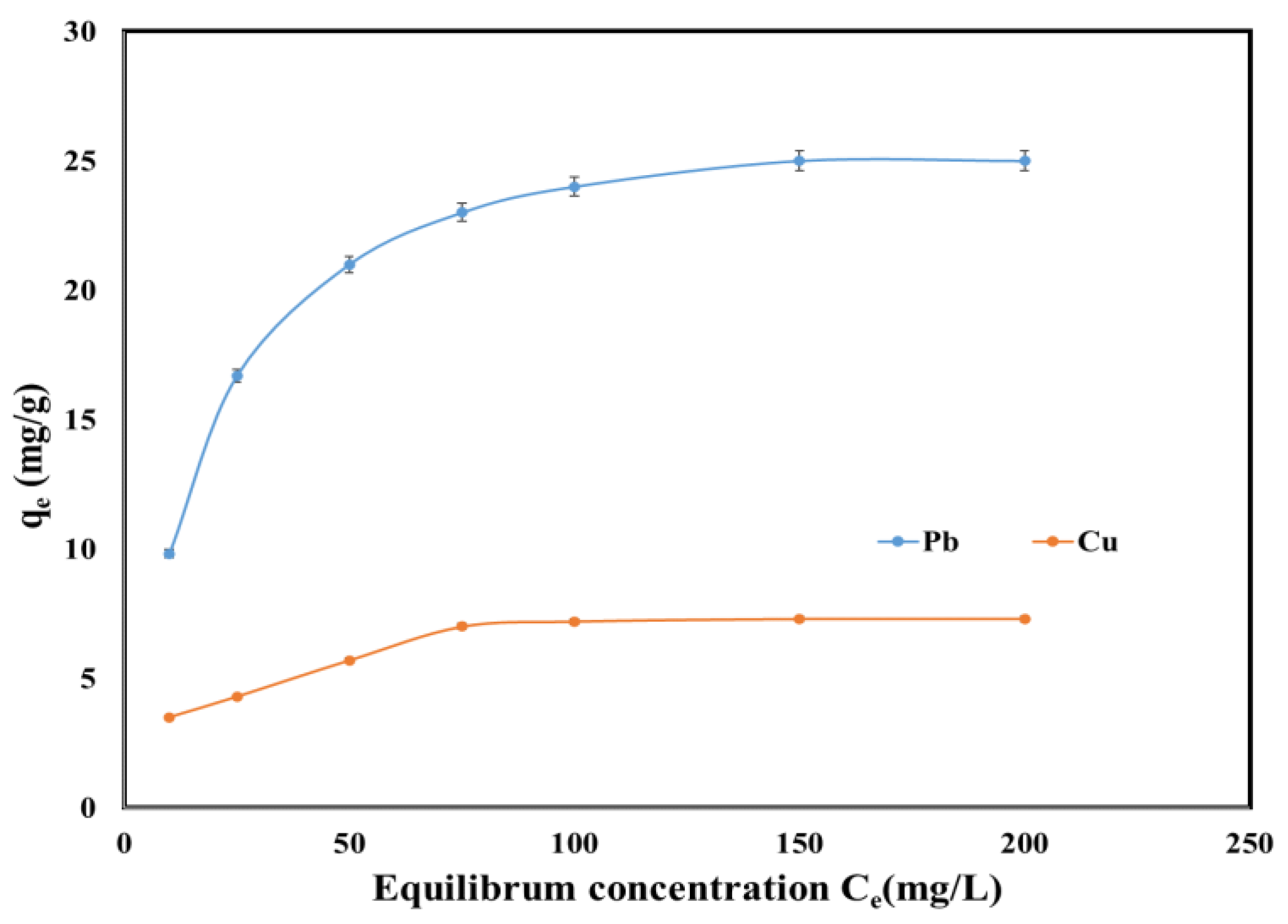
2.5. Equilibrium Studies
2.6. Adsorption Mechanism and Performance
3. Materials and Methods
3.1. Materials
3.2. Heavy Metal Ion Adsorption
3.3. Adsorbent Characterization
4. Conclusions
- capitalization of waste and therefore reduction of waste disposal;
- low-cost and efficiency;
- alkaline effects.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cieslik, B.M.; Namiesnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Jung, J.M.; Oh, J.I.; Kim, J.G.; Kwon, H.H.; Park, Y.K.; Kwon, E.E. Valorization of sewage sludge via non-catalytic transesterification. Environ. Int. 2019, 131, 105035. [Google Scholar] [CrossRef]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å. Singh, Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Đurđević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case Study of Croatia. Energies 2019, 12, 1927. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; San Martín, J.; Rodriguez, J.J.; de la Rubia, M.A. Anaerobic co-digestion of the process water from waste activated sludge hydrothermally treated with primary sewage sludge. A new approach for sewage sludge management. Renew. Energy 2020, 146, 435–443. [Google Scholar] [CrossRef]
- Suleiman, H.; Rorat, A.; Grobelak, A.; Grosser, A.; Milczarek, M.; Płytycz, B.; Kacprzak, M.; Vandenbulcke, F. Determination of the performance of vermicomposting process applied to sewage sludge by monitoring of the compost quality and immune responses in three earthworm species: Eisenia fetida, Eisenia andrei and Dendrobaena veneta. Bio-resour. Technol. 2017, 241, 103–112. [Google Scholar] [CrossRef]
- Ma, W.; Tang, Y.; Wu, P.; Xia, Y. Sewage sludge incineration ash for coimmobilization of lead, zinc and copper: Mechanisms of metal incorporation and competition. Waste Manage. 2019, 99, 102–111. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, G.; Yan, B.; Cheng, Z.; Ma, W. Behaviour of mercury during Co-incineration of sewage sludge and municipal solid waste. J. Clean. Prod. 2020, 253. [Google Scholar] [CrossRef]
- Rezaee, F.; Danesh, S.; Tavakkolizadeh, M.; Khatami, M.M. Investigating chemical, physical and mechanical properties of eco-cement produced using dry sewage sludge and traditional raw materials. J. Clean. Prod. 2019, 214, 749–757. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Li, J.S.; Lu, J.X.; Cheeseman, C.; Poon, C.S. Recycling incinerated sewage sludge ash (ISSA) as a cementitious binder by lime activation. J. Clean. Prod. 2020, 244. [Google Scholar] [CrossRef]
- Chakraborty, S.; Jo, B.W.; Jo, J.H.; Baloch, Z. Effectiveness of sewage sludge ash combined with waste pozzolanic minerals in developing sustainable construction material: An alternative approach for waste management. J. Clean. Prod. 2017, 153, 253–263. [Google Scholar] [CrossRef]
- Semerci, N.; Kunt, B.; Calli, B. Phosphorus recovery from sewage sludge ash with bioleaching and electrodialysis. Int. Biodeterior. Biodegrad. 2019, 144. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.S.; Poon, C.S. Using incinerated sewage sludge ash as a high-performance adsorbent for lead removal from aqueous solutions: Performances and mechanisms. Chemosphere 2019, 226, 587–596. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.S.; Poon, C.S. Recycling of incinerated sewage sludge ash as an adsorbent for heavy metals removal from aqueous solutions. J. Environ. Manag. 2019, 247, 509–517. [Google Scholar] [CrossRef]
- Tsai, C.K.; Doong, R.; Hung, H.Y. Sustainable valorization of mesoporous aluminosilicate composite from display panel glasses waste for adsorption of heavy metal ions. Sci. Total Environ. 2019, 673, 337–346. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Ali, O.; Wei, Y.; Zhang, S.; Zhang, Y.; Cai, T.; Liu, C.; Luo, S. Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J. Hazard. Mater. 2018, 344, 1034–1042. [Google Scholar] [CrossRef]
- Wang, S.; Terdkiatburana, T.; Tadé, M.O. Single and co-adsorption of heavy metals and humic acid on fly ash. Sep. Purif. Technol. 2008, 58, 353–358. [Google Scholar] [CrossRef]
- Pan, S.C.; Lin, C.C.; Tseng, D.H. Reusing sewage sludge ash as adsorbent for copper removal from wastewater. Resour. Conserv. Recycl. 2003, 39, 79–90. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/ipcs/assessment/public_health/lead/en/ (accessed on 5 April 2020).
- Onundi, Y.B.; Mamun, A.A.; Al Khatib, M.F.; Ahmed, Y.M. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Tech. 2010, 7, 751–758. [Google Scholar] [CrossRef]
- Militaru, B.A.; Pode, R.; Manea, F.; Linul, P.A. Simple and Combined Acidic Extraction of Phosphorus from Sewage Sludge Ash. Rev. Chim. 2019, 70, 133–139. [Google Scholar] [CrossRef]
- Nikolaychuk, P.A. The revised potential – pH diagram for Pb – H2O system. Ovidius Univ. Ann. Chem. 2018, 29, 55–67. [Google Scholar] [CrossRef]
- Escudero, R.G.; Espinoza Estrada, E.; Miranda, F. Precipitation of Lead Species in a Pb – H 2 O System. Res. J. Recent Sci. 2013, 2, 1–8. [Google Scholar]
- Abdullah, S.R.S.; Rahman, R.A.; Mohamad, A.B.; Mustafa, M.M.; Khadum, A.A.H. Removal of Mixed Heavy Metals by Hydroxide Precipitation. J. Kejuruteraan 1999, 11, 85–101. [Google Scholar]
- Huang, H.-H. The Eh-pH Diagram and Its Advances. Metals 2016, 6, 23. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Mater. 2020, 12, 12. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Hefny, M.; El-Chaghaby, G.A.F. Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int. J. Environ. Sci. Tech. 2007, 4, 67–73. [Google Scholar] [CrossRef]
- Gercel, O.; Gercel, H.F. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Eng. J. 2007, 132, 289–297. [Google Scholar] [CrossRef]
- Benzaoui, T.; Selatnia, A.; Djabali, D. Adsorption of copper (II) ions from aqueous solution using bottom ash of expired drugs incineration. Adsorpt. Sci. Technol. 2018, 36, 114–129. [Google Scholar] [CrossRef]
- Aydın, H.; Bulut, Y.; Yerlikaya, C. Removal of copper (II) from aqueous solution by adsorption ontolow-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenemine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Abdelkader, N.B.-H.; Bentouami, A.; Derriche, Z.; Bettahar, N.; de Ménorval, L.-C. Synthesis and characterization of Mg–Fe layer double hydroxides and its application on adsorption of Orange G from aqueous solution. Chem. Eng. J. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Ertugay, N.; Malkoc, E. Adsorption isotherm, kinetic, and thermodynamic studies for methylene blue from aqueous solution by needles of Pinus sylvestris L. Pol. J. Environ. Stud. 2014, 23, 1995–2006. [Google Scholar]
- Golban, A.; Lupa, L.; Cocheci, L.; Pode, R. Synthesis of MgFe Layered Double Hydroxide from Iron-Containing Acidic Residual Solution and Its Adsorption Performance. Crystals 2019, 9, 514. [Google Scholar] [CrossRef]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, Q.; Hu, Y.; He, Z. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Chen, S.B.; Ma, Y.B.; Chen, L.; Xian, K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: Single- and multi-metal competitive adsorption study. Geochem. J. 2010, 44, 233–239. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Removal of Pb(II) and chromium from wastewater using bagasse fly ash-a sugar industry waste. J. Colloid Interface Sci. 2004, 271, 321–328. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Q.; Gong, F.; Sugita, S. Adsorption of Pb(II) and mercury by rice husk ash. J. Colloid Interface Sci. 2004, 278, 1–8. [Google Scholar] [CrossRef]
- Hequet, V.; Ricou, P.; Lecuyer, I.; Cloirec, P.L. Removal of Cu2+ and Zn2+ in aqueous solutions by sorption onto mixed fly ash. Fuel 2001, 80, 851–856. [Google Scholar] [CrossRef]
- Shukla, S.P.; Tiwari, E.M.; Singh, N.B.; Pandey, S.S. Removal of Lead and Copper from Textile Wastewater using Egg Shells. Iran. J. Energy Environ. 2018, 8, 202–209. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
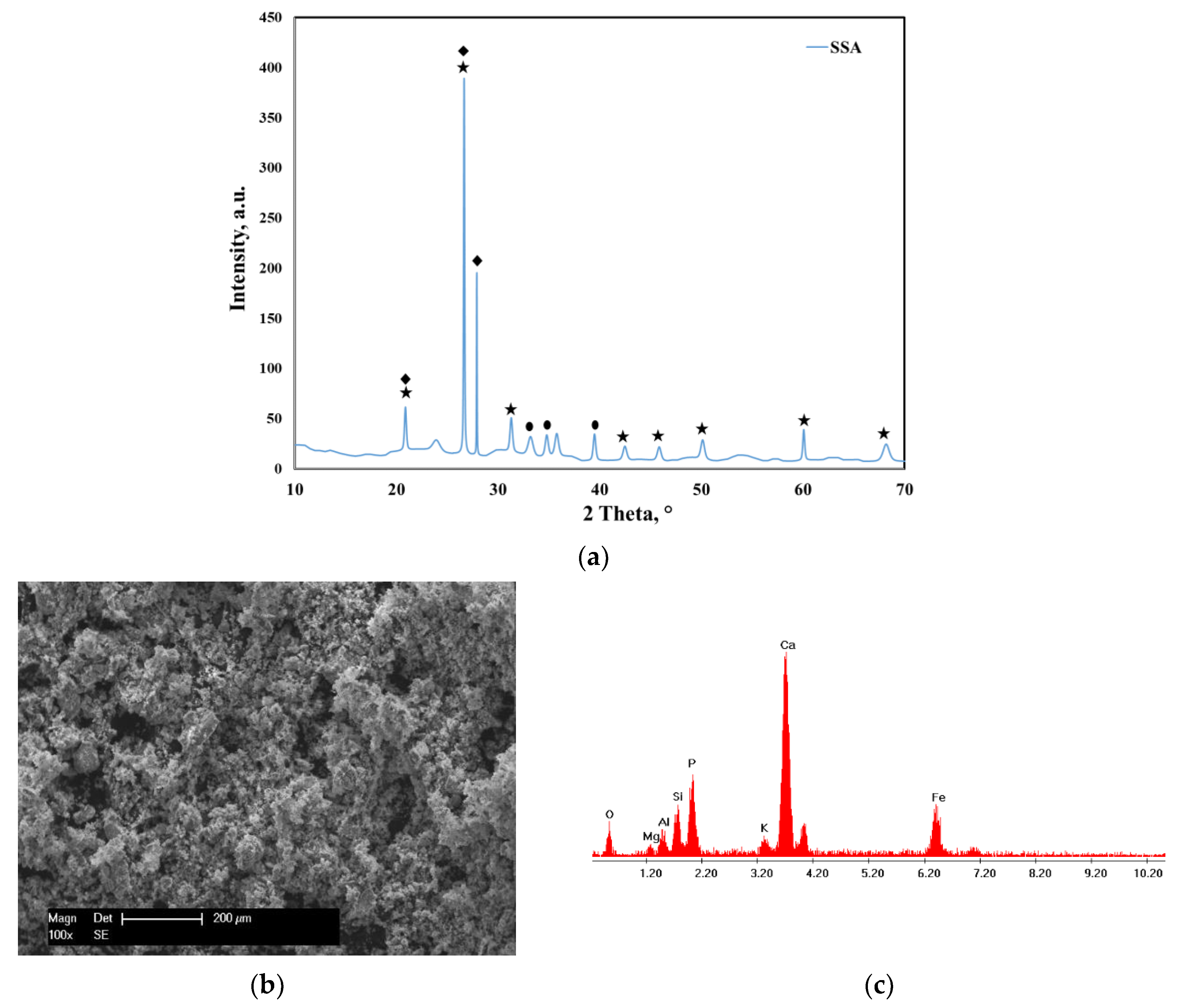

 quartz, SiO2—COD ID: 01-075-6051,
quartz, SiO2—COD ID: 01-075-6051,  calcium phosphate, Ca2P2O7—COD ID: 00-045-1061,
calcium phosphate, Ca2P2O7—COD ID: 00-045-1061,  hematite, Fe2O3—COD ID: 01-076-4579,
hematite, Fe2O3—COD ID: 01-076-4579,  lead phosphate, Pb2P2O7—COD ID: 00-013-0273,
lead phosphate, Pb2P2O7—COD ID: 00-013-0273,  copper oxide, CuO—COD ID: 00-03-0884,
copper oxide, CuO—COD ID: 00-03-0884,  lead silicate, Pb2SiO4—COD ID: 00-037-0203.
lead silicate, Pb2SiO4—COD ID: 00-037-0203.
 quartz, SiO2—COD ID: 01-075-6051,
quartz, SiO2—COD ID: 01-075-6051,  calcium phosphate, Ca2P2O7—COD ID: 00-045-1061,
calcium phosphate, Ca2P2O7—COD ID: 00-045-1061,  hematite, Fe2O3—COD ID: 01-076-4579,
hematite, Fe2O3—COD ID: 01-076-4579,  lead phosphate, Pb2P2O7—COD ID: 00-013-0273,
lead phosphate, Pb2P2O7—COD ID: 00-013-0273,  copper oxide, CuO—COD ID: 00-03-0884,
copper oxide, CuO—COD ID: 00-03-0884,  lead silicate, Pb2SiO4—COD ID: 00-037-0203.
lead silicate, Pb2SiO4—COD ID: 00-037-0203.
| Parameters | Values |
|---|---|
| BET surface area (m2/g) | 7.24 |
| Adsorption average pore diameter (nm) | 17.2 |
| Pore volume (cm3/g) | 0.029 |
| Model | Parameter | Pb | Pb + Cu | Cu | Cu + Pb |
|---|---|---|---|---|---|
| Pseudo-first-order | K1, min–1 | 0.003 | 0.004 | 0.003 | 0.004 |
| qe calc, mg/g | 4.81 | 3.55 | 2.05 | 1.17 | |
| qe exp, mg/g | 9.90 | 4.80 | 3.55 | 1.61 | |
| R2 | 0.814 | 0.916 | 0.869 | 0.910 | |
| Pseudo-second-order | K2, min/(mg/g) | 0.087 | 0.007 | 0.020 | 0.02 |
| qe calc, mg/g | 10.3 | 5.27 | 3.69 | 1.76 | |
| qe exp, mg/g | 9.90 | 4.80 | 3.55 | 1.61 | |
| R2 | 0.999 | 0.997 | 0.999 | 0.996 | |
| Intraparticle diffusion | Kint | 0.628 | 0.274 | 0.213 | 0.071 |
| R2 | 0.995 | 0.991 | 0.924 | 0.983 |
| Type of Isotherm | Parameter | Pb (II) | Cu (II) |
|---|---|---|---|
| Langmuir | KL, L/mg | 0.280 | 0.079 |
| qLcalc, mg/g | 25.5 | 7.86 | |
| R2 | 0.999 | 0.998 | |
| Freundlich | KF, mg/g | 12.6 | 2.21 |
| 1/n | 0.142 | 0.240 | |
| R2 | 0.993 | 0.962 | |
| Temkin | KT, L/g | 299 | 2.06 |
| bT, J/mol | 1066 | 1949 | |
| R2 | 0.982 | 0.955 | |
| Dubinin–Radushkevich | Kad, mol2/kJ2 | 4 × 10−8 | 5 × 10−6 |
| qs, mg/g | 22.1 | 6.36 | |
| R2 | 0.825 | 0.658 |
| Metal | Pb | Cu | Pb | Cu | Pb | Cu |
|---|---|---|---|---|---|---|
| Ce (mg/L) | 10 | 75 | 150 | |||
| qe (mg/g) | 9.63 | 3.50 | 19 | 6.00 | 25 | 7.30 |
| Ca (mg/L) | 6.20 | 4.42 | 6.24 | 4.48 | 6.58 | 4.92 |
| K (mg/L) | 0.565 | 0.470 | 0.795 | 0.520 | 0.801 | 0.580 |
| Mg(mg/L) | 0.720 | 0.190 | 0.700 | 0.220 | 0.740 | 0.240 |
| Initial pH | Zn (mg/L) | Ni (mg/L) | Co (mg/L) | Mn (mg/L) | Cr (mg/L) | Al (mg/L) |
|---|---|---|---|---|---|---|
| 7 | UDL | UDL | 0.02 | 0.07 | 0.06 | 0.11 |
| 6 | UDL | UDL | 0.02 | 0.08 | 0.06 | 0.11 |
| 5 | UDL | UDL | 0.02 | 0.08 | 0.06 | 0.06 |
| 4 | UDL | UDL | 0.03 | 0.09 | 0.06 | 0.07 |
| 3 | 0.18 | UDL | 0.02 | 0.27 | 0.06 | 0.1 |
| 2 | 0.98 | UDL | 0.01 | 3.41 | 0.06 | 13.6 |
| Pb Removal | Cu Removal | ||||
|---|---|---|---|---|---|
| Adsorbent | qe (mg/g) | References | Adsorbent | qe (mg/g) | References |
| SSA, Hong Kong, China | 62.4 | [14] | SSA, Hong Kong, China | 7.62 | [15] |
| Fly ash | 2.5 | [38] | SSA | 4.10 | [19] |
| Ash of rice husk | 10.86 | [39] | Fly ash | 4.20 | [40] |
| Egg shells | 4.33 | [41] | Egg shells | 3.54 | [41] |
| SSA, Timis County, Romania | 25.0 | Present paper | SSA, Timis County, Romania | 7.50 | Present paper |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militaru, B.A.; Pode, R.; Lupa, L.; Schmidt, W.; Tekle-Röttering, A.; Kazamer, N. Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems. Molecules 2020, 25, 2559. https://doi.org/10.3390/molecules25112559
Militaru BA, Pode R, Lupa L, Schmidt W, Tekle-Röttering A, Kazamer N. Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems. Molecules. 2020; 25(11):2559. https://doi.org/10.3390/molecules25112559
Chicago/Turabian StyleMilitaru, Bogdan Adrian, Rodica Pode, Lavinia Lupa, Winfried Schmidt, Agnes Tekle-Röttering, and Norbert Kazamer. 2020. "Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems" Molecules 25, no. 11: 2559. https://doi.org/10.3390/molecules25112559
APA StyleMilitaru, B. A., Pode, R., Lupa, L., Schmidt, W., Tekle-Röttering, A., & Kazamer, N. (2020). Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems. Molecules, 25(11), 2559. https://doi.org/10.3390/molecules25112559








