Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste
Abstract
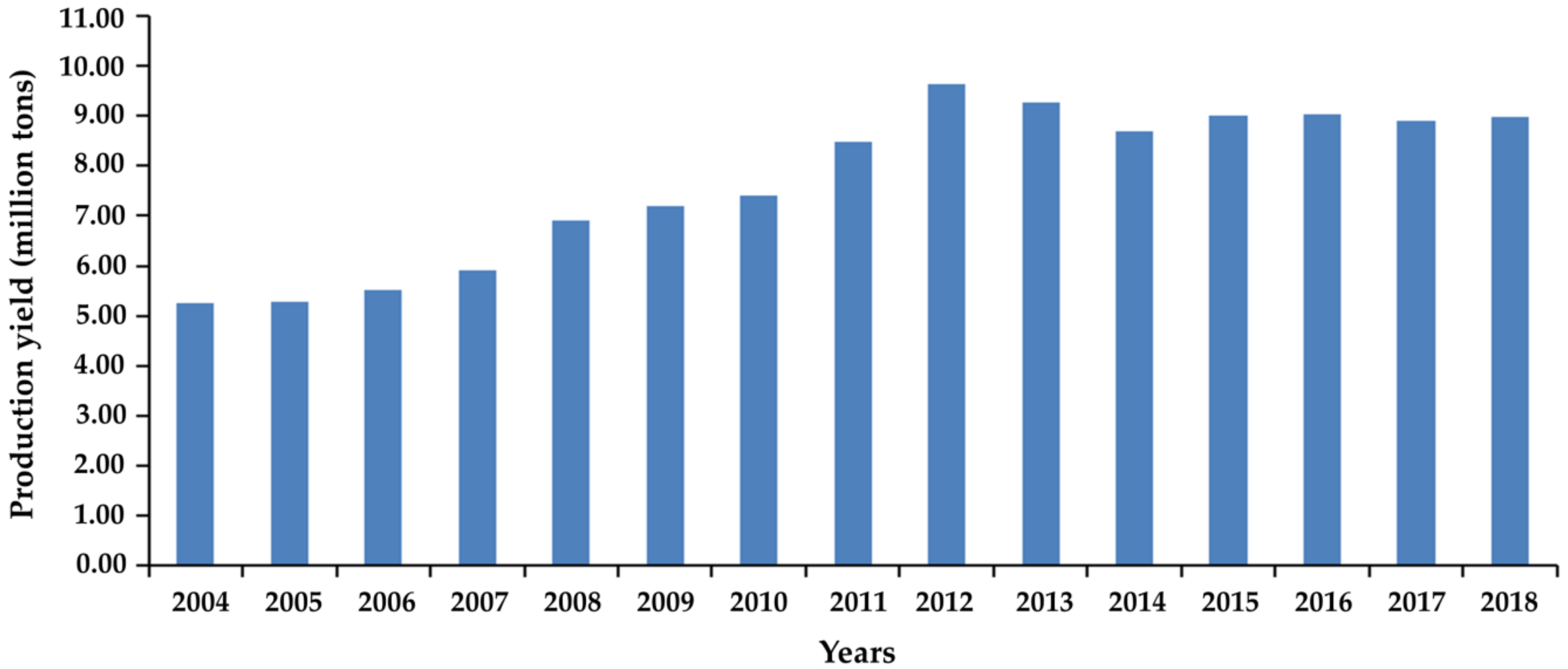
:1. Introduction
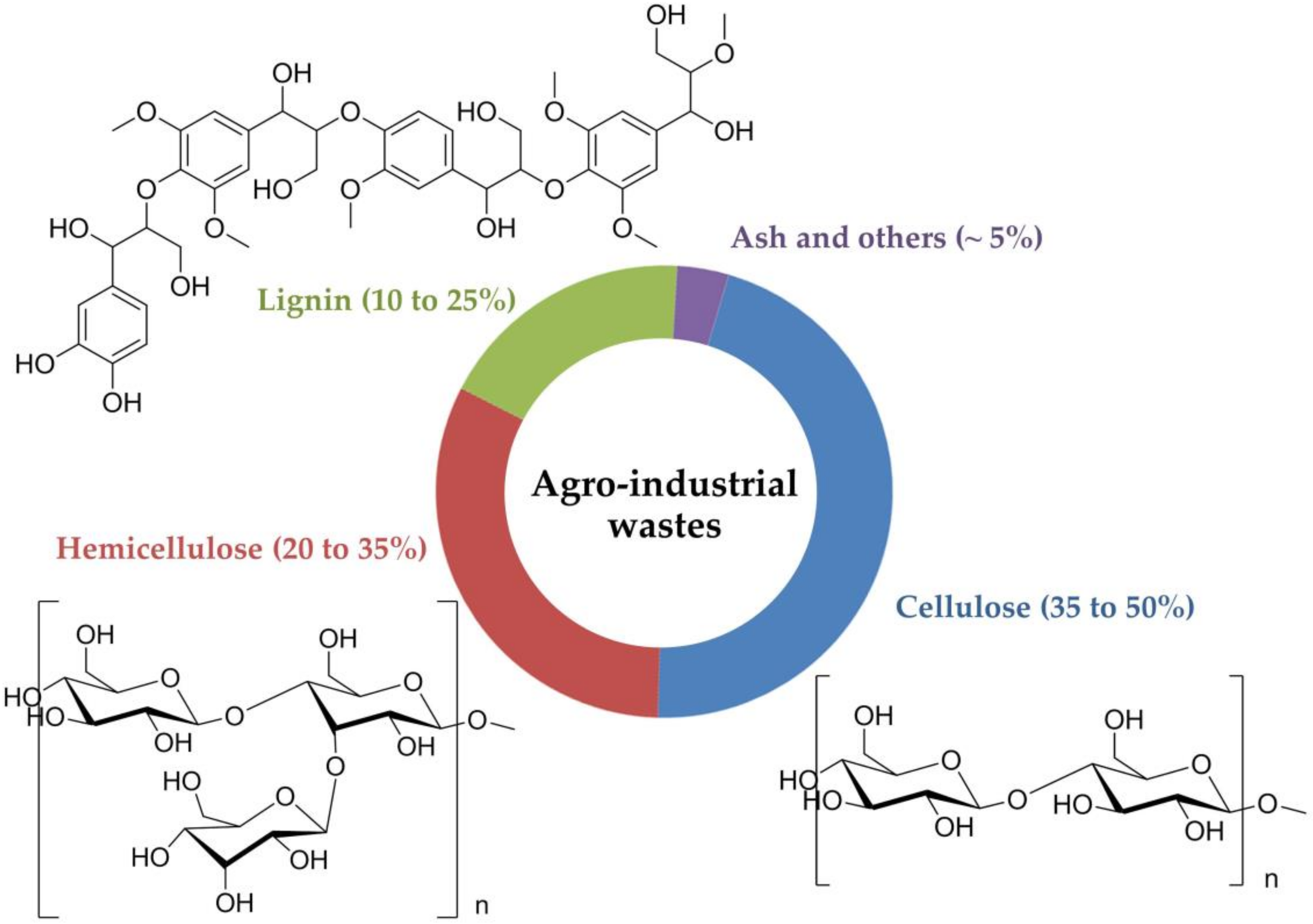
2. The Composition of Agro-Industrial Wastes
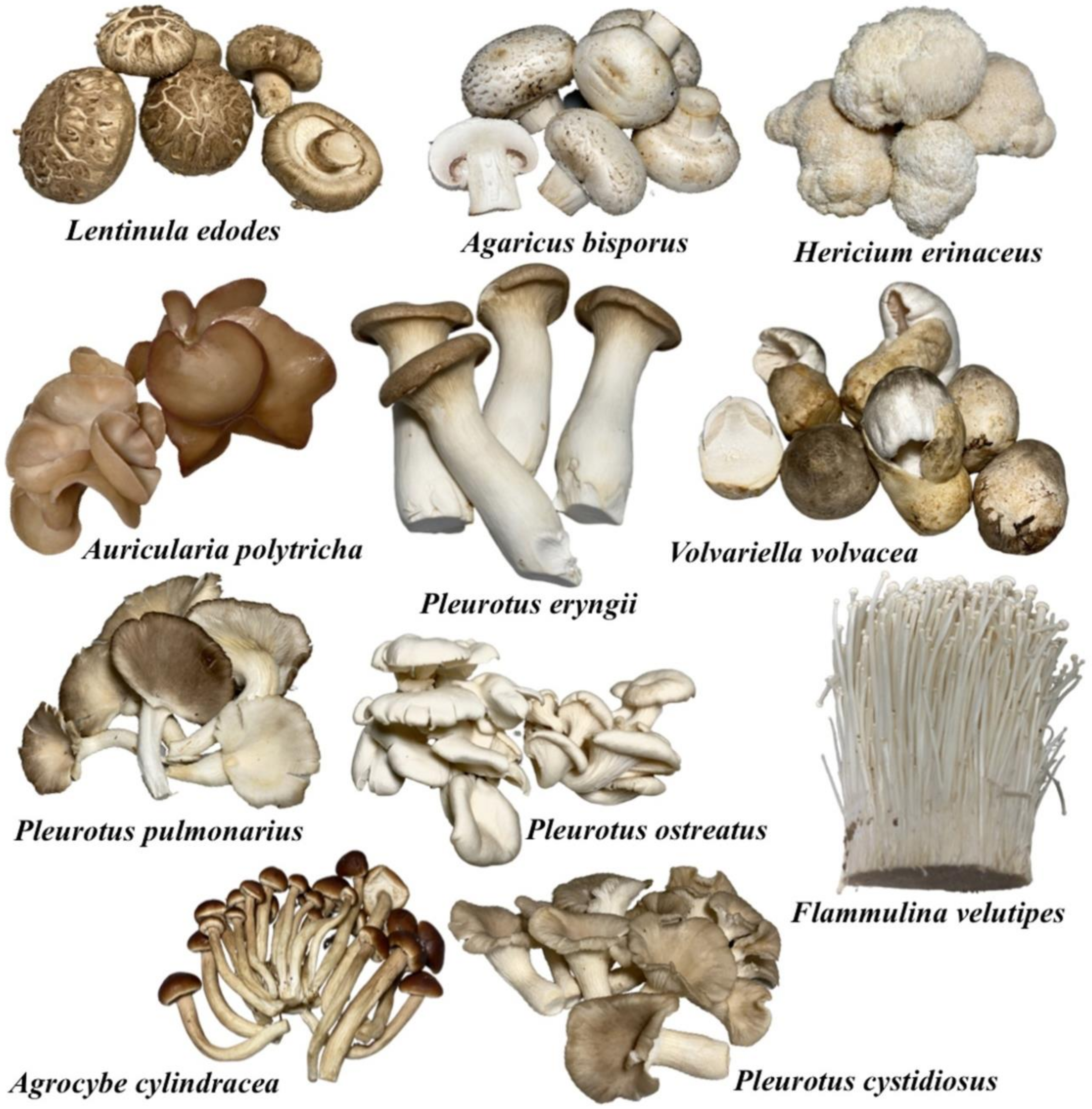
3. Mushroom Cultivation on Agro-Industrial Wastes
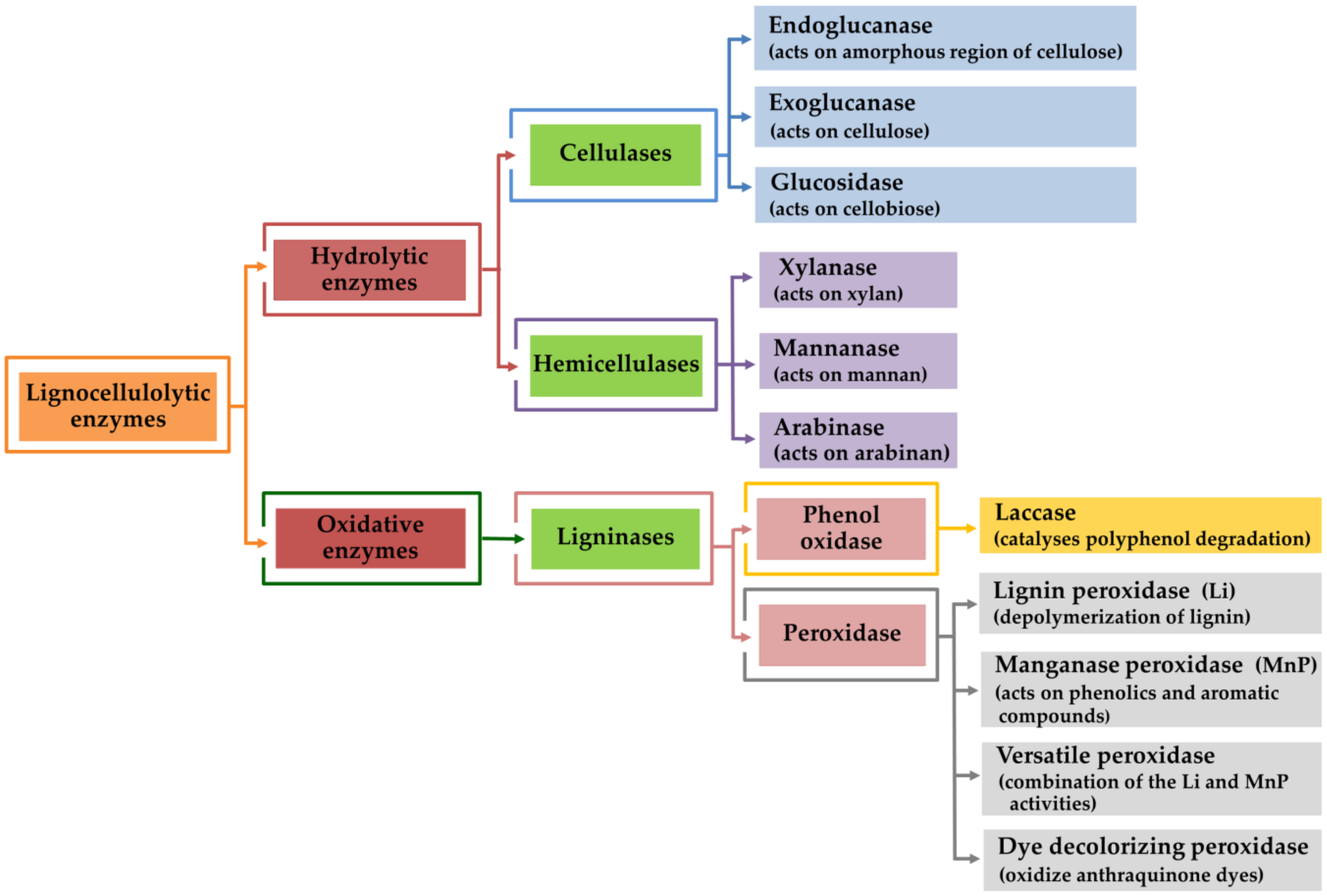
4. Lignocellulolytic Enzyme Production by Mushroom Using Agro-Industrial Wastes
4.1. Cellulose Degradation Enzymes
4.2. Hemicellulose Degradation Enzymes
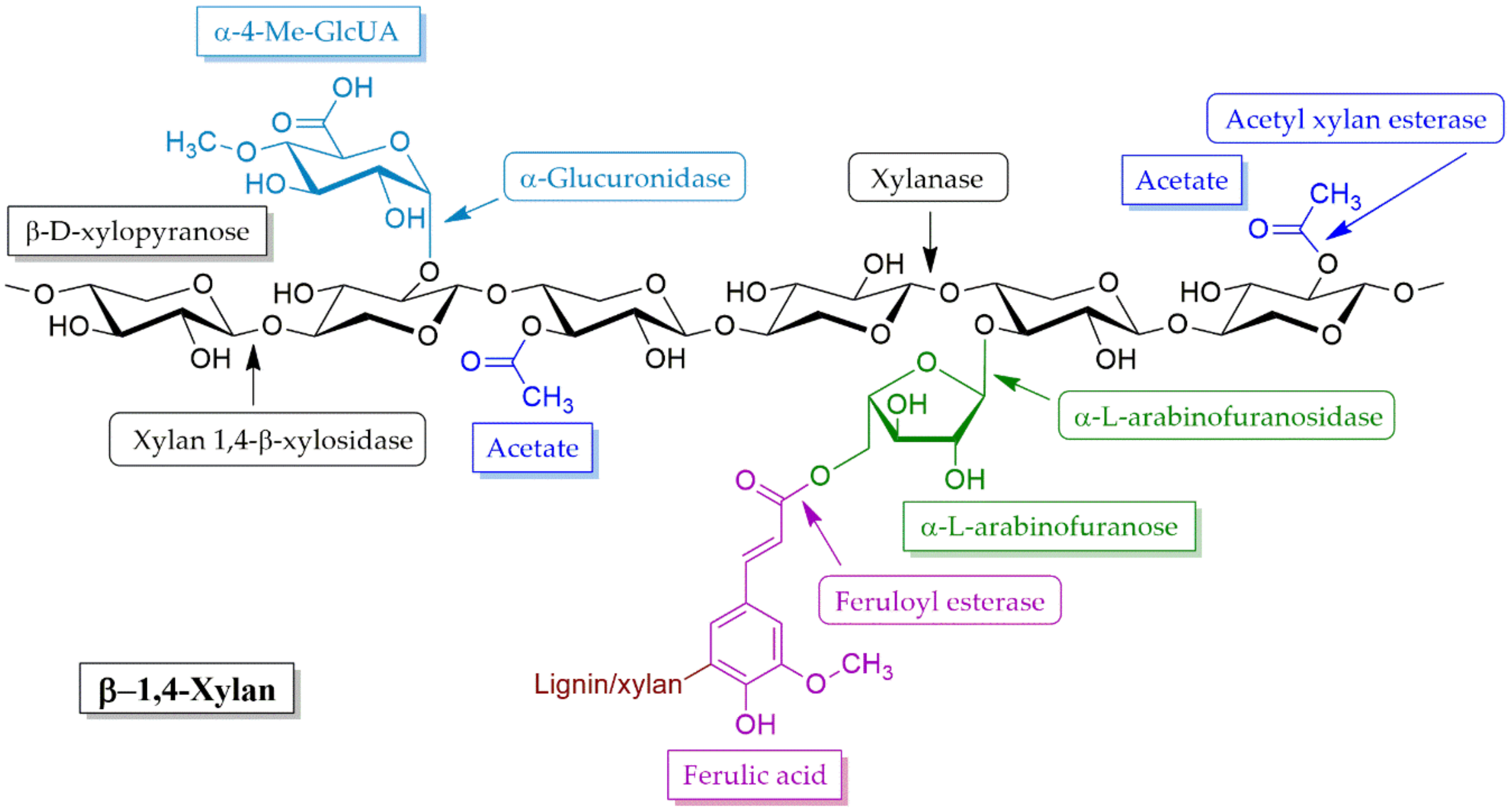
4.2.1. Xylanases
4.2.2. Mananases
4.2.3. Arabinanases
4.3. Lignin Degradation Enzymes
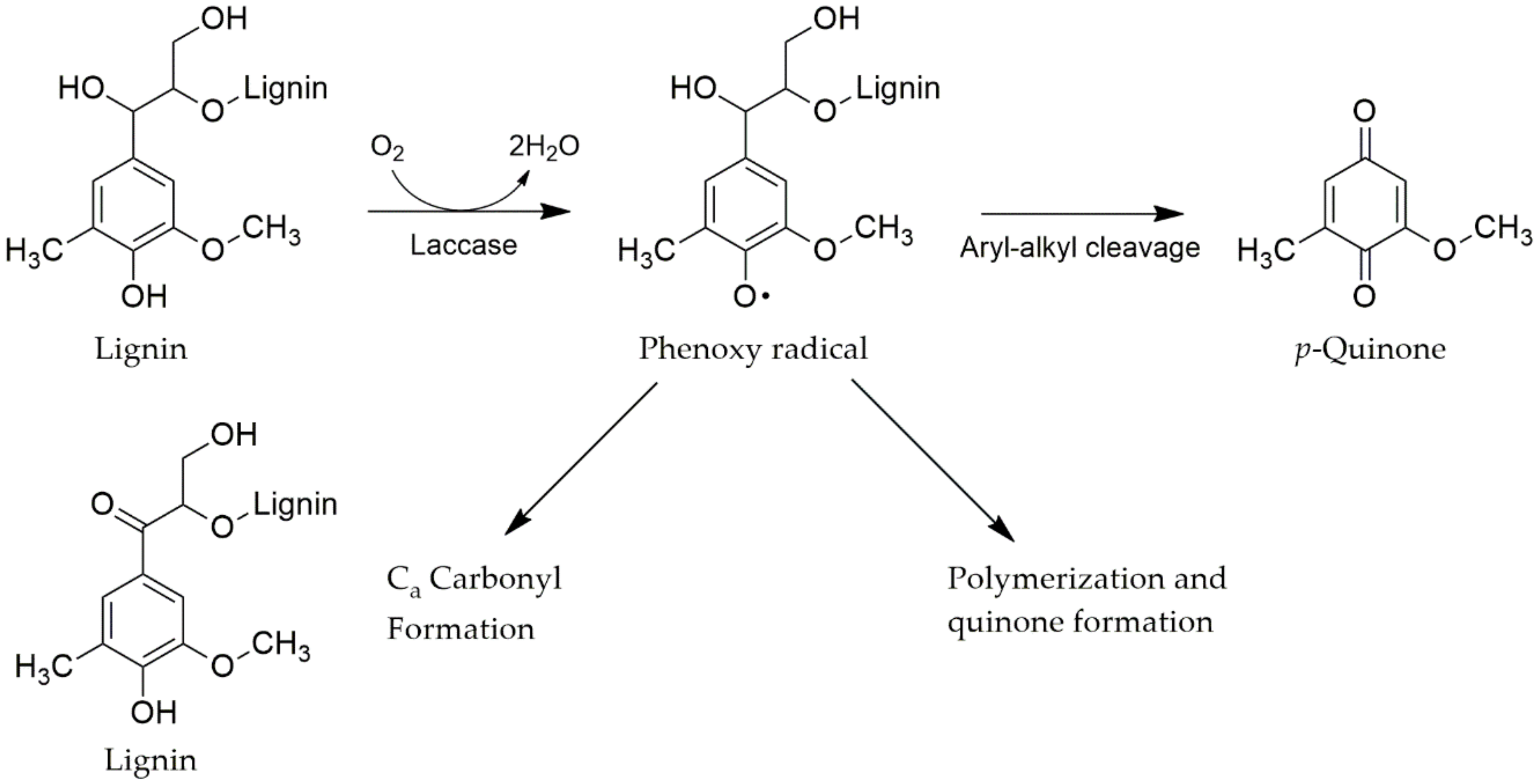
4.3.1. Laccases
4.3.2. Lignin Peroxidases
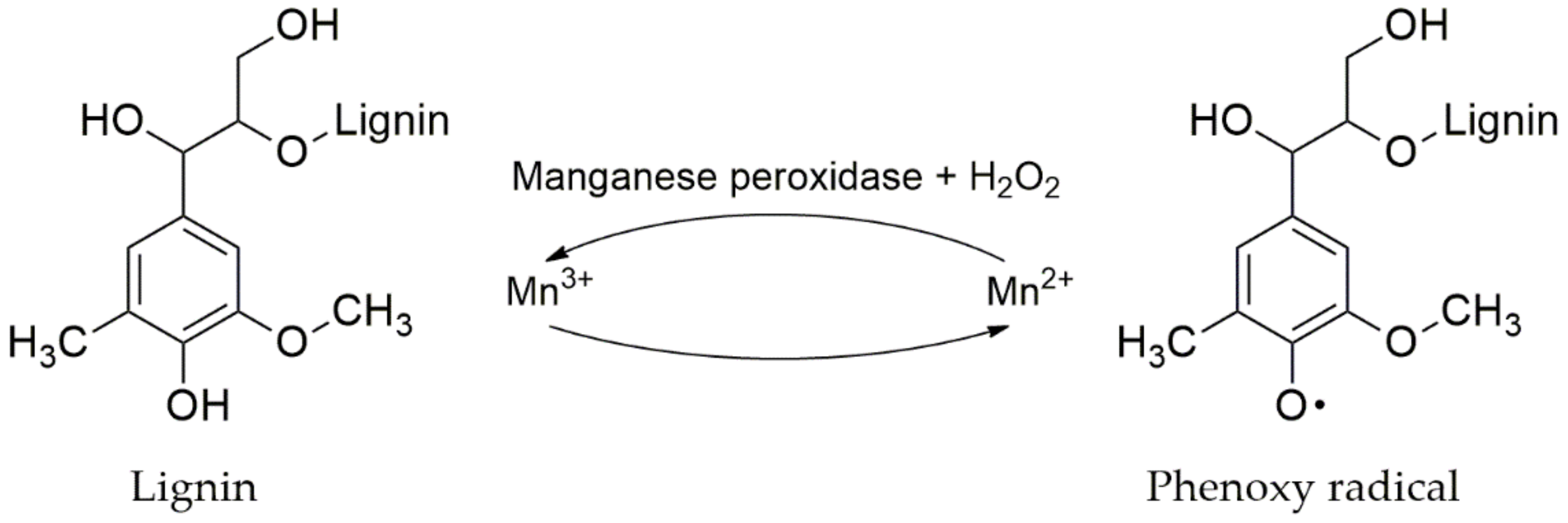
4.3.3. Manganese Peroxidase
4.3.4. Versatile Peroxidase
4.3.5. Dye Decolorizing Peroxidases
4.4. Application of Lignocellulolytic Enzymes in Bioprocessing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Panesar, P.S.; Kaur, R.; Singla, G.; Sangwan, R.S. Bio-processing of agro-industrial wastes for production of food-grade enzymes: Progress and prospects. Appl. Food Biotechnol. 2016, 3, 4. [Google Scholar]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sc. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Oliveira, D.M.D.S.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V.; et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agricola 2018, 75, 255–272. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.L. Adding value to agro-Industrial wastes. Ind. Chem. 2016, 2, e103. [Google Scholar] [CrossRef]
- Hongzhang, C. Biotechnology of Lignocellulose: Theory and Practice; Springer: New York, NY, USA, 2016. [Google Scholar]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Knob, A.; Forthamp, D.; Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as alternative for xylanase production by filamentous fungi. BioResources 2014, 9, 5738–5773. [Google Scholar]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Broadbelt, L.J.; Vinu, R. Mechanistic understanding of thermochemical conversion of polymers and lignocellulosic biomass. Adv. Chem. Eng. 2016, 49, 95–198. [Google Scholar]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O., Ed.; Springer: Cham, Switzerland, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Heinze, T. Hemicellulose. Adv. Polym. Sci. 2005, 186, 1–67. [Google Scholar]
- Geneau-Sbartai, C.; Leyris, J.; Slivestre, F.; Rigal, L. Sunflower cake as a natural composite: Composition and plastic properties. J. Agric. Food Chem. 2008, 56, 11198–11208. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-based hydrogels: Synthesis and applications. Polymers 2020, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Davin, L.B.; Lewis, N.G. Lignin primary structures and diligent sites. Curr. Opin. Biotechnol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Silveira, M.L.L.; Furlan, S.A.; Ninow, J.L. Development of an alternative technology for the oyster mushroom production using liquid inoculum. Cienc. Technol. Aliment. 2008, 28, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Aguilar, M.D. The suitability of banana leaf residue as raw material for the production of high lignin content micro/nano fibers: From residue to value-added products. Ind. Crop. Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Nigam, P.S.; Gupta, N.; Anthwal, A. Pre-treatment of agro-industrial residues. In Biotechnology for Agro-Industrial Residues Utilization; Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Nederlands, 2009; pp. 13–33. [Google Scholar]
- Adapa, P.K.; Tabil, L.G.; Schoenau, G.J.; Canam, T.; Dumonceaux, T. Quantitative ananlysis of lignocellulosic companents of non-treated and stream exploded barley, canola, oat and wheat straw using fourier transform infrared spectroscopy. J. Agric. Sci. Technol. 2011, B1, 177–188. [Google Scholar]
- Carrijo, O.A.; Liz, R.S.; Makishima, N. Fiber of green coconut shell as an agricultural substrate. Hortic. Bras. 2002, 20, 533–535. [Google Scholar] [CrossRef]
- Graminha, E.B.N.; Gonçalvez, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Rofiqah, U.; Kurniawan, A.; Aji, R.W.N. Effect of temperature in ionic liquids pretreatment on structure of lignocellulose from corncob. J. Phys. Conf. Ser. 2019, 1373, 1–7. [Google Scholar] [CrossRef]
- Pointner, M.; Kuttner, P.; Obrlik, T.; Jager, A.; Kahr, H. Composition of corncobs as a substrate for fermentation of biofuels. Agron. Res. 2014, 12, 391–396. [Google Scholar]
- El-Tayeb, T.S.; Abdelhafez, A.A.; Ali, S.H.; Ramadan, E.M. Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Braz. J. Microbiol. 2012, 43, 1523–1535. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, J. Hydrolysis of lignocellulosic material for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Abbas, A.; Ansumali, S. Global potential of rice husk as a renewable feedstock for ethanol biofuel production. Bioenerg. Res. 2010, 3, 328–334. [Google Scholar] [CrossRef]
- Limayema, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Comb. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Buzala, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Comparison of digestibility of wood pulps produced by the sulfate and TMP methods and woodchips of various botanical origins and sizes. Cellulose 2015, 22, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Chuayplod, P.; Aht-ong, D. A study of microcrystalline cellulose prepared from parawood (Hevea brasiliensis) sawdust waste using different acid types. J. Met. Mater. Miner. 2018, 28, 106–114. [Google Scholar]
- Da Silva Neta, J.M.; Oliveira, L.S.C.; da Silva Flavio, L.H.; Tabosa, J.N.; Pacheco, J.G.A.; da Silva, M.J.V. Use of sweet sorghum bagasse (Sorghum bicolor (L.) Moench) for cellulose acetate synthesis. BioResources 2019, 14, 3534–3553. [Google Scholar]
- Dong, M.; Wang, S.; Xu, F.; Wang, J.; Yang, N.; Li, Q.; Chen, J.; Li, W. Pretreatment of sweet sorghum straw and its enzymatic digestion: Insight into the structural changes and visualization of hydrolysis process. Biotechnol. Biofuels. 2019, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, W.S.; Tardin, F.D.; Tavares, G.; Queiroz, P.V.; Mota, S.S.; Kasuya, M.C.M.; de Queiroz, J.H. Use of sorghum straw (Sorghum bicolor) for second generation ethanol production: Pretreatment and enzymatic hydrolysis. Quim. Nova 2013, 36, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.M.; Neto, W.P.F.; Silverio, H.A.; Martins, D.F. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Choonut, A.; Saejong, M.; Sangkharak, K. The Production of ethanol and hydrogen from pineapple peel by Saccharomyces cerevisiae and Enterobacter aerogenes. Energy Procedia 2014, 52, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Taher, I.B.; Fickers, P.; Chnitit, S.; Hassouna, M. Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol. Prog. 2017, 33, 397–406. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food. Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Szymánska-Chargot, M.; Chylińska, M.; Gdula, K.; Koziol, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.C.; Trably, E.; Escudié, R.; Hamelin, J.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; Dumas, C. Total solids content: A key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol. Biofuels 2013, 6, 164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Peng, F.; Cheng, K.; Liu, D. Enhancement of enzymatic digestibility of sugarcane bagasse by alkali-peracetic acid pretreatment. Enzyme Microbial. Technol. 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Moutta, R.O.; Chandel, A.K.; Rodrigues, R.C.L.B.; Silva, M.B.; Rocha, G.J.M.; da Silva, S.S. Statistical optimization of sugarcane leaves hydrolysis into simple sugars by dilute sulfuric acid catalyzed process. Sugar Technol. 2012, 13, 53–60. [Google Scholar] [CrossRef]
- Saad, M.B.W.; Oliveira, L.R.M.; Candido, R.G.; Quintana, G.; Rocha, G.J.M.; Goncalves, A.R. Preliminary studies on fungal treatment of sugarcane straw for organosolv pulping. Enzyme Microbial. Technol. 2008, 45, 220–225. [Google Scholar] [CrossRef]
- Ariffin, H.; Hassan, M.A.; Umi Kalsom, M.S.; Abdullah, N.; Ghazali, F.M.; Shirai, Y. Production of bacterial endoglucanase from oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci. Bioeng. 2008, 3, 231–2236. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, M.H.M.; Rahman, N.A.; Abd-Aziz, S.; Funaoka, M.; Shinano, T.; Shirai, Y. Utilization of glucose recovered by phase separation system from acid-hydrolysed oil palm empty fruit bunch for bioethanol production. Sci. Pertanika J. Trop. Agric. 2012, 35, 117–126. [Google Scholar]
- Tufail, T.; Saeed, F.; Imran, M.; Arshammad, M.U.; Anjum, F.M.; Afzaal, M.; Ain, H.B.U.; Shahbaz, M.; Gondal, T.A.; Hussain, S. Biochemical characterization of wheat straw cell wall with special reference to bioactive profile. Int. J. Food Prop. 2018, 21, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of almond shell characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef] [Green Version]
- Xinyuan, J.; Yuanyuan, L.; Zhong, G.; An, M.; Zecai, H.; Suwen, Y. Pyrolysis characteristics and correlation analysis with the major components of seven kinds of nutshell. Sci. Silvae Sin. 2015, 51, 79–86. [Google Scholar]
- Akgül, M.; Korkut, S.; Camlibel, O.; Ayata, Ü. Some chemical properties of Luffa and its suitability for medium density fiberboard (MDF) production. Bioresurse 2013, 8, 1709–1717. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernandez-Bolanos, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Ndika, E.V.; Chidozie, U.S.; Ikechukwu, U.K. Chemical modification of cellulose from palm kernel de-oiled cake to microcrystalline cellulose and its evaluation as a pharmaceutical excipient. Afr. J. Pure Appl. Chem. 2019, 13, 49–57. [Google Scholar]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 20 May 2020).
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernándea-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbial. 2015, 376–387. [Google Scholar] [CrossRef]
- Cheung, P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2007; pp. 5–13. [Google Scholar]
- Hoa, H.T.; Wang, C.; Wang, C. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Cueva, M.B.R.; Hernáadez, A.; Niňo-Ruiz, Z. Influence of C/N ratio on productivity and the protein contents of Pleurotus ostreatus grown in differents residue mixtures. Rev. FCA Uncuyo. 2017, 49, 331–334. [Google Scholar]
- Ragunathan, R.; Swaminathan, K. Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chem. 2003, 80, 371–375. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Gimenez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Expr. 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moonmoon, M.; Shelly, N.J.; Khan, M.A.; Uddin, M.N.; Hossain, K.; Tania, M.; Ahmed, S. Effects of different levels of wheat bran, rice bran and maize powder supplementation with saw dust on the production of shiitake mushroom (Lentinus edodes (Berk.) Singer). Saudi J. Biol. Sci. 2011, 18, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippoussis, A. Production of mushrooms using agro-industrial residues as substrates. In Biotechnology for Agro-Industrial Residues Processing; Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–196. [Google Scholar]
- Shekhar, H.S.; Kilpatrick, M. Mushroom (Agaricus bisporus) compost quality factor for predicting potential yield of fruiting bodies. Can. J. Microbiol. 2000, 46, 515–519. [Google Scholar]
- Oei, P. Mushroom Cultivation, 3rd ed.; Backhuys Publishers: Leiden, The Netherlands, 2003; 429. [Google Scholar]
- Lisiecka, J.; Sobieralski, K.; Siwulski, M.; Jasinska, A. Almond mushroom Agaricus brasiliensis (Wasser et al.)–properties and culture condition. Acta Sci. Pol. Hortorum Cultus 2013, 12, 27–40. [Google Scholar]
- Cies, L. Resultados de dos ciclos de cultivo de champiñón Portobello. El Champiñón en Castilla-La Mancha 2009, 29, 1. [Google Scholar]
- Kopytowski, F.J.; Minhoni, M.T.A. C/N ratio on yield of Agaricus blazei Murrill ss. Heinemann. Mushroom Sci. 2004, 16, 213–220. [Google Scholar]
- Zied, D.C.; Savoie, J.; Pardo-Giménez, A. Soybean the main nitrogen source in cultivation substrates of edible and medicinal mushrooms. In Soybean and Nutrition; El-Shemy, H., Ed.; Janeza Trdine: Rijeka, Croatia, 2011; pp. 434–452. [Google Scholar]
- Poppe, J.; Höfte, M. Twenty wastes for twenty cultivated mushroom. Mushroom Sci. 1995, 14, 171–179. [Google Scholar]
- Chang, S.T.; Milles, P.G. Edible Mushroom and Their Cultivation; CRC Press: Florida, FL, USA, 1989; p. 345. [Google Scholar]
- Chang-Ho, Y.; Ho, T.M. Effect of nitrogen amendment on the growth of Volvariella volvacea. Mushroom Sci. 1979, 10, 619–625. [Google Scholar]
- Kaul, T.; Khurana, M.; Kachroo, J. Chemical composition of cereal straw of the Kashmir valley. Mushroom Sci. 1981, 11, 19–25. [Google Scholar]
- Heltay, I.; Zavodi, I. Rice straw compost. Mushroom Sci. 1960, 4, 393–399. [Google Scholar]
- Shi, L.; Chen, D.; Xu, C.; Ren, A.; Yu, H.; Zhao, M. Highly-efficient liposome-mediated transformation system for the basidiomycetous fungus Flammulina velutipes. J. Gen. Appl. Microbiol. 2017, 63, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.; Yang, F.C. Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresur. Technol. 2004, 91, 105–109. [Google Scholar] [CrossRef]
- Wakchaure, G.C. Production and marketing of mushrooms: Global and national scenario. In Mushrooms Cultivation, Marketing and Consumption; Singh, M., Vijay, B., Kamal, S., Wakchaure, G.C., Eds.; ICAR Publishing: Solan, India, 2011; pp. 15–22. [Google Scholar]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Expr. 2016, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Toker, H.; Baysal, E.; Yigibasi, O.N.; Colak, M.; Perker, H.; Simsek, H.; Yilmaz, F. Cultivation of Agaricus bisporus on wheat straw and waste tea leaves based composts using poplar leaves as activator material. Afr. J. Biotechnol. 2007, 6, 204–212. [Google Scholar]
- Tsai, S.Y.; Wu, T.P.; Huang, S.J.; Mau, J.L. Nonvolatile taste components of Agaricus bisporus harvested at different stages of maturity. Food Chem. 2007, 103, 1457–1464. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; Dias, E.S.; Rinker, D.L.; Caitano, C.E.C.; Zied, D.C. Optimization of cultivation techniques improves the agronomic behavior of Agaricus subrufescens. Sci. Rep. 2020, 10, 8154. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi–assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.R.H. Cultivation of the monkey head mushroom (Hericium erinaceus) in Egypt. J. App. Sci. Res. 2007, 3, 1229–1233. [Google Scholar]
- Gaitán-Hernández, R.; Cortés, N.; Mata, G. Improvement of yield of the edible and medicinal mushroom Lentinula edodes on wheat straw by use of supplemented spawn. Braz. J. Microbiol. 2014, 45, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.S. Productivity and proximate content of Pleurotus sajor-caju. Biosci. Discov. 2013, 4, 169–172. [Google Scholar]
- Medany, G.M. Cultivation possibility of golden oyster mushroom (Pleurotus citrinopileatus) under the Egyptian conditions. Egypt. J. Agric. Res. 2014, 92, 749–761. [Google Scholar]
- Ana, S.; Aak, A.; Aa, H.; Ea, S. Effect of residues agricultural wastes on the productivity and quality of Pleurotus colombinus l. by using polyethylene bags wall technique. Adv. Plants Agric. Res. 2016, 5, 528–536. [Google Scholar]
- Telang, S.M.; Patil, S.S.; Baig, M.M.V. Comparative study on yield and nutritional aspect of Pleurotus eous mushroom cultivated on different substrate. Food Sci. Res. J. 2010, 1, 60–63. [Google Scholar]
- Sardar, H.; Ali, M.A.; Anjum, M.A.; Nawaz, F.; Hussain, S.; Naz, S.; Karimi, S.M. Agro-industrial residues influence mineral elements accumulation and nutritional composition of king oyster mushroom (Pleurotus eryngii). Sci. Hort. 2017, 225, 327–334. [Google Scholar] [CrossRef]
- Prasad, S.; Rathore, H.; Sharma, S.; Tiwari, G. Yield and proximate composition of Pleurotus florida cultivated on wheat straw supplemented with perennial grasses. Indian J. Agric. Sci. 2018, 88, 91–94. [Google Scholar]
- Nasreen, Z.; Ali, S.; Usman, S.; Nazir, S.; Yasmeen, A. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on lignocellulosic by-products. Int. J. Adv. Res. Bot. 2016, 2, 42–49. [Google Scholar]
- Telang, S.M.; Patil, S.S.; Baig, M.M.V. Comparative study on yield and nutritional aspect of Pleurotus sapidus mushroom cultivated on different substrate. Food Sci. Res. J. 2010, 1, 127–129. [Google Scholar]
- De Andrade, M.C.N.; Zied, D.C.; Minhoni, M.T.A.; Filho, J.K. Yield of four Agaricus bisporus strains in three compost formulations and chemical composition analyses of the mushrooms. Braz. J. Microbial. 2008, 39, 593–598. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, C.S.M.; Sales-Campos, C.; de Carvalho, L.P.; Minhoni, M.T.A.; Saad, A.L.M.; Alquati, G.P.; de Andrade, M.C.N. Cultivation and bromatological analysis of medicinal mushroom Ganoderma lucidum (Curt.: Fr.) P. Karst cultivated in agricultural waste. Afr. J. Biotechnol. 2015, 14, 412–418. [Google Scholar]
- Gao, S.; Huang, Z.; Feng, X.; Bian, Y.; Huang, W.; Lui, Y. Bioconversion of rice straw agroresidues by Lentinula edodes and evaluation of non-volatile taste compounds in mushrooms. Sci. Rep. 2020, 10, 1814. [Google Scholar] [CrossRef] [PubMed]
- Adenipekun, C.O.; Omolaso, P.O. Comparative study on cultivation, yield performance and proximate composition of Pleurotus pulmonarius Fries. (Quelet) on rice straw and banana leaves. World J. Agric. Sci. 2015, 11, 151–158. [Google Scholar]
- Emriru, B.; Zenebech, K.; Kebede, F. Effect of substrates on the yield, yield attribute and dietary values of oyster mushroom (Pleurotus ostreatus) in the pastoral regions of northern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11199–11218. [Google Scholar]
- Ashraf, J.; Ali, M.A.; Ahmad, M.; Ayyub, C.M.; Shafi, J. Effect of different substrate supplements on oyster mushroom (Pleurotus spp.) production. Food Sci. Technol. 2013, 1, 44–51. [Google Scholar]
- Biswas, M.K.; Layak, M. Techniques for increasing the biological efficiency of paddy straw mushroom (Volvariella volvacea) in eastern India. Food Sci. Technol. 2014, 2, 52–57. [Google Scholar]
- Ahlawat, O.P.; Ahlawat, K.; Dhar, B.L. Influence of lignocellulolytic enzymes on substrate colonization and yield in monosporous isolates and parent strains of Volvariella volvacea (Bull. Fr.). Sing. India J. Microbiol. 2005, 45, 205–210. [Google Scholar]
- Reyes, R.G.; Lopez, L.L.M.A.; Kumakura, K.; Kalaw, S.P.; Kikukawa, T.; Eguchi, F. Coprinus comatus, a newly domesticated wild nutriceutical mushroom in the Philippines. J. Argic. Tecnhol. 2009, 5, 299–316. [Google Scholar]
- Stojkovic, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.I.; Sokovic, M.; Ferreira, I.C.F.R. Nutrients and non-nutrients composition and bioactivity of wild and cultivated Coprinus comatus (O.F.Müll.). Pers. Food Chem. Toxicol. 2013, 59, 289–296. [Google Scholar] [CrossRef]
- Salami, A.O.; Bankole, F.A.; Salako, Y.A. Nutrient and mineral content of oyster mushroom (Pleurotus florida) grown on selected lignocellulosic agro-waste substrates. Virol. Mycol. 2016, 5, 2. [Google Scholar]
- Adedokun, O.M.; Akuma, A.H. Maximizing agricultural residues: Nutritional properties of straw mushroom on maize husk, wastes cotton and plantain leaves. Nat. Res. 2013, 4, 534–537. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Iqdal, J.; Salim, M.; Ahmad, I.; Sarwar, M.A.; Shehzad, M.A.; Rafiq, M.A. Performance of oyster mushroom (Pleurotus ostreatus) on cotton waste amended with maize and banana leaves. Pak. J. Nutr. 2011, 10, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Garuba, T.; Abdukkareem, K.A.; Ibrahim, I.A.; Oyebamiji, O.I.; Shoyooye, O.A.; Ajibade, T.D. Influence of substrates on the nutritional quality of Pleurotus pulmonarius and Pleurotus ostreatus. Ceylon J. Sci. 2017, 46, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Haq, I.U.; Khan, M.A.; Khan, S.A.; Ahmad, M. Biochemical analysis of fruiting bodies of Volvariella volvacea strain Vv pk, grown on six different substrates. Soil Environ. 2011, 30, 146–150. [Google Scholar]
- Ahmed, S.A.; Kadam, J.A.; Mane, V.P.; Patil, S.S.; Baig, M.M.V. Biological efficiency and nutritional contents of Pleurotus florida (Mont.) Singer cultivated on different agro-wastes. Nat. Sci. 2009, 7, 44–48. [Google Scholar]
- Herawati, E.; Arung, E.T.; Amirta, R. Domestication and nutrient analysis of Schizopyllum commune, alternative natural food sources in East Kalimantan. Agric. Agric. Sci. Procedia 2016, 9, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Triyono, S.; Haryanto, A.; Telaumbanua, M.; Lumbanraja, D.J.; To, F. Cultivation of straw mushroom (Volvariella volvacea) on oil palm empty fruit bunch growth medium. Int. J. Recycl. Org. Waste Agric. 2019, 8, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Familoni, T.V.; Ogidi, C.O.; Akinyele, B.J.; Onifade, A.K. Evaluation of yield, biological efficiency and proximate composition of Pleurotus species cultivated on different wood dusts. Czech Mycol. 2018, 70, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Salmones, D.; Mata, G.; Ramos, L.M.; Waliszewski, K.N. Cultivation of shiitake mushroom, Lentinula edodes, in several lignocellulosic materials originating from the subtropics. Agron. EDP Sci. 1999, 19, 13–19. [Google Scholar] [CrossRef]
- Selvakumar, P.; Rajasekar, S.; Babu, A.G.; Periasamy, K.; Raaman, N.; Reddy, M.S. Improving biological efficiency of Pleurotus strain through protoplast fusion between P. ostreatus var. florida and P. djamor var. roseus. Food Sci. Biotechnol. 2015, 24, 1741–1748. [Google Scholar]
- Iqbal, B.; Khan, H.; Saifullah, L.; Khan, I.; Shah, B.; Naeem, A.; Ullah, W.; Khan, N.; Adnan, M.; Shah, S.R.A.; et al. Substrates evaluation for the quality, production and growth of oyster mushroom (Pleurotus florida Cetto). J. Entomol. Zool. Stud. 2016, 4, 98–107. [Google Scholar]
- Sardar, A.; Satankar, V.; Jagajanantha, P.; Mageshwaran, V. Effect of substrates (cotton stalks and cotton seed hulls) on growth, yield and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus florida). J. Cotton Res. Dev. 2020, 34, 135–145. [Google Scholar]
- Kortei, N.K.; Dzogbefia, V.P.; Obodai, M. Assessing the effect of composting cassava peel based substrates on the yield, nutritional quality, and physical characteristics of Pleurotus ostreatus (Jacq. ex Fr.) Kummer. Biotechnol. Res. Int. 2014, 571520, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetorgbor, M.M.; Apetorgbor, A.K. Comparative studies on yield of Volvariella volvacea using root and tuber peels for improved livelihood of communities. J. Ghana Sci. Assoc. 2015, 16, 35–43. [Google Scholar]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A. Valorization of olive by-products as substrates for the cultivation of Ganoderma lucidum and Pleurotus ostreatus mushrooms with enhanced functional and prebiotic properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.H.; Wu, C.Y.; Lu, P.L.; Kuo, Y.C.; Liang, Z.C. Biological efficiency and nutritional value of the culinary-medicinal mushroom Auricularia cultivated on a sawdust basal substrate supplement with different proportions of grass plants. Saudi J. Biol. Sci. 2019, 26, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Pati, S.S.; Ahmed, S.A.; Telang, S.M.; Baig, M.M.V. The nutritional value of Pleurotus ostreatus (Jacq.; Fr.) Kumm cultivated on different lignocellulosis agro-wastes. Innov. Rom. Food Biotechnol. 2010, 7, 66–76. [Google Scholar]
- De Siqueira, F.G.; Martos, E.T.; da Silva, G.; da Silva, R.; Dias, E.S. Biological efficiency of Agaricus brasiliensis cultivated in compost with nitrogen concentrations. Hortic. Bras. 2011, 29, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Harith, N.; Abdullah, N.; Sabaratnam, V. Cultivation of Flammulina velutipes mushroom using various agro-residues as a fruiting substrate. Pesq. Agropec. Bras. 2014, 49, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Wiafe-Kwagyan, M.; Obadai, M.; Odamtten, G.T.; Kortei, N.K. The potential use of rice waste lignocellulose and its amendments as substrate for the cultivation of Pleurotus eous strain P-3 in Ghana. Int. J. Adv. Phar. Biol. Chem. 2016, 5, 116–130. [Google Scholar]
- Bernardi, E.; Volcão, L.M.; Melo, L.G.; Nascimento, J.S. Productivity, biological efficiency and bromatological composition of Pleurotus sajor-caju growth on different substrates in Brazil. Agric. Nat. Resour. 2019, 53, 99–105. [Google Scholar]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.H.; Daniel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degration mechanisms across the tree of life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredon, M.; Dittmer, J.; Noël, C.; Moumen, B.; Bouchon, D. Lignocellulose degradation at the holobiont level: Teamwork in a keystone soil invertebrate. Microbiome 2018, 6, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Santek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda, R.H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef] [PubMed]
- Madeira, J.V., Jr.; Contesini, F.J.; Calzado, F.; Rubio, M.V.; Zubieta, M.P.; Lopes, D.B.; de Melo, R.R. Agro-industrial residues and microbial enzymes: An overview on the eco-friendly bioconversion into high value-added products. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 475–511. [Google Scholar]
- Ritota, M.; Manzi, P. Pleurotus spp. cultivation on different agri-food by-products: Example of biotechnological application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef] [Green Version]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Sajith, S.; Priji, P.; Sreedevi, S.; Benjamin, S. An overview on fungal cellulases with an industrial perspective. J. Nutr. Food. Sci. 2016, 6, 461. [Google Scholar]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K. Thermostable enzymes as biocatalysts in the biofuel industry. Adv. Appl. Microbiol. 2010, 70, 1–55. [Google Scholar]
- Zhang, Y.H.P.; Himmel, M.E.; Mielenz, J.R. Outlook for cellulase improvement, screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.J.; Saini, R.; Lakshmi Tewari, L. 2015. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Jun, H.S.; Forsberg, C.W. Cel9D, an atypical 1,4-β-d-glucan glucohydrolase from Fibrobacter succinogenes: Characteristics, catalytic residues and synergistic interactions with other cellulases. J. Bacteriol. 2008, 190, 1976–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothiraj, C.; Balaji, P.; Eyini, M. Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. Afr. J. Biotechnol. 2006, 5, 1882–1885. [Google Scholar]
- Bansal, N.; Tewari, R.; Soni, R.; Soni, S.K. Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manag. 2012, 32, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, H.N.; Ramanjaneyulu, G.; Rajasekhar Reddy, B. Optimization of cellulase production by Penicillium sp. 3 Biotech 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellilä, S.; Fonseca, L.; Uchima, C.; Cota, J.; Goldman, G.H.; Saloheimo, M.; Sacon, V.; Siika-aho, M. Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol. Biofuels 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Reddy, G.V.; Babu, P.R.; Komaraiah, P.; Roy, K.R.R.M.; Kothari, I.L. Utilization of banana waste for the production of lignolytic and cellulolytic enzymes by solid substrate fermentation using two Pleurotus species (P. ostreatus and P. sajor-caju). Process. Biochem. 2003, 38, 1457–1462. [Google Scholar] [CrossRef]
- Balaraju, K.; Park, K.; Jahagirdar, S.; Kaviyarasan, V. Production of cellulase and laccase enzymes by Oudemansiella radicata using agro wastes under solid-state and submerged conditions. Res. Biotechnol. 2010, 1, 21–28. [Google Scholar]
- Pandey, V.K.; Singh, M.P. Biodegradation of wheat straw by Pleurotus ostreatus. Cell. Mol. Biol. 2014, 60, 29–34. [Google Scholar]
- Elisashvili, V.; Chichua, D.; Kachlishvili, E.; Tsiklauri, N.; Khardziani, T. Lignocellulolytic enzyme activity during growth and fruiting of the edible and medicinal mushroom Pleurotus ostreatus (Jacq.: Fr.) Kumm. (Agaricomycetideae). Int. J. Med. Mushrooms 2003, 5, 193–198. [Google Scholar] [CrossRef]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Kharziani, T.; Kvesitadze, G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol. 2008, 99, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Montoya, S.; Orrego, C.E.; Levin, L. Growth, fruiting and lignocellulolytic enzyme production by the edible mushroom Grifola frondosa (maitake). World J. Microbiol. Biotechnol. 2012, 28, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, W.S.; Queiroz, P.V.; Tavares, G.P.; Santos, F.A.; Soares, F.E.D.F.; Kasuya, M.C.M.; Queiroz, J.H.D. Multi-enzyme complex of white rot fungi in saccharification of lignocellulosic material. Braz. J. Microbiol. 2018, 49, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Khardziani, T.; Agathos, S.N. Lignocellulose-degrading enzyme production by white-rot basidiomycetes isolated from the forests of Georgia. World J. Microbiol. Biotechnol. 2009, 25, 331–339. [Google Scholar] [CrossRef]
- Chuwech, M.; Rakariyatham, N. Potential of peanut hulls as substrates for fungal cellulase bioproduction through solid state fermentation. Asia. Pac. J. Sci. Technol. 2014, 19, 235–343. [Google Scholar]
- Lechner, B.E.; Papinutti, V.L. Production of lignocellulosic enzymes during growth and fruiting of the edible fungus Lentinus tigrinus on wheat straw. Process. Biochem. 2006, 41, 594–598. [Google Scholar] [CrossRef]
- Valášková, V.; Baldrian, P. Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res. Microbiol. 2006, 157, 119–124. [Google Scholar] [CrossRef]
- Wu, Y.; Shin, H. Cellulase from the fruiting bodies and mycelia of edible mushrooms: A review. J. Mushrooms 2016, 14, 127–135. [Google Scholar] [CrossRef]
- Deswal, D.; Khasa, Y.P.; Kuhad, R. Optimization of cellulose production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Penninckx, M.J.; Tsiklauri, N.; Elisashvili, V. Effect of nitrogen source on lignocellulolytic enzyme production by white rot basidiomycetes under solid state cultivation. World J. Microbial. Biotechnol. 2005, 224, 391–397. [Google Scholar]
- Machuca, A.; Ferraz, A. Hydrolytic and oxidative enzymes produced by white- and brown-rot fungi during Eucalyptus grandis decay in solid medium. Enzym. Microb. Technol. 2001, 29, 386–391. [Google Scholar] [CrossRef]
- Pandit, N.P.; Maheshwari, S.K. Optimization of cellulase enzyme production from sugarcane pressmud using oyster mushroom-Pleurotus sajor-caju by solid state fermentation. J. Bioremed. Biodegrad. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hoque, M.M.; Basunia, M.A.; Alam, N.; Khan, M.A. Production of cellulase by Pleurotus ostreatus and Pleurotus sajor-caju in solid state fermentation of lignocellulosic biomass. Turk. J. Agric. For. 2011, 35, 333–341. [Google Scholar]
- Levin, L.; Herrmann, C.; Papinutti, V.L. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem. Eng. J. 2008, 39, 207–214. [Google Scholar] [CrossRef]
- Giorgio, E.M.; Fonseca, M.I.; Tejerina, M.R.; Ramos-Hryb, A.B.; Sanabria, N.; Zapata, P.D.; Villalba, L.L. Chips and sawdust substrates application for lignocellulolytic enzymes production by solid state fermentation. Int. Res. J. Microbiol. 2012, 3, 120–127. [Google Scholar]
- Nguyen, K.A.; Kumla, J.; Suwannarach, N.; Penkhrue, W.; Lumyong, S. Optimization of high endoglucanase yields production from polypore fungus, Microporus xanthopus strain KA038 under solid-state fermentation using green tea waste. Bio 2019, 8, bio047183. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Ma, F.; Zhang, X. Lignocellulose degradation and enzyme production by Irpex lacteus CD2 during solid-state fermentation of corn stover. J. Biosci. Bioeng. 2009, 108, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Philippoussis, A.; Diamantopoulou, P. Agro-food industry wastes and agricultural residues conversion into high value products by mushroom cultivation. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Institute National de la Recherche Agronomique (INRA), Arcachon, France, 4–7 October 2011; pp. 339–351. [Google Scholar]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Critical. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Mandels, M.; Andreotti, R.; Roche, C. Measurement of saccharifying cellulase. Biotechnol. Bioeng. Symp. 1976, 6, 21–33. [Google Scholar]
- Kubicek, C.P. Release of carboxymethyl-cellulase and β-glucosidase from cell walls of Trichoderma reesei. Eur. J. Appl. Biotechnol. 1981, 13, 226–231. [Google Scholar] [CrossRef]
- Korotkova, O.G.; Semenova, M.V.; Morozova, V.V.; Zorov, I.N.; Sokolova, L.M.; Bubnova, T.M.; Okunev, O.N.; Sinitsyn, A.P. Isolation and properties of fungal beta-glucosidases. Biochemistry 2009, 74, 569–577. [Google Scholar] [PubMed]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal beta-glucosidases: A bottleneck in industrial use of lignocellulosic materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.R. Microbial Degradation of Lignocellulosic Biomass. In Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; Chandel, A.K., da Silva, S.S., Eds.; IntechOpen: London, UK, 2012; pp. 207–247. [Google Scholar]
- Ahmed, S.; Jabeen, A.; Jamil, A. Xylanase from Trichoderma harzianum: Enzyme characterization and gene isolation. J. Chem. Soc. Pak. 2011, 29, 176. [Google Scholar]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, A.M.; Jurak, E.; de Gijsel, P.; Ohm, R.A.; Henrissat, B.; Lugones, L.G.; Kabel, M.A.; Wosten, H.A.B. Production of α-1,3-l-arabinofuranosidase active on substituted xylan does not improve compost degradation by Agaricus bisporus. PLoS ONE 2018, 13, e0201090. [Google Scholar] [CrossRef]
- Dos Santos, C.R.; de Giuseppe, P.O.; de Souza, F.H.M.; Zanphorlin, L.M.; Domingues, M.N.; Pirolla, R.A.S.; Honorato, R.V.; Tonoli, C.C.C.; de Morais, M.A.B.; Martins, V.P.M.; et al. Murakami, M.T. The mechanism by which a distinguishing arabinofuranosidase can cope with internal di-substitutions in arabinoxylans. Biotechnol. Biofuels 2018, 11, 223. [Google Scholar] [CrossRef] [Green Version]
- Gómez, S.; Payne, A.M.; Savko, M.; Fox, G.C.; Shepard, W.E.; Fernandez, F.J.; Vega, M.C. Structural and functional characterization of a highly stable endo-β-1,4-xylanase from Fusarium oxysporum and its development as an efficient immobilized biocatalyst. Biotechnol. Biofuels 2016, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, P.; Mahajan, R. Cellulase and xylanase synergism in industrial biotechnology. Appl. Microbiol. Biot. 2019, 103, 8711–8724. [Google Scholar] [CrossRef]
- Burlacu, A.; Cornea, C.P.; Israel-Roming, F. Screening of xylanase producing microorganisms. Res. J. Agric. Sci. 2016, 48, 8–15. [Google Scholar]
- Meddeb-Mouelhi, F.; Moisan, J.K.; Beauregard, M. A comparison of plate assay methods for detecting extracellular cellulase and xylanase activity. Enzyme Microb. Technol. 2014, 66, 16–19. [Google Scholar] [CrossRef]
- Lim, S.H.; Lee, Y.H.; Kang, H.W. Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster Mushrooms, Pleurotus spp., and potential use in dye decolorization. Mycobiology 2013, 41, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amore, A.; Amoresano, A.; Birolo, L.; Henrissat, B.; Leo, G.; Palmese, A.; Faraco, V. A family GH51 α-l-arabinofuranosidase from Pleurotus ostreatus: Identification, recombinant expression and characterization. Appl. Microbiol. Biotechnol. 2011, 94, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of Somogyi methods for determination of glucose. J. Biol. Chem. 1994, 153, 375–380. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar]
- Azeri, C.; Tamer, A.U.; Oskay, M. Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr. J. Biotechnol. 2010, 9, 63–72. [Google Scholar]
- Driss, D.; Bhiri, F.; Elleuch, L.; Bouly, N.; Stals, I.; Miled, N.; Blibech, M.; Ghorbel, R.; Chaabouni, S.E. Purification and properties of an extracellular acidophilic endo-1,4-β-xylanase, naturally deleted in the ‘‘thumb’’, from Penicillium occitanis Pol6. Proc. Biochem. 2012, 46, 1299–1306. [Google Scholar] [CrossRef]
- Hatanaka, K. Incorporation of fluorous glycosides to cell membrane and saccharide chain elongation by cellular enzymes. Top. Curr. Chem. 2012, 308, 291–306. [Google Scholar]
- Kuhad, R.C.; Sing, A. Lignocellulose biotechnology: Current and future prospects. Crit. Rev. Biotechnol. 1993, 13, 151–172. [Google Scholar] [CrossRef]
- Soni, H.; Rawat, H.K.; Pletschke, B.I.; Kango, N. Purification and characterization of β-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass. Biotech 2016, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Sherief, A.A.; El-Tanash, A.B.; Temraz, A.M. Lignocellulolytic enzymes and substrate utilization during growth and fruiting of Pleurotus ostreatus on some solid wastes. J. Environ. Sci. Technol. 2010, 3, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Manavalan, T.; Manavalan, A.; Thangavelu, K.P.; Heese, K. Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. J. Proteom. 2012, 77, 298–309. [Google Scholar] [CrossRef]
- Iandolo, D.; Piscitelli, A.; Sannia, G.; Faraco, V. Enzyme production by solid substrate fermentation of Pleurotus ostreatus and Trametes versicolor on tomato pomace. Appl. Biochem. Biotechnol. 2010, 163, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Liu, J.; Yang, J.; Lin, Y.; Yang, Y.; Ji, L.; Li, M.; Yuan, H. Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol. Biofuels 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, K.T.; Cajthaml, T.; Snajdr, J.; Baldrian, P. Differential degradation of oak (Quercus petraea) leaf litter by litter-decomposing basidiomycetes. Res. Microbiol. 2007, 158, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, M.; Levin, L.; Albertó, E.; Lechner, B. Effect of co-cultivation of two Pleurotus species on lignocellulolytic enzyme production and mushroom fructification. Int. Biodeterior. 2012, 66, 71–76. [Google Scholar] [CrossRef]
- Heidorne, F.O.; Magalhães, P.O.; Ferraz, A.L.; Milagres, A.M.F. Characterization of hemicellulases and cellulases produced by Ceriporiopsis subvermispora grown on wood under biopulping conditions. Enzyme Microb. Technol. 2006, 38, 436–442. [Google Scholar] [CrossRef]
- Papinutti, V.L.; Forchiassin, F. Lignocellulolytic enzymes from Fomes sclerodermeus growing in solid-state fermentation. J. Food Eng. 2007, 81, 54–59. [Google Scholar] [CrossRef]
- Boonrung, S.; Mongkolthanaruk, W.; Aimi, T.; Boonlue, S. Cellulase and xylanase acting at alkaline pH from mushroom, Leucoagaricus meleagris KKU-C1. Chiang Mai J. Sci. 2014, 41, 84–96. [Google Scholar]
- De Oliveira Rodrigues, P.; Gurgel, L.V.A.; Pasquini, D.; Badotti, F.; Góes-Neto, A.; Baffi, M.A. Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among ascomycetes and basidiomycetes. Renew. Energy 2020, 145, 2683–2693. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biot. 2012, 93, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ayaşan, T.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Patra, A.K. The role of β-mannanase (Hemicell) in improving poultry productivity, health and environment. Braz. J. Poultry Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Olaniyi, O.O.; Bankefa, E.O.; Folasade, I.O.; Familoni, T.V. Nutrient enrichment of mannanase-treated cassava peels and corn cob. Res. J. Microbiol. 2015, 10, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Pinho, G.P.; Matoso, J.R.M.; Silvério, F.O.; Mota, W.C.; Lopes, P.S.N.; Ribeiro, L.M. A new spectrophotometric method for determining the enzymatic activity of endo-β-mannanase in seeds. J. Braz. Chem. Soc. 2014, 25, 1246–1252. [Google Scholar] [CrossRef]
- Titapoka, S.; Keawsompong, S.; Haltric, D.; Nitisinprasert, S. Selection and characterization of mannanase-producing bacteria useful for the formation of prebiotic manno-ligosaccharides from copra meal. World J. Microbiol. Biotechnol. 2008, 24, 1425–1433. [Google Scholar] [CrossRef]
- Maijala, P.; Kango, N.; Szijarto, N.; Viikari, L. Characterization of hemicellulases from thermophilic fungi. Anton. Leeuw. 2012, 101, 905–917. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Zhang, J.; Sun, J.; Matsukawa, S.; Xie, J.; Wei, D. Characterization of abnZ2 (yxiA1) and abnZ3 (yxiA3) in Paenibacillus polymyxa, encoding two novel endo-1,5-α-l-arabinanases. Bioresour. Bioprocess. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Seiboth, B.; Metz, B. Fungal arabinan and l-arabinose metabolism. Appl. Microbiol. Biotechnol. 2011, 89, 1665–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numan, M.T.; Bhosle, N.B. α-l-Arabinofuranosidases: The potential applications in biotechnology. J. Ind. Microbiol. Biotechnol. 2006, 33, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Yanay, T.; Sato, M. Purification and characterization of a novel α-l-arabinofuranosidase from Pichia capsulata X91. Biosci. Biotechnol. Biochem. 2000, 64, 1181–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurak, E.; Patyshakuliyeva, A.; de Vries, R.P.; Gruppen, H.; Kabel, M.A. Compost grown Agaricus bisporus lacks the ability to degrade and consume highly substituted xylan fragments. PLoS ONE 2015, 10, e0134169. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Akin, D.E. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J. Ind. Microbiol. Biotechnol. 2008, 35, 355–366. [Google Scholar] [CrossRef]
- Scharf, M.E.; Tartar, A. Termite digestomes as sources for novel lignocellulases. Biofuels Bioprod. Biorefin. 2008, 2, 540–552. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- Niladevi, K.N. Ligninolytic enzymes. In Biotechnology for Agro-Industrial Residues Utilisation; Nigam, P.S., Pandey, A., Eds.; Springer: Amsterdam, The Netherlands, 2009; pp. 397–414. [Google Scholar]
- Rogalski, J.; Lundell, T.; Leonowicz, A.; Hatakka, A. Production of laccase, lignin peroxidase and manganese-dependent peroxidase by various strains of Trametes versicolor depending on culture conditions. Acta Microbiol. Pol. 1991, 40, 221–234. [Google Scholar]
- Tripathi, A.; Upadhyay, R.C.; Singh, S. Extracellular Ligninolytic Enzymes in Bjerkandera adusta and Lentinus squarrosulus. Indian J. Microbiol. 2012, 52, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Saeki, N.; Takeda, H.; Tanesaka, E.; Yoshida, M. Induction of manganese peroxidase and laccase by Lentinula edodes under liquid culture conditions and their isozyme detection by enzymatic staining on native-PAGE. Mycoscience 2011, 52, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Caramelo, L.; Martinez, M.J.; Martinez, A.T. A search for ligninolytic peroxidases in the fungus Pleurotus eryngii involving alpha-keto-gamma-thiomethylbutyric acid and lignin model dimers. Appl. Environ. Microbiol. 1999, 65, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Chmelová, D.; Ondrejovič, M. Effect of potential inductors on laccase production by white-rot fungus Ceriporiopsis subvermispora. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 84–87. [Google Scholar]
- Tovar-Herrera, O.E.; Martha-Paz, A.M.; Pérez-LLano, Y.; Aranda, E.; Tacoronte-Morales, J.E.; Pedroso-Cabrera, M.T.; Arévalo-Niño, K.; Folch-Mallol, J.L.; Batista-García, R.A. Schizophyllum commune: An unexploited source for lignocellulose degrading enzymes. MicrobiologyOpen 2018, 7, e00637. [Google Scholar] [CrossRef] [PubMed]
- Kalra, K.; Chauhan, R.; Shaves, M.; Sachdeva, S. Isolation of laccase producing Trichoderma spp. and effect of pH and temperature on its activity. Int. J. Chem. Environ. Technol. 2013, 5, 2229–2235. [Google Scholar]
- Zeng, S.; Zhao, J.; Xia, L. Simultaneous production of laccase and degradation of bisphenol A with Trametes versicolor cultivated on agricultural wastes. Bioprocess Biosyst. Eng. 2017, 40, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adekunle, A.E.; Zeng, C.; Guo, C.; Liu, C. Laccase production from Trametes versicolor in solid-state fermentation of steam-exploded pretreated cornstalk. Waste Biomass. Valori. 2017, 8, 153–159. [Google Scholar] [CrossRef]
- Aâssi, D.; Zouari-Mechichi, H.; Frikha, F.; Rodriguez-Couto, S.; Mechichi, T. Sawdust waste as a low-cost support- substrate for laccases production and adsorbent for azo dyes decolorization. J. Environ. Health Sci. 2016, 14, 1–12. [Google Scholar]
- Karp, S.G.; Faraco, V.; Amore, A.; Letti, L.A.J.; Soccol, V.T.; Soccol, C.R. Statistical optimization of laccase production and delignification of sugarcane bagasse by Pleurotus ostreatus in solid-state fermentation. Biomed. Res. Int. 2015, 2015, 181–204. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, U.; Winquist, E.; Mattila, T.; Hatakka, A.; Eerikäinen, T. Production of manganese peroxidase and laccase in a solid-state bioreactor and modeling of enzyme production kinetics. Bioprocess Biosyst. Eng. 2015, 38, 57–68. [Google Scholar] [CrossRef]
- Hariharan, S.; Padma, N. Optimization of lignin peroxidase, manganese peroxidase, and Lac production from Ganoderma lucidum under solid state fermentation of pineapple leaf. Bioresouyces 2013, 8, 250–271. [Google Scholar] [CrossRef] [Green Version]
- Usha, K.Y.; Praveen, K.; Rajasekhar Reddy, B. Enhanced production of ligninolytic enzymes by a mushroom Stereum ostrea. Biotechnol. Res. Int. 2014, 2014, 815495. [Google Scholar] [CrossRef]
- Asgher, M.; Asad, M.J.; Legge, R.L. Enhanced lignin peroxidase synthesis by Phanerochaete chrysosporium in solid state bioprocessing of a lignocellulosic substrate. World J. Microbiol. Biot. 2006, 22, 449–453. [Google Scholar] [CrossRef]
- Silva, E.M.; Martins, S.F.; Milagres, A.M.F. Extraction of manganese peroxidase produced by Lentinula edodes. Bioresour. Technol. 2008, 99, 2471–2475. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Nigam, P.S. Remediation of textile dye waste water using a white-rot fungus Bjerkandera adusta through solid-state fermentation (SSF). Appl. Biochem. Biotechnol. 2008, 151, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, I.; Rodrigues da Luz, J.M.; Oliveira, S.F.; Humberto de Queiroz, J.; Kasuya, M.C.M. High-yield cellulase and LiP production after SSF of agricultural wastes by Pleurotus ostreatus using different surfactants. Biocatal. Agric. Biotechnol. 2019, 22, 101428. [Google Scholar] [CrossRef]
- Mehboob, N.; Asad, M.; Imran, M.; Gulfraz, M.; Wattoo, F.H.; Hadri, S.H.; Asghar, M. Production of lignin peroxidase by Ganoderma leucidum using solid state fermentation. Afr. J. Biotechnol. 2011, 10, 9880–9887. [Google Scholar]
- Coconi-Linares, N.; Magaña-Ortíz, D.; Guzmán-Ortiz, D.A.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. High-yield production of manganese peroxidase, lignin peroxidase, and versatile peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef]
- Gochev, V.K.; Krastanov, A.I. Fungal laccases. Bulg. J. Agric. Sci. 2007, 13, 75–83. [Google Scholar]
- Zheng, Y.; Guo, M.; Zhou, Q.; Liu, H. Effect of lignin degradation product sinapyl alcohol on laccase catalysis during lignin degradation. Ind. Crops Prod. 2019, 139, 111544. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, N.; Li, W.; Li, J.; Li, Z.; Wang, J.; Tang, X. Laser mutagenesis of Phellinus igniarius protoplasts for the selective breeding of strains with high laccase activity. Appl. Biochem. Biotechnol. 2020, 190, 584–600. [Google Scholar] [CrossRef]
- Palma, C.; Lloret, L.; Sepúlveda, L.; Contreras, E. Production of versatile peroxidase from Pleurotus eryngii by solid-state fermentation using agricultural residues and evaluation of its catalytic properties. Prep. Biochem. Biotechnol. 2016, 46, 200–207. [Google Scholar] [CrossRef]
- Rich, J.O.; Anderson, A.M.; Berhow, M.A. Laccase-mediator catalyzed conversion of model lignin compounds. Biocat. Agric. Biotechnol. 2016, 5, 111–115. [Google Scholar] [CrossRef]
- Song, Q.; Deng, X.; Song, R. Expression of Pleurotus ostreatus laccase gene in Pichia pastoris and Its degradation of corn stover lignin. Microorganisms 2020, 8, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios-Estrada, C.; de Jesus Rostro-Alanis, M.; Munoz-Gutierrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldivar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation - A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Cho, N.; Luterek, J.; Wilkolazka, A.; Wojtas-Wasilewska, M.; Matuszewska, A.; Hofrichter, M.; Wesenberg, D.; Rogalski, J. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar] [CrossRef]
- Shraddha Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 217861. [Google Scholar] [CrossRef] [Green Version]
- Ardon, O.; Kerem, Z.; Hadar, Y. Enhancement of lignin degradation and laccase activity in Pleurotus ostreatus by cotton stalk extract. Can. J. Microbiol. 1998, 44, 676–680. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Patil, N.N.; Shinde, S.S. Ligninase in Degradation of Lignocellulosic Wastes. In Enzymes in Degradation of the Lignocellulosic Wastes; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.; Dai, Y.; Si, J.; Cui, B. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int. J. Biol. Macromol. 2017, 102, 758–770. [Google Scholar] [CrossRef]
- Dias, A.A.; Matos, A.J.S.; Fraga, I.; Sampaio, A.; Bezerra, R.M.F. An easy method for screening and detection of laccase activity. Open Biotechnol. J. 2017, 11, 89–93. [Google Scholar] [CrossRef]
- Dias, A.A.; Bezerra, R.M.; Pereira, A.N. Activity and elution profile of laccase during biological decolorization of olive mill wastewater. Bioresour. Technol. 2004, 92, 7–13. [Google Scholar] [CrossRef]
- Minussi, R.C.; Pastore, G.M.; Duran, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.S.; Gill, P.K. Comparison of two assay procedures for lignin peroxidase. Enzyme Microb. Technol. 2001, 28, 602–605. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, C.; Zeng, G.; Huang, D.; Xu, P.; Cheng, M. Growth, metabolism of Phanerochaete chrysosporium and route of lignin degradation in response to cadmium stress in solid-state fermentation. Chemosphere 2015, 138, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, H.; Lyu, S.; Ma, F.; Yu, H.; Zhang, X. Characterization of a novel manganese peroxidase from white-rot fungus Echinodontium taxodii 2538, and its use for the degradation of lignin-related compounds. Process Biochem. 2016, 51, 1776–1783. [Google Scholar] [CrossRef]
- Burlacu, A.; Israel-Roming, F.; Cornea, C.P. Depolymerization of kraft lignin with laccase and peroxidase: A review. Sci. Bull. Ser. F Biotechnol. 2018, 22, 172–179. [Google Scholar]
- Brink, D.P.; Ravi, K.; Lidén, G.; Gorwa-Grauslund, M.F. Mapping the diversity of microbial lignin catabolism: Experiences from the eLignin database. Appl. Microbiol. Biotechnol. 2019, 103, 3979–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, V.; Fahimi, H.D. A new sensitive colorimetric assay for peroxidase using 3,3′-diaminobenzidine as hydrogen donor. Anal. Biochem. 1973, 55, 554–562. [Google Scholar] [CrossRef]
- De Jong, E.; Field, J.A.; de Bont, J.A. Evidence for a new extracellular peroxidase manganese-inhibited peroxidase from the white-rot fungus Bjerkandera sp. BOS 55. FEBS Lett. 1992, 299, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Rajan, A.; Kurup, J.G.; Abraham, T.E. Solid state production of manganese peroxidases using arecanut husk as substrate. Braz. Arch. Biol. Technol. 2010, 53, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Kuhar, F.; Castiglia, V.C.; Zamora, J.C. Detection of manganese peroxidase and other exoenzymes in four isolates of Geastrum (Geastrales) in pure culture. Rev. Argent. Microbiol. 2016, 48, 274–278. [Google Scholar] [CrossRef]
- Busse, N.; Wagner, D.; Kraume, M.; Czermak, P. Reaction kinetics of versatile peroxidase for the degradation of lignin compounds. Am. J. Biochem. Biotechnol. 2013, 9, 365–394. [Google Scholar] [CrossRef]
- Giardina, P.; Palmieri, G.; Fontanella, B.; Rivieccio, V.; Sannia, G. Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch. Biochem. Biophys. 2000, 376, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.; Sridhar, M. Versatile peroxidases: Super peroxidases with potential biotechnological applications-A mini review. J. Dairy Vet. Anim. Res. 2016, 4, 277–280. [Google Scholar]
- Chen, M.; Yao, S.; Zhang, H.; Liang, X. Purification and characterization of a versatile peroxidase from edible mushroom Pleurotus eryngii. Chin. J. Chem. Eng. 2010, 18, 824–829. [Google Scholar] [CrossRef]
- Fisher, A.B.; Fong, S.S. Lignin biodegradation and industrial implications. AIMS Bioeng. 2014, 1, 92–112. [Google Scholar] [CrossRef]
- Sugano, Y.; Muramatsu, R.; Ichiyanagi, A.; Sato, T.; Shoda, M. DyP, a unique dye-decolorizing peroxidase, represents a novel heme peroxidase family: ASP171 replaces the distal histidine of classical peroxidases. J. Biol. Chem. 2007, 282, 36652–36658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Shrestha, R.; Jia, K.; Gao, P.F.; Geisbrecht, B.V.; Bossmann, S.H.; Shi, J.; Li, P. Characterization of dye-decolorizing peroxidase (DyP) from Thermomonospora curvata reveals unique catalytic properties of A-type DyPs. J. Biol. Chem. 2015, 290, 23447–23463. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Shoda, M. Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl. Environ. Microbiol. 1999, 65, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Liers, C.; Pecyna, M.J.; Kellner, H.; Worrich, A.; Zorn, H.; Steffen, K.T.; Hofrichter, M.; Ullrich, R. Substrate oxidation by dye-decolorizing peroxidases (DyPs) from wood- and litter-degrading agricomycetes compared to other fungal and heme-proteins. Appl. Microbiol. Biotechnol. 2013, 97, 5839–5849. [Google Scholar] [CrossRef]
- Lauber, C.; Schwarz, T.; Nguyen, Q.K.; Lorenz, P.; Lochnit, G.; Zorn, H. Identification, heterologous expression and characterization of a dye-decolorizing peroxidase of Pleurotus sapidus. AMB Express 2017, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Luo, H.; Zhang, X.; Yao, B.; Ma, F.; Su, X. Dye-decolorizing peroxidases in Irpex lacteus combining the catalytic properties of heme peroxidases and laccase play important roles in ligninolytic system. Biotechnol. Biofuels 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Lončar, N.; Draškovic, N.; Božić, N.; Romero, E.; Simić, S.; Opsenica, I.; Vujcic, Z.; Fraaije, M.W. Expreesion and characterization of a dye-decolorizing peroxidase from Pseudomonas fluorescens Pf0-1. Catalysts 2019, 9, 463. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, M.; Ray, R.R. Current trends in research and application of microbial cellulases. Res. J. Microbiol. 2011, 6, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, J.; Lei, L.; Jiang, Y.; Gao, F.; Zhou, G.H. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different Sections of the gastrointestinal tract of broilers fed wheat-based diets. Asian Aust. J. Anim. Sci. 2014, 27, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Van Zyl, W.H.; Rosea, S.H.; Trollopeb, K.; Gorgensb, J.F. Fungal β-mannanases: Mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem. 2010, 45, 1203–1213. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial cellulases: An overview and applications. In Cellulose; Pascual, A.R., Martin, M.E.E., Eds.; Intechopen: London, UK, 2019; pp. 1–21. [Google Scholar]
- Daba, A.S.; Youssef, G.A.; Kabeil, S.S.; Hafez, E.E. Production of recombinant cellulase enzyme from Pleurotus ostreatus (Jacq.) P. Kumm. (type NRRL-0366). Afr. J. Microbiol. Res. 2011, 5, 1197–1202. [Google Scholar]
- Sharma, H.P.; Patel, H.; Sharma, S. Enzymatic extraction and clarification of juice from various fruits—A review. Trends Post Harvest Technol. 2014, 2, 1–14. [Google Scholar]
- Shi, H.; Ding, H.; Huang, Y.; Wang, L.; Zhang, Y.; Li, X.; Wang, F. Expression and characterization of a GH43 endo-arabinanase from Thermotoga thermarum. BMC Biotechnol. 2014, 14, 35. [Google Scholar] [CrossRef] [Green Version]
- Saleem, F.; Ahmed, S.; Jamil, A. Isolation of a xylan degrading gene from genomic DNA library of a thermophilic fungus Chaetomium thermophile ATCC 28076. Pak. J. Bot. 2008, 40, 1225–1230. [Google Scholar]
- Khanongnuch, C.; Sanguansook, C.; Lumyong, S. Nutritive quality of β-mannanase treated copra meal in broiler diets and effectiveness on some fecal bacteria. Int. J. Poult. Sci. 2006, 5, 1087–1091. [Google Scholar]
- Järvinen, J.; Taskila, S.; Isomäki, R.; Ojamo, H. Screening of white-rot fungi manganese peroxidases: A comparison between the specific activities of the enzyme from different native producers. AMB Express 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, D.; Larrondo, L.F.; Putnam, N.; Gelpke, M.D.S.; Huang, K.; Chapman, J.; Helfenbein, K.G.; Ramaiya, P.; Detter, C.J.; Larimerm, F.; et al. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nature 2004, 22, 695–700. [Google Scholar]
- Iimura, Y.; Sonoki, T.; Habe, H. Heterologous expression of Trametes versicolor laccase in Saccharomyces cerevisiae. Protein Expr. Purif. 2018, 141, 39–43. [Google Scholar] [CrossRef] [PubMed]





| Agro-Industrial Wastes | Composition (% Dry Weight Basis) | C/N Ratio | Reference | ||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | |||
| Apple pomace | 43 | 24 | 20 | 48/1 | [19] |
| Banana straw | 53 | 29 | 15 | 40/1 | [20] |
| Banana leaves | 55 | 20 | 25 | 38/1 | [21] |
| Barley straw | 23–33 | 21–22 | 14–19 | 82–120/1 | [22,23] |
| Canola straw | 22 | 17 | 18 | 33–45/1 | [23] |
| Coconut husk | 24–43 | 3–12 | 25–45 | 75–186/1 | [24,25] |
| Coffee husk | 43 | 7 | 9 | 40/1 | [26] |
| Corn bran | 34 | 39 | 49 | ND | [25] |
| Corn cob | 35–45 | 35–44 | 11–15 | 50–123/1 | [27,28] |
| Corn stalk | 34–61 | 19–24 | 7–9 | 57–80/1 | [25,29] |
| Corn straw | 30 | 25 | 8 | 50/1 | [25] |
| Cotton stalk | 58 | 14 | 22 | 70–78/1 | [22] |
| Grasses | 25–41 | 25–50 | 7–30 | 16–42/1 | [30] |
| Hardwoods | 40–55 | 24–40 | 18–25 | 150–450/1 | [30] |
| Oat bran | 49 | 25 | 18 | 12/1 | [25] |
| Oat straw | 25–40 | 21–27 | 17–18 | 48–83/1 | [22,23] |
| Rice bran | 35 | 25 | 17 | 12–48/1 | [25] |
| Rice husk | 35 | 25 | 20 | 30–80/1 | [31] |
| Rice straw | 32–39 | 23–24 | 18–36 | 35–72/1 | [29,32] |
| Rye straw | 38 | 31 | 19 | 82/1 | [22] |
| Beech sawdust | 41 | 33 | 22 | 100–331/1 | [33] |
| Birch sawdust | 40 | 36 | 20 | 700/1 | [33] |
| Oak sawdust | 25–38 | 18–29 | 18–25 | 162–200/1 | [31,33] |
| Pine sawdust | 42 | 25 | 28 | 724–1070/1 | [33] |
| Poplar sawdust | 44 | 32 | 21 | 46–71/1 | [33] |
| Rubber tree sawdust | 38 | 25 | 15 | 177/1 | [34] |
| Spruce sawdust | 42 | 26 | 28 | 763–1000/1 | [33] |
| Softwood | 45–50 | 25–35 | 25–35 | 310–520/1 | [30] |
| Sorghum stalk | 17 | 25 | 11 | 45/1 | [25] |
| Sorghum straw | 36 | 26 | 8 | 20–46/1 | [35,36] |
| Pineapple leaf | 36 | 23 | 27 | 49/1 | [37] |
| Pineapple peel | 22 | 75 | 3 | 77/1 | [38] |
| Potato peel | 35 | 5 | 4 | 25/1 | [39] |
| Orange peel | 9–14 | 6–11 | 1–2 | 102/1 | [40,41] |
| Lemon peel | 12 | 5 | 2 | ND | [41] |
| Tomato pomace | 9 | 5 | 5 | ND | [42] |
| Banana peel | 12 | 10 | 3 | 18–29/1 | [22] |
| Soya stalk | 35 | 25 | 20 | 20–40/1 | [43] |
| Sugarcane bagasse | 30–45 | 26–36 | 11–23 | 50/1 | [22,29,44] |
| Sugarcane straw | 36–41 | 21–31 | 16–26 | 70–120/1 | [45,46] |
| Sunflower stalk | 42 | 30 | 13 | 97/1 | [43] |
| Oil palm empty fruit bunch | 45–51 | 28–29 | 12–15 | 77/1 | [47,48] |
| Water hyacinth | 21 | 34 | 7 | 11/1 | [10] |
| Wheat bran | 30 | 50 | 15 | 19/1 | [25] |
| Wheat straw | 27–38 | 21–29 | 18–21 | 50–80/1 | [22,25,49] |
| Walnut shell | 36 | 28 | 43 | 175/1 | [50] |
| Almond shell | 38 | 29 | 30 | 61/1 | [51] |
| Chestnut shell | 21 | 16 | 36 | 8/1 | [51] |
| Pistachio shell | 43 | 25 | 16 | 43/1 | [51] |
| Hazelnut shell | 55 | 34 | 35 | 50–58/1 | [52] |
| Olive oil cake | 31 | 21 | 26 | 14–17/1 | [53] |
| Oil palm cake | 64 | 15 | 5 | ND | [54] |
| Sunflower oil cake | 25 | 12 | 8 | ND | [54] |
| Cotton seed hull | 31 | 20 | 18 | 59–67/1 | [55] |
| Mushroom Species | C/N Ratio (%) | Reference | ||
|---|---|---|---|---|
| Minimum | Optimum | Maximum | ||
| Agaricus bisporus | 16/1 | 19/1 | 22/1 | [68] |
| Agaricus bitorquis | 16/1 | 19/1 | 22/1 | [69] |
| Agaricus brasiliensis | 10/1 | 26–28/1 | 50/1 | [70] |
| Agaricus brunescens | 16/1 | 19/1 | 21/1 | [71] |
| Agaricus subrufescens | 16/1 | 27/1 | 33/1 | [72] |
| Lentinula edodes | 25/1 | 30–35/1 | 55/1 | [73] |
| Lentinus sajor-caju | 40/1 | 45–55/1 | 90/1 | [74] |
| Pleurotus cornucopiae | 40/1 | 45–55/1 | 97/1 | [75] |
| Pleurotus eryngii | 40/1 | 45–55/1 | 70/1 | [75] |
| Pleurotus flabellatus | 40/1 | 45–60/1 | 100/1 | [76] |
| Pleurotus florida | 40/1 | 45–60/1 | 150/1 | [77,78] |
| Pleurotus ostreatus | 40/1 | 45–60/1 | 90/1 | [78] |
| Flammulina velutipes | ND | 30/1 | ND | [79] |
| Ganoderma lucidum | ND | 70–80/1 | ND | [80] |
| Volvariella volvacea | ND | 40–60/1 | ND | [81] |
| Agro-Industrial Wastes | Mushroom Species | Biological Efficacy (%) | Chemical Composition (% Dry Weight) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Crude Protein | Carbohydrate | Fat | Fiber | Ash | ||||
| Wheat straw | Agaricus bisporus | 47.2–51.1 | 21.0–27.0 | 38.0–48.0 | 3.0–4.0 | 17.0–23.3 | 8.0–11.0 | [83,84] |
| Agaricus subrufescens | 53.7 | 28.4 | 63.2 | 1.6 | 6.2 | 6.8 | [85] | |
| Agrocybe cylindracea | 61.4 | 1.5 | 89.6 | 0.3 | 40.4 | 8.6 | [86] | |
| Hericium erinaceus | 39.4–43.5 | 26.8 | 58.9 | 3.7 | ND | 10.5 | [87] | |
| Lentinula edodes | 66.0–93.1 | 15.2–15.4 | 63.7–65.7 | 1.1–1.5 | ND | 3.8–4.4 | [88] | |
| Lentinus sajor-caju | 74.9 | 22.9 | 56.0 | 2.6 | 7.1 | 6.6 | [89] | |
| Pleurotus citrinopileatus | 98.3–105.6 | 25.3 | 64.0 | 2.7 | ND | 8.1 | [90] | |
| Pleurotus columbinus | 69.2 | 2.9 | 25.9 | 0.42 | 5.4 | 8.5 | [91] | |
| Pleurotus eous | 75.1 | 19.5 | 50.2 | 2.6 | 7.8 | 6.0 | [92] | |
| Pleurotus eryngii | 48.2 | 21.5 | 56.0 | 2.4 | 13.5 | 7.6 | [93] | |
| Pleurotus florida | 66.4 | 27.9 | 51.2 | 2.4 | 12.2 | 8.7 | [94] | |
| Pleurotus ostreatus | 22.6–52.6 | 11.6–14.6 | 47.5–74.4 | 1.8–2.5 | 19.1–27.1 | 8.6–12.0 | [86,95] | |
| Pleurotussapidus | 62.2 | 14.9 | 48.5 | 2.0 | 7.3 | 6.2 | [96] | |
| Barley straw | Lentinula edodes | 64.1–88.6 | 15.1–16.8 | 75.1–77.7 | 1.9–2.2 | ND | 5.2–5.8 | [88] |
| Pleurotus ostreatus | 21.3 | 12.8 | 54.7 | 29.9 | 0.9 | 1.2 | [95] | |
| Oat straw | Agaricus bisporus | 47.2–52.9 | 26.8–36.2 | ND | 2.3–3.1 | 6.6–10.3 | 9.8–11.3 | [97] |
| Ganoderma lucidum | 2.3 | 9.9 | ND | ND | ND | 1.0 | [98] | |
| Rice straw | Lentinula edodes | 48.7 | 16.2 | 78.0 | 6.0 | 1.5 | 3.4 | [99] |
| Lentinus sajor-caju | 78.3 | 23.4 | 55.0 | 2.4 | 7.9 | 6.8 | [89] | |
| Hericium erinaceus | 33.9 | 24.1 | 60.5 | 4.2 | ND | 11.3 | [87] | |
| Pleurotus citrinopileatus | 76.5–89.2 | 22.8 | 64.9 | 3.2 | ND | 91 | [90] | |
| Pleurotus columbinus | 71.4 | 4.8 | 27.3 | 0.3 | 5.0 | 7.7 | [91] | |
| Pleurotus eous | 79.8 | 29.3 | 48.0 | 2.4 | 8.0 | 6.2 | [92] | |
| Pleurotus eryngii | 45.9 | 21.8 | 53.0 | 1.9 | 13.8 | 8.7 | [93] | |
| Pleurotus pulmonarius | 23.5 | 21.1 | ND | 5.2 | 7.0 | 6.9 | [100] | |
| Pleurotus ostreatus | 25.6–84.6 | 12.5–23.4 | 55.3–57.4 | 2.8–16.2 | 7.7–0.7 | 6.3–13.6 | [95,101] | |
| Pleurotussapidus | 64.7 | 23.4 | 45.6 | 1.6 | 8.0 | 6.4 | [96] | |
| Pleurotus djamor | 82.7 | 24.8 | 37.7 | 3.1 | 22.0 | 8.3 | [102] | |
| Volvariella volvacea | 10.2–15.0 | 36.9–38.1 | 42.8–42.3 | 0.8–1.0 | 4.4–6.0 | 9.0–10.3 | [103,104,105] | |
| Corprinus comatus | 18.0 | 10.9 | 76.6 | 1.9 | ND | 20.5 | [106] | |
| Corn straw | Pleurotus florida | 31.6 | 26.3 | 31.3 | 0.5 | 19.6 | 5.2 | [107] |
| Volvariella volvacea | ND | 23.0 | 13.9 | 1.4 | 36.6 | 11.9 | [108] | |
| Corn cob | Agrocybe cylindracea | 33.5 | 14.8 | 72.4 | 2.9 | 17.0 | 10.1 | [86] |
| Pleurotus columbinus | 79.1 | 1.9 | 28.5 | 0.2 | 4.12 | 9.3 | [91] | |
| Pleurotus cystidiosus | 50.1 | 24.5 | 40.6 | 3.0 | 24.3 | 7.57 | [109] | |
| Pleurotus eryngii | 51.8 | 23.8 | 54.8 | 1.9 | 9.7 | 7.0 | [93] | |
| Pleurotus florida | 55.0 | 29.1 | 38.2 | 0.9 | 22.8 | 3.5 | [107] | |
| Pleurotus ostreatus | 31.7–66.1 | 15.4–29.7 | 30.8–73.4 | 2.7–3.4 | 13.8–29.8 | 7.1—8.0 | [86,109] | |
| Banana leaves | Pleurotus ostreatus | ND | 15.0 | 24.9 | 2.2 | 5.1 | 11.2 | [62,110] |
| Pleurotus pulmonarius | 17.9 | 16.9–23.5 | 26.2 | 1.9–5.5 | 5.8–7.2 | 6.4–10.3 | [62,100] | |
| Volvariella volvacea | 15.2 | 23.9 | ND | ND | 8.1 | 6.1 | [111] | |
| Soya stalk | Lentinus sajor-caju | 83.0 | 25.8 | 52.2 | 2.8 | 6.7 | 7.3 | [89] |
| Pleurotus eous | 82.3 | 30.5 | 50.5 | 2.6 | 9.0 | 6.5 | [92] | |
| Pleurotus ostreatus | 85.2 | 24.7 | 53.2 | 2.8 | 7.2 | 6.7 | [101] | |
| Pleurotus columbinus | 90.6 | 7.4 | 33.3 | 0.4 | 5.1 | 9.2 | [91] | |
| Pleurotus florida | 87.6 | 23.5 | 57.8 | 2.5 | 8.0 | 8.0 | [112] | |
| Pleurotus sapidus | 72.7 | 26.8 | 24.9 | 2.1 | 7.5 | 7.0 | [96] | |
| Sunflower stalk | Lentinus sajor-caju | 63.1 | 21.0 | 50.7 | 2.8 | 7.7 | 6.9 | [89] |
| Pleurotus eous | 61.5 | 27.4 | 52.0 | 2.2 | 7.9 | 5.2 | [92] | |
| Pleurotus sapidus | 45.9 | 20.1 | 48.5 | 2.4 | 7.3 | 6.2 | [96] | |
| Oil palm empty fruit bunch | Schizopyllum commune | 3.7 | 6.1 | 37.4 | 4.5 | 0.01 | 1.94 | [113] |
| Volvariella volvacea | 3.6–6.5 | 33.5–41.0 | 27.9–45.7 | 3.7–5.1 | 7.7–16.0 | 9.4–9.9 | [114] | |
| Cotton stalk | Pleurotus florida | 25.1 | 29.8 | 37.3 | 2.2 | 19.4 | 8.7 | [115] |
| Pleurotus pulmonarius | 42.3 | 29.3 | 44.5 | 3.1 | 11.3 | 9.2 | [115] | |
| Pleurotus ostreatus | 44.3 | 30.1 | 40.2 | 2.1 | 17.2 | 8.4 | [115] | |
| Rice husk | Pleurotus ostreatus | 9.5 | 5.9 | 48.5 | 30.9 | 0.3 | 14.3 | [95] |
| Sugarcane bagasse | Lentinula edodes | 130.0–133.0 | 13.1–13.8 | 73.0–78.9 | 0.9–1.0 | ND | 6.2–7.1 | [116] |
| Pleurotus cystidiosus | 49.5 | 22.1 | 45.2 | 2.3 | 22.8 | 7.5 | [109] | |
| Pleurotus djmor | 101.7 | 25.1 | 45.2 | 2.1 | 9.1 | 4.1 | [117] | |
| Pleurotus eryngii | 41.3 | 20.5 | 49.0 | 3.1 | 8.0 | 7.8 | [93] | |
| Pleurotus florida | 75.6 | 8.7 | ND | 4.0 | 2.5 | 0.3 | [118] | |
| Pleurotus ostreatus | 65.7 | 27.1 | 34.9 | 2.0 | 29.3 | 6.7 | [109] | |
| Sugarcane straw | Lentinula edodes | 83.0–98.0 | 14.4 | 72.5–78.2 | 0.7–0.9 | NR | 6.4–6.5 | [116] |
| Cottonseed hull | Pleurotus florida | 13.6 | 20.0 | 61.2 | 11.9 | 11.9 | 5.5 | [119] |
| Pleurotus ostreatus | 8.9 | 17.5 | 65.9 | 1.2 | 10.2 | 5.2 | [119] | |
| Cassava peel | Pleurotus ostreatus | 24.0–26.1 | 10.5–10.7 | 73.0–74.6 | 2.1–2.2 | 8.5–8.9 | 7.5–7.7 | [120] |
| Volvariella volvacea | 0.6-2.3 | 11.5–14.3 | 51.4–53.4 | 2.4–2.6 | 0.4–0.5 | 5.0–6.2 | [121] | |
| Hardwood sawdust | Hericium erinaceus | 47.5–50.3 | 24.8 | 60.9 | 3.6 | ND | 10.6 | [87] |
| Acacia sawdust | Pleurotus cystidiosus | 36.3 | 15.7 | 55.9 | 2.1 | 20.1 | 6.3 | [109] |
| Pleurotus ostreatus | 46.4 | 19.5 | 51.3 | 1.3 | 22.0 | 5.9 | [109] | |
| Beech sawdust | Agrocybe cylindracea | 38.3 | 18.4 | 70.3 | 3.4 | 15.0 | 8.2 | [86] |
| Ganoderma lucidum | 61.2 | 16.8 | 77.9 | 2.2 | 47.9 | 3.1 | [122] | |
| Pleurotus ostreatus | 46.8 | 16.1 | 73.6 | 3.5 | 15.8 | 6.2 | [86] | |
| Sawdust | Auricularia polytricha | 13.9–44.6 | 10.2 | 78.4 | 0.9 | ND | 4.2 | [123] |
| Pleurotus columbinus | 89.1 | 1.7 | 25.0 | 0.2 | 4.6 | 9.1 | [91] | |
| Pleurotus citrinopileatus | 38.4–51.6 | 24.1 | 65.6 | 2.6 | ND | 7.8 | [90] | |
| Pleurotus eryngii | 35.5 | 19.5 | 52.5 | 2.4 | 7.8 | 7.5 | [93] | |
| Agro-Industrial Wastes | Mushroom Species | Biological Efficacy (%) | Chemical Composition (% Dry Weight) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Crude Protein | Carbohydrate | Fat | Fiber | Ash | ||||
| Soya stalk (50%) + rice straw (50%) | Pleurotus florida | 85.2 | 22.7 | 54.9 | 2.6 | 7.6 | 6.5 | [111] |
| Pleurotus ostreatus | 81.7 | 23.0 | 50.5 | 2.7 | 7.7 | 6.4 | [124] | |
| Soya stalk (50%) + wheat straw (50%) | Pleurotus florida | 78.2 | 22.4 | 57.1 | 2.3 | 7.5 | 6.4 | [111] |
| Pleurotus ostreatus | 77.7 | 21.1 | 52.0 | 2.6 | 7.4 | 6.2 | [124] | |
| Wheat straw (50%) + Rice straw (50%) | Hericium erinaceus | 32.5–37.2 | 25.6 | 60.6 | 3.9 | ND | 9.7 | [87] |
| Pleurotus florida | 72.3 | 20.2 | 53.9 | 2.3 | 7.4 | 6.5 | [111] | |
| Pleurotus ostreatus | 71.8 | 20.3 | 56.0 | 2.6 | 7.5 | 5.9 | [124] | |
| Oat straw (80%) + wheat bran (20%) | Ganoderma lucidum | 2.0–2.5 | 10.6–12.5 | ND | ND | 47.8–57.7 | 1.3–1.5 | [98] |
| Cotton stalk (50%) + Cottonseed hull (50%) | Pleurotus florida | 17.3 | 24.5 | 52.0 | 3.2 | 13.2 | 7.1 | [119] |
| Pleurotus ostreatus | 20.2 | 22.8 | 58.0 | 2.9 | 10.8 | 5.5 | [119] | |
| Acacia sawdust (50%) + corn cob (50%) | Pleurotus cystidiosus | 43.6 | 21.4 | 44.8 | 2.8 | 23.6 | 7.3 | [109] |
| Pleurotus ostreatus | 58.8 | 18.7 | 46.9 | 3.3 | 24.5 | 6.7 | [109] | |
| Acacia sawdust (50%) + sugarcane bagasse (50%) | Pleurotus cystidiosus | 41.1 | 25.6 | 37.5 | 1.8 | 28.5 | 6.8 | [109] |
| Pleurotus ostreatus | 58.9 | 24.2 | 37.8 | 2.5 | 28.8 | 6.7 | [109] | |
| Sugarcane bagasse (50%) + grasses (50%) | Agaricus brasiliensis | 44.3 | 28.3 | ND | 1.6 | 5.8 | 6.7 | [125] |
| Rubber tree sawdust (50%) + rice straw (50%) | Flammulina velutipes | 123.9 | 17.0–27.0 | 58.0–87.0 | 1.8–7.3 | ND | 7.3–10.4 | [126] |
| Beech sawdust (50%) + olive pruning residues (50%) | Ganoderma lucidum | 20.5 | 15.3 | 79.3 | 2.0 | 43.8 | 3.4 | [122] |
| Wheat straw (50%) + olive pruning residues (50%) | Pleurotus ostreatus | 56.8 | 19.9 | 71.7 | 1.9 | 16.5 | 6.5 | [122] |
| Sawdust (90%) + rice bran (10%) | Pleurotus eous | 48.4–68.1 | 27.8 | 28.6 | 5.6 | 17.3 | 4.9 | [127] |
| Sugarcane bagasse (50%) + rice straw (50%) | Lentinus sajor-caju | 83.9 | 30.9 | 33.8 | ND | 24.5 | 6.9 | [128] |
| Cassava peel (50%) + corn cobs (50%) | Pleurotus ostreatus | 31.1–33.7 | 10.6–10.8 | 73.6–74.8 | 2.1–2.2 | 8.6–8.9 | 7.3–7.8 | [120] |
| Hard wood sawdust (50%) + rice straw (50%) | Hericium erinaceus | 36.5–44.2 | 25.1 | 59.8 | 4.0 | ND | 11.0 | [87] |
| Hard wood sawdust (50%) + wheat straw (50%) | Hericium erinaceus | 41.4–46.5 | 24.7 | 60.8 | 4.2 | ND | 10.3 | [87] |
| Hardwood sawdust (30%) + corn stalk (60%) + rice bran (10%) | Auricularia polytricha | 27.3–41.0 | 11.1 | 76.1 | 0.9 | ND | 4.8 | [129] |
| Enzyme | Agro-Industrial Wastes | Mushroom Species | Activity | Reference |
|---|---|---|---|---|
| Total cellulase | Wheat straw | Lentinula edodes | 45–60 U/mL | [151] |
| Pleurotus dryinus | 41–120 U/mL | [151,160] | ||
| Pleurotus ostreatus | 665–1185 U/mL | [151] | ||
| Pleurotus tuber-regium | 505 U/mL | [151] | ||
| Fomitopsis sp. | 3.5 U/gds | [159] | ||
| Tree leaves (Fagus sylvatica) | Lentinula edodes | 40–45 U/mL | [151] | |
| Pleurotus dryinus | 205 U/mL | [151] | ||
| Pleurotus ostreatus | 14–15 U/mL | [151] | ||
| Pleurotus tuber-regium | 20 U/mL | [151] | ||
| Sorghum straw | Pycnosporus sanguineus | 0.8 U/gds | [153] | |
| Pleurotus ostreatus | 1.3 U/gds | [153] | ||
| Pleurotus eryngii | 0.7 U/gds | [153] | ||
| Phanerochaete chrysosporium | 1.1 U/gds | [153] | ||
| Trametes versicolor | 1.0 U/gds | [153] | ||
| Eucalyptus wood chip | Wolfiporia cocos | 1.4–8.3 U/gds | [161] | |
| Laetiporeus sulfureus | 0.8–15.4 U/gds | [161] | ||
| Poria medulla-panis | 0.4–3.4 U/gds | [161] | ||
| Pycnoporus coccineus | 1.8–3.7 U/gds | [161] | ||
| Phlebia tremellosa | 0.6–2.0 U/gds | [161] | ||
| Trametes versicolor | 0.8–4.0 U/gds | [161] | ||
| Endoglucanase | Wheat straw | Lentinus tigrinus | 1230 U/gds | [156] |
| Lentinula edodes | 180–345 U/mL | [151] | ||
| Pleurotus dryinus | 910 U/mL | [151] | ||
| Pleurotus ostreatus | 185-245 U/mL | [151] | ||
| Pleurotus tuber-regium | 150 U/mL | [151] | ||
| Piptoporus betulinus | 83.5 U/gds | [157] | ||
| Tree leaves (Fagus sylvatica) | Lentinula edodes | 40–65 U/mL | [151] | |
| Pleurotus dryinus | 1130 U/mL | [151] | ||
| Pleurotus ostreatus | 25–1300 U/mL | [151] | ||
| Pleurotus tuber-regium | 150 U/mL | [151] | ||
| Sorghum straw | Pycnosporus sanguineus | 2.0 U/gds | [153] | |
| Pleurotus ostreatus | 2.3 U/gds | [153] | ||
| Pleurotus eryngii | 1.4 U/gds | [153] | ||
| Phanerochaete chrysosporium | 4.0 U/gds | [153] | ||
| Trametes versicolor | 2.2 U/gds | [153] | ||
| Sugarcane bagasse | Lentinus sajor-caju | 13.9–18.9 U/gds | [162] | |
| Pleurotus ostreatus | 3.0 U/gds | [163] | ||
| Sawdust | Trametes trogii | 504 U/gds | [164] | |
| Coriolus versicolor | 0.6 U/gds | [165] | ||
| Ganoderma applanatum | 0.1 U/gds | [165] | ||
| Pycnoporus sanguineus | 0.6 U/gds | [165] | ||
| Trametes villosa | 0.2 U/gds | [165] | ||
| Pleurotus ostreatus | 3.0 U/gds | [163] | ||
| Lentinus sajor-caju | 0.9 U/gds | [163] | ||
| Rice straw | Pleurotus ostreatus | 7.1 U/gds | [163] | |
| Lentinus sajor-caju | 1.9 U/gds | [163] | ||
| Oak sawdust | Grifola frondosa | 12.3 U/gds | [152] | |
| Pine chip | Coriolus versicolor | 2.4 U/gds | [165] | |
| Ganoderma applanatum | 2.8 U/gds | [165] | ||
| Pycnoporus sanguineus | 4.8 U/gds | [165] | ||
| Trametes villosa | 3.9 U/gds | [165] | ||
| Green tea waste | Microporus xanthopus | 38.6 U/gds | [166] | |
| Wheat straw | Fomitopsis sp. | 53.6 U/gds | [159] | |
| Exoglucanase | Oak sawdust | Grifola frondosa | 16.2 U/gds | [152] |
| Rice straw | Pleurotus ostreatus | 2.0 U/gds | [163] | |
| Lentinus sajor-caju | 1.8 U/gds | [163] | ||
| Sugarcane bagasse | Pleurotus ostreatus | 7.0 U/gds | [163] | |
| Lentinus sajor-caju | 2.0 U/gds | [163] | ||
| Sawdust | Pleurotus ostreatus | 2.8 U/gds | [163] | |
| Lentinus sajor-caju | 0.6 U/gds | [163] | ||
| Corn stover | Irpex lacteus | 69.3 U/gds | [167] | |
| β -Glucosidase | Sorghum straw | Pycnosporus sanguineus | 0.4 U/gds | [153] |
| Pleurotus ostreatus | 0.2 U/gds | [153] | ||
| Pleurotus eryngii | 0.2 U/gds | [153] | ||
| Phanerochaete chrysosporium | 1.1 U/gds | [153] | ||
| Trametes versicolor | 1.9 U/gds | [153] | ||
| Eucalyptus wood chip | Wolfiporia cocos | 8.3–42.0 U/gds | [161] | |
| Laetiporeus sulfureus | 7.6–37 U/gds | [161] | ||
| Poria medulla-panis | 2.7–10.5 U/gds | [161] | ||
| Pycnoporus coccineus | 8.0–22.0 U/gds | [161] | ||
| Phlebia tremellosa | 3.8–15.6 U/gds | [161] | ||
| Trametes versicolor | 3.8–20.0 U/gds | [161] | ||
| Oak sawdust | Grifola frondosa | 2.3 U/gds | [152] | |
| Rice straw | Pleurotus ostreatus | 2.5 U/gds | [163] | |
| Lentinus sajor-caju | 1.2 U/gds | [163] | ||
| Sugarcane bagasse | Pleurotus ostreatus | 3.5 U/gds | [163] | |
| Lentinus sajor-caju | 2.6–12.3 U/gds | [162,163] | ||
| Sawdust | Pleurotus ostreatus | 2.2 U/gds | [163] | |
| Lentinus sajor-caju | 0.2 U/gds | [163] | ||
| Coriolus versicolor | 0.5 U/gds | [165] | ||
| Ganoderma applanatum | 0.4 U/gds | [165] | ||
| Pycnoporus sanguineus | 0.4 U/gds | [165] | ||
| Trametes villosa | 0.5 U/gds | [165] | ||
| Trametes trogii | 0.89 U/gds | [164] | ||
| Pine chip | Coriolus versicolor | 0.3 U/gds | [165] | |
| Ganoderma applanatum | 0.1 U/gds | [165] | ||
| Pycnoporus sanguineus | 0.8 U/gds | [165] | ||
| Trametes villosa | 0.5 U/gds | [165] | ||
| Wheat straw | Piptoporus betulinus | 78.8 U/gds | [157] | |
| Pleurotus dryinus | 401 U/gds | [160] | ||
| Lentinula edodes | 0.1 U/gds | [168] | ||
| Sorghum straw | Pleurotus eryngii | 0.23 U/gds | [153] |
| Enzyme | Agro-Industrial Wastes | Mushroom Species | Activity | Reference |
|---|---|---|---|---|
| Total xylanase | Tree leaves (Fagus sylvatica) | Lentinula edodes | 85–200 U/mL | [151] |
| Pleurotus dryinus | 2145 U/mL | [151] | ||
| Pleurotus ostreatus | 160–1400 U/mL | [151] | ||
| Pleurotus tuber-regium | 155 U/mL | [151] | ||
| Wheat straw | Lentinula edodes | 195–275 U/mL | [151] | |
| Pleurotus dryinus | 1450 U/mL | [151] | ||
| Pleurotus ostreatus | 260–735 U/mL | [151] | ||
| Pleurotus tuber-regium | 260 U/mL | [151] | ||
| Wheat bran | Fomes fomentarius | 7–63 U/mL | [154] | |
| Ganoderma applanatum | 3 U/mL | [154] | ||
| Pleurotus ostreatus | 16 U/mL | [154] | ||
| Trametes hirsuta | 8 U/mL | [154] | ||
| Trametes ochracea | 35 U/mL | [154] | ||
| Trametes versicolor | 21 U/mL | [154] | ||
| Trametes pubescens | 32 U/mL | [154] | ||
| Trametes biforme | 19 U/mL | [154] | ||
| Endo-1,4-β-xylanase | Rice straw | Pleurotus ostreatus | 21 U/gds | [195] |
| Sawdust | Pleurotus ostreatus | 9 U/gds | [195] | |
| Sugarcane bagasse | Ganoderma lucidum | 33 U/gds | [196] | |
| Tomato pomace | Pleurotus ostreatus | 9 U/gds | [197] | |
| Trametes versicolor | 50 U/gds | [197] | ||
| Jerusalem artichoke stalk | Schizophyllum commune | 106 U/gds | [198] | |
| Oak leaves | Marasmius quercophilus | 73 U/gds | [199] | |
| Mycena inclinata | 105 U/gds | [199] | ||
| Pholiota lenta | 83.2 U/gds | [199] | ||
| Wheat straw | Pleurotus citrinopileatus | 0.12 U/gds | [200] | |
| Pleurotus ostreatus | 0.14 U/gds | [200] | ||
| Pine wood chip | Ceriporiopsis subvermispora | 0.25 U/gds | [201] | |
| Eucalyptus wood chip | Ceriporiopsis subvermispora | 0.12 U/gds | [201] | |
| Soya bran | Fomes sclerodermeus | 31 U/gds | [202] | |
| Rice bran mixed rice husk | Leucoagaricus meleagris | 0.8 U/gds | [203] | |
| Sugarcane bagasse mixed wheat bran | Pleurotus ostreatus | 8.7 U/gds | [204] | |
| Ganoderma lucidum | 16.3 U/gds | [204] | ||
| Trametes versicolor | 36.7 U/gds | [204] | ||
| 1,4-β-Xylosidase | Oak leaves | Marasmius quercophilus | 1.7 U/gds | [199] |
| Mycena inclinata | 5.8 U/gds | [199] | ||
| Pholiota lenta | 1.6 U/gds | [199] | ||
| Pine wood chip | Ceriporiopsis subvermispora | 4.4 U/gds | [201] | |
| Eucalyptus wood chip | Ceriporiopsis subvermispora | 2.6 U/gds | [201] | |
| Wheat straw | Pleurotus citrinopileatus | 11.5 U/gds | [200] | |
| Pleurotus ostreatus | 14.3 U/gds | [200] | ||
| Sugarcane bagasse mixed wheat bran | Ganoderma lucidum | 0.4 U/gds | [204] | |
| Trametes versicolor | 1.5 U/gds | [204] | ||
| Endo-1,4-β-mannanase | Oak leaves | Marasmius quercophilus | 3.4 U/gds | [199] |
| Mycena inclinata | 3.2 U/gds | [199] | ||
| Pholiota lenta | 11.8 U/gds | [199] | ||
| Pine wood chip | Ceriporiopsis subvermispora | 90.4 U/gds | [201] | |
| Eucalyptus wood chip | Ceriporiopsis subvermispora | 52.2 U/gds | [201] | |
| 1,4-β-Mannosidase | Oak leaves | Marasmius quercophilus | 5.9 U/gds | [199] |
| Mycena inclinata | 4.2 U/gds | [199] |
| Enzyme | Agro-Industrial Wastes | Mushroom Species | Activity | Reference |
|---|---|---|---|---|
| Laccase | Tree leaves (Fagus sylvatica) | Lentinula edodes | 7–52 U/L | [151] |
| Pleurotus dryinus | 16 U/L | [151] | ||
| Pleurotus ostreatus | 6.3–8.0 U/L | [151] | ||
| Pleurotus tuber-regium | 2.1 U/L | [151] | ||
| Wheat straw | Lentinula edodes | 3.6–5.2 U/L | [151] | |
| Pleurotus dryinus | 5.7 U/L | [151] | ||
| Pleurotus ostreatus | 1.1–10.1 U/L | [151] | ||
| Pleurotus tuber-regium | 10 U/L | [151] | ||
| Pleurotus citrinopileatus | 1.2–3.7 U/gds | [200] | ||
| Wheat bran | Fomes fomentarius | 7430–17510 U/L | [154] | |
| Ganoderma applanatum | 1910 U/L | [154] | ||
| Pleurotus ostreatus | 9210 U/L | [154] | ||
| Trametes hirsuta | 7350 U/L | [154] | ||
| Trametes ochracea | 3930 U/L | [154] | ||
| Trametes versicolor | 17860 U/L | [154] | ||
| Trametes pubescens | 5319 U/L | [154] | ||
| Trametes biforme | 4960 U/L | [154] | ||
| Wheat bran mixed corn straw | Trametes versicolor | 32.1 U/gds | [227] | |
| Laccase | Corn stalk | Trametes versicolor | 2,765.81 U/L | [228] |
| Sawdust | Coriolopsis gallica | 200 U/gds | [229] | |
| Sugarcane bagasse | Pleurotus ostreatus | 151.6 U/gds | [230] | |
| Oat husk | Cerrena unicolor | 28.2 U/gds | [231] | |
| Pineapple leaves | Ganoderma lucidum | 42.7 U/gds | [232] | |
| Rice bran mixed wheat bran | Stereum ostrea | 24962 U/L | [233] | |
| Rice straw | Schizophyllum commune | 431.2 U/gsd | [234] | |
| Sugarcane bagasse mixed wheat bran | Ganoderma lucidum | 9.4 U/gds | [204] | |
| Pleurotus ostreatus | 2.1 U/gds | [204] | ||
| Trametes versicolor | 1.9 U/gds | [204] | ||
| Soya bran | Fomes sclerodermeus | 14.5 U/gds | [202] | |
| Manganese peroxidase | Tree leaves (Fagus sylvatica) | Lentinula edodes | 1.0–6.7 U/L | [151] |
| Pleurotus dryinus | 5.7 U/L | [151] | ||
| Pleurotus ostreatus | 7–15 U/L | [151] | ||
| Pleurotus tuber-regium | 20 U/L | [151] | ||
| Wheat straw | Lentinula edodes | 20–55 U/L | [151] | |
| Pleurotus dryinus | 13 U/L | [151] | ||
| Pleurotus ostreatus | 7–12 U/L | [151] | ||
| Pleurotus tuber-regium | 2.2 U/L | [151] | ||
| Pleurotus citrinopileatus | 4.9 U/gds | [200] | ||
| Wheat bran | Fomes fomentarius | 350 U/L | [154] | |
| Pleurotus ostreatus | 20 U/L | [154] | ||
| Trametes versicolor | 20–50 U/L | [154] | ||
| Trametes biforme | 570 U/L | [154] | ||
| Oat husk | Cerrena unicolor | 20.4 U/gds | [231] | |
| Pineapple leaves | Ganoderma lucidum | 82.7 U/gds | [232] | |
| Rice bran mixed wheat bran | Stereum ostrea | 3895 U/L | [233] | |
| Rice straw | Schizophyllum commune | 1964 U/gsd | [234] | |
| Eucalyptus sawdust | Lentinula edodes | 700 U/gds | [235] | |
| Soya bran | Fomes sclerodermeus | 14.5 U/gds | [202] | |
| Sugarcane bagasse mixed wheat bran | Ganoderma lucidum | 1.9 U/gds | [204] | |
| Pleurotus ostreatus | 2.3 U/gds | [204] | ||
| Tramets versicolor | 2.1 U/gds | [204] | ||
| Barley husk | Bjerkandera adusta | 510 U/kgds | [236] | |
| Lignin peroxidases | Jatropha waste | Pleurotus ostreatus | 49916 U/L | [237] |
| Corn cob | Ganoderma lucidum | 561.4 U/gds | [238] | |
| Pineapple leaves | Ganoderma lucidum | 287.5 U/gds | [232] | |
| Rice bran mixed wheat bran | Stereum ostrea | 72.8 U/L | [233] | |
| Rice straw | Schizophyllum commune | 1467.3 U/gsd | [234] | |
| Barley husk | Bjerkandera adusta | 1700 U/kgds | [236] | |
| Sugarcane bagasse mixed wheat bran | Ganoderma lucidum | 0.6 U/gds | [204] | |
| Pleurotus ostreatus | 0.5 U/gds | [204] | ||
| Trametes versicolor | 0.7 U/gds | [204] | ||
| Versatile peroxidase | Banana peel | Pleurotus eryngii | 36 U/gds | [239] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. https://doi.org/10.3390/molecules25122811
Kumla J, Suwannarach N, Sujarit K, Penkhrue W, Kakumyan P, Jatuwong K, Vadthanarat S, Lumyong S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules. 2020; 25(12):2811. https://doi.org/10.3390/molecules25122811
Chicago/Turabian StyleKumla, Jaturong, Nakarin Suwannarach, Kanaporn Sujarit, Watsana Penkhrue, Pattana Kakumyan, Kritsana Jatuwong, Santhiti Vadthanarat, and Saisamorn Lumyong. 2020. "Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste" Molecules 25, no. 12: 2811. https://doi.org/10.3390/molecules25122811
APA StyleKumla, J., Suwannarach, N., Sujarit, K., Penkhrue, W., Kakumyan, P., Jatuwong, K., Vadthanarat, S., & Lumyong, S. (2020). Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules, 25(12), 2811. https://doi.org/10.3390/molecules25122811








