Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Gut Microbiota Analysis
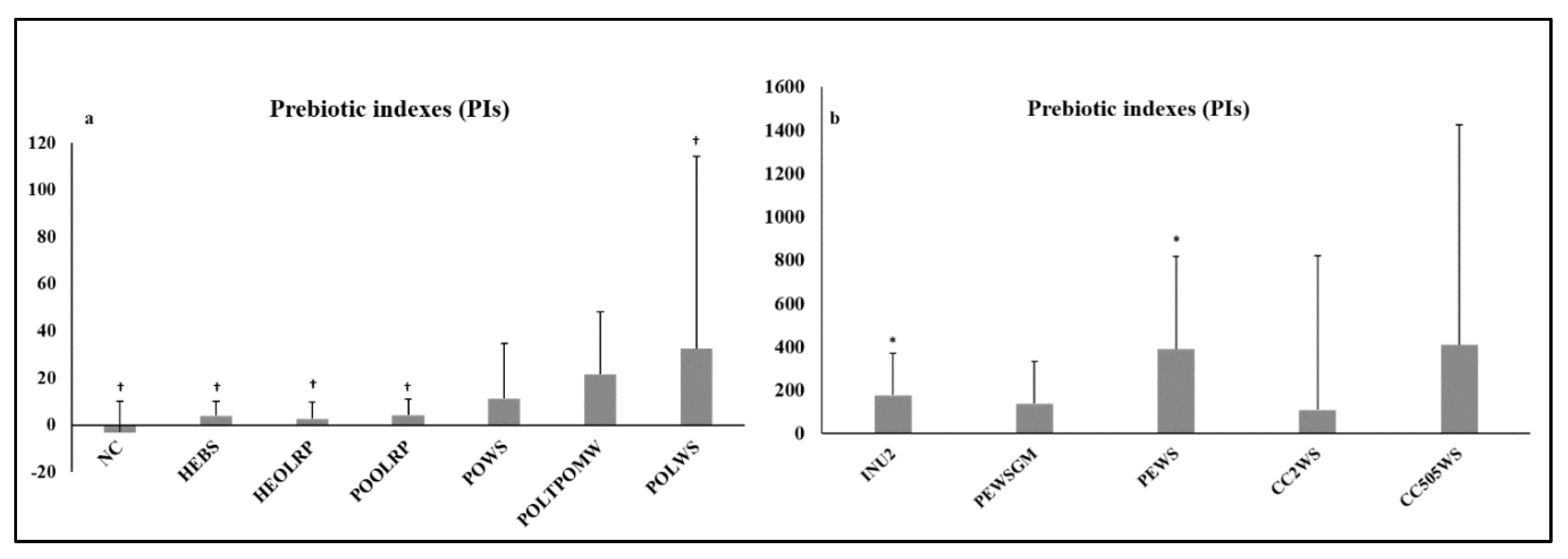
2.2. Prebiotic Indexes
2.3. Short Chain Fatty Acids (SCFAs) Analysis
3. Discussion
4. Materials and Methods
4.1. Fungal Strains, Cultivation Substrates, Mushroom Production and Glucans Content
4.2. Faecal Donors’ Characteristics
4.3. Ethical Standards
4.4. Fecal Sample Collection and In Vitro Static Batch Culture Fermentations
4.5. Gut Microbiota Analysis
4.6. Prebiotic Indexes
4.7. Measurement of SCFAs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidjani Alou, M.; Lagier, J.-C.; Raoult, D. Diet Influence on the Gut Microbiota and Dysbiosis related to Nutritional Disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food. Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.; Rosa, F.; Rossi, N.; Martin, T.; Mohney, R.; Li, W.; Rinaldis, E.; Bell, J.; Venter, J.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [Green Version]
- Pickard, J.; Zeng, M.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.; Luminos, L. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-Y.; Ning, M.-X.; Chen, D.-K.; Ma, W. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Belizario, J.; Faintuch, J. Microbiome and Gut Dysbiosis. In Metabolic Interaction in Infection. Experientia Supplementum; Silvestre, R., Torrado, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 109, pp. 459–476. [Google Scholar]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-S.; Chang, C.-J.; Lu, C.-C.; Martel, J.; Ojcius, D.; Ko, Y.-F.; Young, J.; Lai, H.-C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed. J. 2014, 37, 259–268. [Google Scholar]
- Gibson, G.; Probert, H.; Loo, J.; Rastall, R.; Roberfroid, M.; Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2005, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.T.; Seifert, N.; Richard, N.; Raederstorff, D.; Steinert, R.E.; Prudence, K.; Mohajeri, M.H. The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. Peer J. 2018, 6, e5288. [Google Scholar] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciecierska, A.; Drywień, M.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [Google Scholar] [PubMed]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of Olive Mill Wastewater and Bioconversion of Olive Crop Residues into High-Value-Added Biomass by the Choice Edible Mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C.; Michaud, P. New Developments and Prospective Applications for β (1,3) Glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef]
- Besten, G.; van Eunen, K.; Groen, A.; Venema, K.; Reijngoud, D.-J.; Bakker, B. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Takagi, R.; Sasaki, K.; Fukuda, I.; Tanaka, K.; Yoshida, K.-i.; Kondo, A.; Osawa, R. A Single-Batch Fermentation System to Simulate Human Colonic Microbiota for High-Throughput Evaluation of Prebiotics. PLoS ONE 2016, 11, e0160533. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2017, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.; Hoyles, L.; McCartney, A.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, H.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.; Mohajeri, H. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Benef. Microbes 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Gibson, G.; Walton, G. An In Vitro Approach to Study Effects of Prebiotics and Probiotics on the Faecal Microbiota and Selected Immune Parameters Relevant to the Elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, W.; Pei, F.; Zhao, L.; Hu, Q. In vitro fermentation of six kinds of edible mushrooms and its effects on fecal microbiota composition. LWT-Food Sci. Technol. 2018, 96, 627–635. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, B.; Mukherjee, P.; Newburg, D. Trametes versicolor Extract Modifies Human Fecal Microbiota Composition In vitro. Plant. Food Hum. Nutr. 2013, 68, 107–112. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Sârbu, I. In Vitro Ecological Response of the Human Gut Microbiome to Bioactive Extracts from Edible Wild Mushrooms. Molecules 2018, 23, 2128. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.; Walton, G.; Sousa, S.; Rocha-Santos, T.; Duarte, A.; Freitas, A.; Gomes, A. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT-Food Sci. Technol. 2016, 73, 131–139. [Google Scholar] [CrossRef]
- Chaikliang, C.; Wichienchot, S.; Youravong, W.; Graidist, P. Evaluation on prebiotic properties of β-glucan and oligo-β-glucan from mushrooms by human fecal microbiota in fecal batch culture. Funct. Food Health Dis. 2015, 5, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Jungles, T.; Ruthes, A.; El-Hindawy, M.; Moreno, R.; Zhang, X.; Cordeiro, L.; Hamaker, B.; Iacomini, M. In vitro fermentation of Cookeina speciosa glucans stimulates the growth of the butyrogenic Clostridium cluster XIVa in a targeted way. Carbohydr. Polym. 2017, 183, 219–229. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmi, A.; Shuhaimi, M.; Abd Manap, Y.; Maaruf, A.G. Mushroom as a potential source of prebiotics: A review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar]
- Gargano, M.; Van Griensven, L.; Isikhuemhen, O.; Lindequist, U.; Venturella, G.; Wasser, S.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant. Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Gavini, C. Differences in the Distribution of Bifidobacterial and Enterobacterial Species in Human Faecal Microflora of Three Different (Children, Adults, Elderly) Age Groups. Microb. Ecol. Health Dis. 2001, 13, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef] [Green Version]
- Toward, R.; Montandon, S.; Walton, G.; Gibson, G. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Juric, A.; Walton, G.; Claus, S.; Tzortzis, G.; Toward, R.; Gibson, G. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Rochat, F.; Perruisseau-Carrier, G.; Rochat, I.; Schiffrin, E.J. Effects of oligosaccharide on the faecal flora and non-specific immune system in elderly people. Nutr. Res. 2002, 22, 13–25. [Google Scholar] [CrossRef]
- Bouhnik, y.; Achour, L.; Paineau, D.; Riottot, M.; Attar, A.; Bornet, F.R.J. Four-week short chain fructo-oligosaccharides ingestion leads to increasing fecal bifidobacteria and cholesterol excretion in healthy elderly volunteers. Nutr. J. 2007, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonsky, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Broek, T.; Schuren, F.; Steinert, R.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Muinde, B.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. In vivo fermentation of Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, Y. A Potent Pharmacological Mushroom: Pleurotus eryngii. Fungal Genet. Biol. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Lu, J.; Qin, J.-Z.; Chen, P.; Chen, X.; Zhang, Y.-Z.; Zhao, S.-J. Quality Difference Study of Six Varieties of Ganoderma lucidum with Different Origins. Front. Pharmacol. 2012, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef]
- Flint, H.; Louis, P.; Duncan, S. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. From the Cover: Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Palframan, R.; Gibson, G.R.; Rastall, R.A. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalari, G.; Faulks, R.; Bisignano, C.; Waldron, K.; Narbad, A.; Wickham, M. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoddusi, H.; Grandison, M.A.; Grandison, A.; Tuohy, K. In vitro study on gas generation and prebiotic effects of some carbohydrates and their mixtures. Anaerobe 2007, 13, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; de Wouters, T.; Lacroix, C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci. Rep. 2018, 8, 4318. [Google Scholar] [CrossRef]
- Dalile, B.; Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Morrison, D.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Moens, F.; Selak, M.; Rivière, A.; Leroy, F. Summer Meeting 2013: Growth and physiology of bifidobacteria. J. Appl. Microbiol. 2014, 116, 477–491. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Zhang, X.; Huang, H. Structure characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its immunomodulatory activities. Food Funct. 2017, 9, 294–306. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Tang, C.-h.; Fan, J.-m.; Shi, X.-m.; Pan, Y.-j. Structural investigation of a novel fucoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Food Chem. 2007, 104, 451–456. [Google Scholar]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi--assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.; Yannakoulia, M.; Panagiotakos, D.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability Measures of the Short International Physical Activity Questionnaire (IPAQ) in Greek Young Adults. Hellenic J.Cardiol. 2009, 50, 283–294. [Google Scholar]
- Olano-Martin, E.; Gibson, G.R.; Rastell, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Rycroft, C.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosacharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzeloo-Moghadam, M.; Taiebi, N.; Mosaddegh, M.; Tehrani, B.; Esmaeili, S. The effect of some cosolvents and surfactants on viability of cancerous cell lines. RJP 2014, 1, 41–45. [Google Scholar]
- Zhang, N.; Huang, X.; Zeng, Y.; Wu, X.; Peng, X. Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci. Hum. Wellness 2013, 2, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Palframan, R.; Gibson, G.; Rastall, R. Effect of pH and Dose on the Growth of Gut Bacteria on Prebiotic Carbohydrates in vitro. Anaerobe 2002, 8, 287–292. [Google Scholar] [CrossRef]
- Nadkarni, M.; Martin, F.; Jacques, N.; Hunter, N.; Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology (Reading, Engl.) 2002, 148, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Lyra, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rRNA-targeted primers for quantification of pathogenic and indigenous bacteria in fecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota. Stimulation of Bifidobacterium adolescentis and Fecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phong, S.F.; Shanmugavelu, S.; Thayalini, K.; Noraini, S.; Wong, H.K. Detection of Lactobacillus, Bacteroides and Clostridium perfringens in the gastrointestinal contents of chicken fed different diets by real-time PCR. J. Trop. Agric. Sci. 2010, 38, 81–87. [Google Scholar]
- Walker, A.; Ince, J.; Duncan, S.; Webster, L.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tang, H.; Li, M.; Pang, X.; Wang, L.; Zhang, M.; Zhao, Y.; Zhang, X.; Shen, J. The abundance of fecal Faecalibacterium prausnitzii in relation to obesity and gender in Chinese adults. Arch. Microbiol. 2013, 196, 73–77. [Google Scholar] [CrossRef]
- Suau, A.; Rochet, V.; Sghir, A.; Gramet, G.; Brewaeys, S.; Sutren, M.; Rigottier-Gois, L.; Dore, J. Fusobacterium Prausnitzii and Related Species Represent a Dominant Group Within the Human Fecal Flora. Syst. Appl. Microbiol. 2001, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [Green Version]
- Mountzouris, K.; Balaskas, C.; Fava, F.; Tuohy, K.; Gibson, G.; Kostas, F. Profiling of composition and metabolic activities of the colonic microflora of growing pigs fed diets supplemented with prebiotic oligosaccharides. Anaerobe 2006, 12, 178–185. [Google Scholar] [CrossRef]
- Mitsou, E.; Kougia, E.; Nomikos, T.; Yannakoulia, M.; Mountzouris, K.; Kyriacou, A. Effect of banana consumption on faecal microbiota: A randomised, controlled trial. Anaerobe 2011, 17, 384–387. [Google Scholar] [CrossRef]
Sample Availability: Samples of the materials used in this study are available from the authors. |


| Description | Abbreviation | Total Glucans (% w/w) | α-Glucans (% w/w) | β-Glucans (% w/w) |
|---|---|---|---|---|
| Pleurotus ostreatus IK 1123 in 100% wheat straw (WS, control substrate) | POWS | 39.4 ± 1.4 | 8.7 ± 1.3 | 30.6 ± 1.9 |
| Pleurotus ostreatus IK 1123 in olive pruning residues (OL) | POOLRP | 38.5 ± 2.1 | 3.4 ± 0.2 | 35.1 ± 1.1 |
| Pleurotus ostreatus LGM 22 in 100% wheat straw (control substrate) | POLWS | 34.3 ± 2.7 | 6.6 ± 1.1 | 27.7 ± 2.4 |
| Pleurotus ostreatus LGM 22 in OL:TPOMW (ratio 3:1, w/w) (TPOMW, two-phase olive mill wastes) | POLTPOMW | 39.9 ± 0.8 | 4.9 ± 1.0 | 35.0 ± 0.4 |
| Pleurotus eryngii LGAM 216 in 100% wheat straw (control substrate) | PEWS | 46.6 ± 3.9 | 7.9 ± 1.3 | 38.7 ± 5.4 |
| Pleurotus eryngii LGAM 216 in WS:GM (ratio 1:1, w/w) (GM, grape marc) | PEWSGM | 49.7 ± 2.9 | 7.6 ± 1.4 | 42.2 ± 5.9 |
| Hericium erinaceus LGAM 4514 in 100% beech sawdust (BS, control substrate) | HEBS | 16.4 ± 0.1 | 1.0 ± 0.1 | 15.4 ± 0.2 |
| Hericium erinaceus LGAM 4514 in olive pruning residues | HEOLRP | 21.8 ± 0.9 | 1.1 ± 0.1 | 20.7 ± 0.3 |
| Cyclocybe cylindracea LGAM 951 in 100% wheat straw (control substrate) | CC2WS | 39.3 ± 1.7 | 6.1 ± 0.5 | 33.2 ± 1.9 |
| Cyclocybe cylindracea LGAM 961 in 100% wheat straw (control substrate) | CC505WS | 40.6 ± 2.9 | 3.4 ± 1.1 | 37.2 ± 3.8 |
| Sociodemographic Parameters | |
|---|---|
| Sex (no. of males/females), n (%) | 4/4 (50.0%/50.0%) |
| Age (years) | 73.50 ± 5.88 |
| Smoking (no. of smokers), n (%) | 1 (12.5%) |
| Educational years | 15.25 ± 4.71 |
| Marital status (no. of married/widowed), n (%) | 4/4 (50.0%/50.0%) |
| Medical history-Clinical evaluation | |
| Diagnosis/drug treatment for hypertension, n (%) | 5 (62.5%) |
| Diagnosis/drug treatment for dyslipidemia, n (%) | 2 (25.0%) |
| Drug treatment, n (%) | 6 (75.0%) |
| Dietary supplements, n (%) | 3 (37.5%) |
| Evacuation frequency (times·d−1) | 1.00 (1.00–1.00) |
| Anthropometric measurements | |
| Body weight (kg) | 63.55 (62.55–73.63) |
| Height (m) | 1.64 ± 0.09 |
| BMI (kg·m−2) | 25.14 ± 3.16 |
| Nutritional analysis-Physical activity | |
| Energy intake (kcal·d−1) | 1585.99 ± 582.31 |
| Carbohydrate (% of energy) | 42.91 ± 6.68 |
| Carbohydrate (g·d−1) | 173.99 ± 69.47 |
| Protein (% of energy) | 18.10 ± 4.24 |
| Protein (g·d−1) | 69.83 ± 20.89 |
| Fat (% of energy) | 36.94 ± 5.54 |
| Fat (g·d−1) | 67.38 ± 30.73 |
| SFA (g·d−1) | 22.41 ± 9.60 |
| MUFA (g·d−1) | 22.83 (19.78–37.70) |
| PUFA (g·d−1) | 8.52 (5.20–9.72) |
| Fiber (g·d−1) | 14.89 ± 10.26 |
| Total Physical Activity (MET-min·wk−1) | 1333.38 ± 876.79 |
| Sitting or resting time (h·wk−1) | 34.13 ± 14.21 |
| Moderate level of physical activity, n (%) | 6 (75.0%) |
| Baseline (t = 0 h) | |||||||
| Total Bacteria | Lactobacillus Group | Bifidobacterium spp. | Bacteroides spp. | Clostridium perfringens group | Roseburia spp.-Eubacterium rectale | Faecalibacterium prausnitzii | |
| NC | 10.11 (10.02–10.21) | 6.13 (5.84–7.20) | 8.79 (7.40–9.07) | 9.48 ± 0.27 | 6.59 (6.35–6.75) | 8.45 (8.17–8.78) | 8.86 (8.60–9.06) |
| INU2 | 10.20 (10.08–10.30) | 6.19 (5.84–7.32) | 8.81 (7.38–9.04) | 9.56 ± 0.24 | 6.62 (6.44–6.83) | 8.53 (8.22–8.72) | 8.97 (8.59–9.05) |
| POWS | 9.99 † (9.94–10.12) | 6.09 (5.82–7.20) | 8.78 (7.31–9.02) | 9.50 ± 0.21 | 6.44 (6.11–6.69) | 8.20 (8.04–8.55) | 8.76 (8.40–8.86) |
| POOLRP | 9.96 *,† (9.81–10.01) | 5.98 (5.73–7.06) | 8.71 (7.28–9.06) | 9.40 ± 0.17 | 6.36 (6.08–6.84) | 8.15† (8.05–8.49) | 8.67 (8.40–8.82) |
| POLWS | 10.14 (10.06–10.22) | 6.12 (5.84–7.26) | 8.74 (7.25–9.08) | 9.59 ± 0.18 | 6.76 (6.51–6.91) | 8.58 (8.26–8.74) | 9.00 (8.64–9.13) |
| POLTPOMW | 10.02 (9.89–10.15) | 6.68 (6.24–7.28) | 7.87 (6.80–8.87) | 9.64 ± 0.31 | 6.86 (6.34–7.13) | 8.60 (8.57–8.61) | 8.79 (8.64–9.13) |
| PEWS | 9.84 *,† (9.67–9.88) | 5.94 (5.60–7.04) | 8.73 (7.29–8.97) | 9.37 ± 0.22 | 6.24 (5.97–6.73) | 8.00 *,† (7.72–8.23) | 8.53 *,† (8.35–8.74) |
| PEWSGM | 10.05 † (9.72–10.09) | 6.03 (5.72–7.21) | 8.71 (6.98–9.10) | 9.51 ± 0.23 | 6.48 (6.21–6.76) | 8.08 *,† (7.96–8.51) | 8.74 (8.42–8.87) |
| HEBS | 10.10 (9.97–10.30) | 6.46 (5.83–7.20) | 7.90 (6.74–9.02) | 9.45 ± 0.22 | 6.11*,† (5.73–6.40) | 8.32 (8.04–8.51) | 8.70 (8.39–8.87) |
| HEOLRP | 10.12 (10.02–10.18) | 5.99 (5.74–7.21) | 8.87 (7.54–9.15) | 9.51 ± 0.29 | 6.36 (5.96–6.65) | 8.33 (8.12–8.50) | 8.84 (8.66–8.98) |
| CC2WS | 9.89 *,† (9.79–9.96) | 5.92 (5.43–6.60) | 8.36 (7.06–8.92) | 9.32 ± 0.28 | 5.83 *,† (5.22–6.12) | 8.09 *,† (7.82–8.38) | 8.52 *,† (8.27–8.74) |
| CC505WS | 10.11 † (9.81–10.15) | 5.98 (5.61–6.89) | 8.53 (7.26–9.06) | 9.47 ± 0.30 | 6.12 *,† (5.66–6.49) | 8.53 (7.26–9.06) | 8.30 (8.19–8.62) |
| 24-h Fermentation (t = 24 h) | |||||||
| Total Bacteria | Lactobacillus Group | Bifidobacterium spp. | Bacteroides spp. | Clostridium perfringens Group | Roseburia spp.-Eubacterium rectale | Faecalibacterium prausnitzii | |
| NC | 9.98 †,a (9.86–10.06) | 5.95 † (5.69–7.05) | 8.73 † (7.34–9.16) | 9.04 ± 0.38 †,a | 6.38 (6.03–6.69) | 7.97 a (7.09–8.15) | 8.29 †,a (7.83–8.62) |
| INU2 | 10.28 * (10.14–10.32) | 7.66 *,a (7.03–9.00) | 9.83 *,a (8.11–9.96) | 9.68 ± 0.23 * | 6.42 (6.22–6.70) | 8.15 a (7.91–8.50) | 9.06 *,a (8.66–9.15) |
| POWS | 10.36 *,a (10.14–10.40) | 6.48 (5.87–7.59) | 9.49 a (8.12–9.66) | 9.53 ± 0.33 * | 6.58 (6.25–6.92) | 8.38 * (7.97–8.46) | 9.06 *,a (8.78–9.39) |
| POOLRP | 10.38 *,a (10.20–10.49) | 6.56 (5.84–7.35) | 9.49 *,a (8.36–9.82) | 9.61 ± 0.16 *,a | 6.71 a (6.60–6.82) | 8.33 * (8.01–8.47) | 9.10 *,a (8.97–9.32) |
| POLWS | 10.39 *,a (10.28–10.46) | 6.59 (5.87–8.26) | 9.55 a (8.27–9.66) | 9.62 ± 0.34 * | 6.75 * (6.57–7.32) | 8.50 *,† (8.40–8.79) | 9.21 *,a (8.96–9.32) |
| POLTPOMW | 10.13 (10.08–10.36) | 7.53 (6.09–8.83) | 8.65 (6.99–9.57) | 9.46 ± 0.47 * | 6.50 (6.44–6.74) | 8.58 *,† (8.34–8.72) | 8.78 (8.54–9.03) |
| PEWS | 10.29 *,a (10.18–10.37) | 8.58 *,a (7.40–9.32) | 9.29 a (8.08–9.54) | 9.50 ± 0.32* | 6.52 a (6.13–6.91) | 7.99 a (7.89–8.33) | 9.02 *,a (8.75–9.58) |
| PEWSGM | 10.44 *,†,a (10.30–10.50) | 7.93 *,a (6.81–9.21) | 9.36 a (8.29–9.74) | 9.69 ± 0.23* | 6.72 (6.28–7.08) | 8.51 *,†,a (8.39–8.58) | 9.18 *,a (8.96–9.79) |
| HEBS | 10.12 (9.78–10.18) | 6.45 (5.86–7.97) | 8.19 † (6.72–9.29) | 9.54 ± 0.27* | 6.09 (5.89–6.66) | 8.01 (7.43–8.25) | 8.49 (8.02–8.97) |
| HEOLRP | 10.19 * (10.10–10.36) | 6.29 † (5.72–7.14) | 9.13 †,a (7.60–9.47) | 9.61 ± 0.27* | 6.50 (6.18–6.80) | 8.34 * (8.06–8.42) | 8.79 * (8.47–9.15) |
| CC2WS | 10.14 a (9.96–10.24) | 6.23 a (5.87–9.14) | 9.38 a (7.64–9.88) | 8.88 ± 0.45 †,a | 6.37 a (5.67–6.52) | 7.85 †,a (7.52–8.14) | 8.74 a (8.48–9.00) |
| CC505WS | 10.25 *,a (10.07–10.35) | 6.27 (5.75–9.22) | 9.69 *,a (7.84–9.83) | 9.07 ± 0.52 †,a | 6.40 (5.92–6.62) | 8.07 (7.90–8.25) | 8.98 *,a (9.00–9.24) |
| P overall | 0.018 | 0.733 | 0.939 | 0.001 | 0.022 | 0.001 | 0.143 |
| Baseline (t = 0 h) | ||||||
| Concentrations (μmol mL−1) | ||||||
| Total VFAs | Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 3.80 ± 1.33 † | 1.55 (1.32–2.14) | 0.43 (0.30–0.59) | 1.14 † (0.61–2.15) | 0.10 (0.06–0.16) | 0.11 (0.09–0.22) |
| INU2 | 2.59 ± 0.71 * | 1.49 (0.81–1.65) | 0.37 (0.21–0.45) | 0.67 * (0.58–0.83) | 0.10 (0.09–0.11) | 0.10 (0.08–0.16) |
| POWS | 3.12 ± 0.68 | 1.56 (1.13–1.69) | 0.32 (0.16–0.53) | 1.14 † (0.87–1.37) | 0.07 † (0.06–0.08) | 0.10 (0.09–0.20) |
| POOLRP | 2.92 ± 0.68 * | 1.48 1.05–1.80) | 0.24 * (0.16–0.39) | 0.97 † (0.90–1.26) | 0.07 † (0.06–0.09) | 0.11 (0.09–0.14) |
| POLWS | 2.90 ± 0.97 * | 1.44 (0.75–1.84) | 0.26 (0.16–0.47) | 0.99 † (0.82–1.36) | 0.07 † (0.05–0.08) | 0.10 (0.08–0.14) |
| POLTPOMW | 2.94 ± 0.52 | 1.60 (1.24–1.80) | 0.29 (0.22–0.46) | 0.95 (0.68–1.04) | 0.07 † (0.06–0.10) | 0.10 (0.09–0.13) |
| PEWS | 2.66 ± 0.72 * | 1.33 (0.89–1.67) | 0.25 * (0.18–0.39) | 0.90 (0.63–0.99) | 0.08 † (0.05–0.08) | 0.10 (0.09–0.15) |
| PEWSGM | 2.82 ± 0.78 | 1.55 (1.03–1.89) | 0.28 * (0.19–0.41) | 0.90 (0.66–0.98) | 0.07 † (0.06–0.09) | 0.10 (0.09–0.14) |
| HEBS | 2.90 ± 0.62 | 1.52 (1.19–1.90) | 0.32 (0.21–0.48) | 0.88 (0.66–0.95) | 0.08 (0.06–0.11) | 0.12 (0.08–0.15) |
| HEOLRP | 3.32 ± 0.93 | 2.15 † (1.36–2.68) | 0.26 (0.21–0.42) | 0.86 (0.62–0.93) | 0.07 (0.06–0.09) | 0.09 (0.08–0.12) |
| CC2WS | 2.80 ± 0.75 * | 1.48 (1.00–1.83) | 0.28 (0.21–0.48) | 0.83 (0.51–1.06) | 0.08 (0.06–0.09) | 0.11 (0.08–0.15) |
| CC505WS | 2.60 ± 0.62 * | 1.43 (0.88–1.65) | 0.30 (0.21–0.41) | 0.82 (0.62–0.89) | 0.08 (0.06–0.09) | 0.10 (0.10–0.13) |
| 8-h Fermentation (t = 8 h) | ||||||
| Concentrations (μmol mL−1) | ||||||
| Total VFAs | Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 20.32 ± 4.57 †,a | 11.31 a (8.40–13.29) | 1.96 a (1.71–3.69) | 4.38 a (3.87–5.35) | 1.27 †,a (0.66–2.25) | 0.98 †,a (0.87–1.15) |
| INU2 | 41.77 ± 12.51 *,a | 22.41 a (19.08–27.55) | 4.15 a (3.02–6.26) | 11.01 a (8.67–13.90) | 0.25 *,a (0.21–0.44) | 0.29 *,a (0.17–0.36) |
| POWS | 66.43 ± 16.33 *,†,a | 31.21 *,a (22.93–38.89) | 9.34 *,†,a (7.56–14.24) | 26.21 *,†,a (17.20–28.04) | 0.62 *,a (0.36–0.83) | 0.41 *,a (0.29–0.67) |
| POOLRP | 77.53 ± 13.90 *,†,a | 36.13 *,†,a (34.86–41.51) | 11.90 *,†,a (9.84–16.50) | 26.40 *,†,a (20.33–29.90) | 0.75 †,a (0.69–0.89) | 0.54 *,†,a (0.37–0.83) |
| POLWS | 67.66 ± 11.90 *,†,a | 32.49 *,a (27.15–37.04) | 10.62 *,†,a (9.90–13.66) | 25.00 *,†,a (18.50–26.36) | 0.58 *,†,a (0.51–0.78) | 0.47 *,†,a (0.29–0.76) |
| POLTPOMW | 71.10 ± 12.02 *,†,a | 36.19 *,†,a (33.44–38.13) | 12.77 *,†,a (11.06–18.89) | 21.24 *,a (12.47–24.28) | 0.86 †,a (0.75–1.23) | 0.70 †,a (0.34–0.97) |
| PEWS | 73.68 ± 19.96 *,†,a | 35.19 *,†,a (28.88–41.37) | 10.82 *,†,a (7.54–17.92) | 25.59 *,†,a (21.29–32.67) | 0.62 *,a (0.36–0.79) | 0.35 *,a (0.22–0.46) |
| PEWSGM | 81.16 ± 24.52 *,†,a | 36.69 *,†,a (29.40–51.05) | 10.75 *,†,a (9.20–18.32) | 28.51 *,†,a (15.61–37.37) | 0.86 †,a (0.42–0.89) | 0.40 *,a (0.28–0.58) |
| HEBS | 95.81 ± 21.96 *,†,a | 32.40 *,a (28.36–33.84) | 23.08 *,†,a (13.49–29.10) | 42.97 *,†,a (19.49–53.54) | 2.11 †,a (1.69–2.80) | 1.74 †,a (0.93–1.89) |
| HEOLRP | 89.70 ± 20.70 *,†,a | 31.84 *,a (25.12–36.75) | 17.66 *,†,a (9.47–23.41) | 36.14 *,†,a (28.32–60.43) | 1.05 †,a (0.70–1.46) | 1.25 †,a (0.94–1.59) |
| CC2WS | 68.56 ± 16.26 *,†,a | 30.75 *,†,a (28.73–43.57) | 8.63 *,†,a (6.90–15.27) | 21.92 *,†,a (16.14–26.49) | 0.55 †,a (0.51–0.98) | 0.30 *,a (0.20–0.45) |
| CC505WS | 68.12 ± 13.63 *,†,a | 32.26 *,†,a (27.92–38.39) | 8.73 *,†, a (7.52–14.72) | 21.82 *,†,a (18.77–25.93) | 0.75 *,†,a (0.42–0.80) | 0.26 *,a (0.23–0.40) |
| 24-h Fermentation (t = 24 h) | ||||||
| Concentrations (μmol mL−1) | ||||||
| Total VFAs | Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 27.41 ± 5.27 †,a,b | 13.19 a,b (11.79–17.55) | 2.90 a (2.35–3.28) | 5.77 a,b (5.38–5.94) | 2.33 †,a,b (1.97–2.94) | 2.16 †,a,b (1.83–2.67) |
| INU2 | 47.58 ± 14.06 *,a | 28.28 a (20.05–32.92) | 4.81 a (3.77–5.93) | 10.74 a (7.60–18.65) | 0.37 *,a (0.32–0.46) | 0.36 *,a (0.24–0.51) |
| POWS | 101.10 ± 15.73 *,†,a,b | 40.32 *,†,a,b (39.42–51.55) | 14.04 *,†,a,b (11.54–19.78) | 41.66 *,†,a,b (34.82–45.80) | 0.93 *,†,a (0.70–1.29) | 0.76 *,†,a,b (0.50–1.06) |
| POOLRP | 105.32 ± 19.75 *,†,a,b | 45.56 *,†,a,b (40.94–50.31) | 15.03 *,†,a,b (12.80–23.32) | 42.17 *,†,a,b (33.37–43.71) | 0.90 *,†,a (0.65–1.21) | 0.97 *,†,a,b (0.67–1.31) |
| POLWS | 94.45 ± 15.41 *,†,a,b | 34.25 *,a,b (31.13–42.87) | 13.81 *,†,a,b (12.37–21.63) | 42.27 *,†,a,b (32.58–44.15) | 1.15 *,†,a,b (0.76–1.30) | 0.73 *,†,a,b (0.45–0.89) |
| POLTPOMW | 107.44 ± 8.88 *,†,a,b | 46.52 *,†,a,b (45.07–50.57) | 20.43 *,†,a,b (16.00–24.83) | 37.71 *,†,a,b (31.31–39.98) | 1.96 †,a,b (1.60–2.20) | 1.44 † (0.56–2.15) |
| PEWS | 98.32 ± 17.41 *,†,a.b | 35.51 *,†,a (31.17–47.60) | 14.51 *,†,a (10.80–19.70) | 45.39 *,†,a,b (44.67–46.11) | 0.61 *,a (0.50–0.84) | 0.39 *,a (0.25–0.58) |
| PEWSGM | 103.18 ± 14.30 *,†,a,b | 41.03 *,†,a (31.12–50.50) | 16.46 *,†,a (10.58–20.58) | 46.56 *,†,a,b (33.08–52.68) | 0.84 *,†,a (0.48–1.10) | 0.46 *,a (0.37–0.62) |
| HEBS | 141.08 ± 15.28 *,†,a,b | 51.40 *,†,a,b (40.02–53.88) | 35.26 *,†,a,b (28.34–38.74) | 50.51 *,†,a,b (32.60–62.29) | 5.41 †,a,b (3.99–8.02) | 4.45 †,a,b (2.50–5.80) |
| HEOLRP | 133.45 ± 15.58 *,†,a,b | 31.59 *,a (24.99–39.75) | 20.95 *,†,a,b (17.96–30.30) | 80.90 *,†,a,b (50.12–89.47) | 1.99 †,a,b (0.70–1.46) | 2.17 †,a,b (1.43–2.64) |
| CC2WS | 102.71 ± 17.32 *,†,a,b | 38.92 *,†,a,b (31.41–51.13) | 15.13 *,†,a,b (14.28–25.54) | 47.17 *,†,a,b (29.88–49.09) | 0.85 *,†,a (0.62–1.26) | 0.44 *,a,b (0.25–0.61) |
| CC505WS | 110.64 ± 14.39 *,†,a,b | 43.06 *,†,a,b (34.91–48.64) | 17.26 *,†,a,b (13.12–28.97) | 46.11 *,†,a,b (42.77–50.71) | 1.16 *,†,a,b (0.79–1.55) | 0.36 *,a (0.20–0.51) |
| p overall | <0.001 | 0.015 | 0.006 | <0.001 | <0.001 | <0.001 |
| Baseline (t = 0 h) | |||||
| Molar Ratio (%) | |||||
| Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 46.20 (41.82–54.41) | 11.70 (11.29–15.87) | 28.83 (21.56–42.37) | 2.14 † (1.90–5.07) | 2.81 (2.15–7.68) |
| INU2 | 50.68 (46.30–53.72) | 13.05 (10.89–14.81) | 26.29 (21.11–32.42) | 3.56 * (3.18–5.32) | 4.59 (2.84–7.95) |
| POWS | 46.34 † (41.52–48.37) | 8.95 *,† (7.28–17.12) | 38.07 † (27.59–45.55) | 2.17 † (1.99–2.35) | 4.51 (2.60–5.72) |
| POOLRP | 48.67 (45.64–51.55) | 7.95 *,† (7.41–11.01) | 35.94 † (29.02–42.04) | 2.48 † (2.02–2.69) | 4.02 (2.68–6.05) |
| POLWS | 44.91 † (40.37–49.66) | 8.63 *,† (8.17–11.75) | 39.15 *,† (34.70–41.18) | 2.32 † (1.92–2.92) | 4.17 (2.65–5.95) |
| POLTPOMW | 53.23 (50.29–54.05) | 9.56 (9.04–13.75) | 29.25 (26.65–34.85) | 2.07 † (1.84–4.11) | 3.16 (2.68–5.49) |
| PEWS | 50.19 (45.62–51.99) | 9.52 *,† (8.96–10.86) | 31.83 (28.39–37.69) | 2.51 † (2.15–2.84) | 4.64 (2.60–6.56) |
| PEWSGM | 53.32 * (49.54–55.39) | 9.51 *,† (8.60–10.84) | 30.90 (28.02–33.52) | 2.66 † (1.95–3.05) | 4.03 (2.69–5.10) |
| HEBS | 52.54 (50.72–54.59) | 10.82 (8.97–13.68) | 28.17 (26.54–31.40) | 2.45 (1.94–4.69) | 4.54 (2.44–6.49) |
| HEOLRP | 61.00 *,† (58.26–63.29) | 8.45 *,† (7.41–9.90) | 24.10 * (22.74–27.69) | 2.15 † (1.74–2.64) | 2.97 (2.22–4.01) |
| CC2WS | 52.94 * (50.32–55.49) | 12.04 (9.67–12.69) | 29.30 (24.37–30.04) | 2.66 (2.00–3.43) | 3.66 (2.86–5.67) |
| CC505WS | 50.72 (46.01–51.65) | 11.75 (10.59–12.44) | 31.80 (29.85–32.22) | 3.14 (2.00–3.78) | 4.14 (2.98–5.85) |
| 8-h Fermentation (t = 8 h) | |||||
| Molar Ratio (%) | |||||
| Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 52.73 (50.42–60.20) | 11.36 (9.36–14.29) | 22.63 a (20.40–25.86) | 6.30 † (3.95–8.88) | 4.91 † (4.03–5.34) |
| INU2 | 59.38 a (50.88–64.45) | 8.96 (8.82–15.10) | 25.40 (19.33–34.05) | 0.65 *,a (0.45–1.38) | 0.62 *,a (0.37–1.13) |
| POWS | 46.89 *,† (45.82–53.30) | 14.96 *,†,a (13.50–19.14) | 34.93 *,† (31.98–39.32) | 0.93 *,a (0.60–1.37) | 0.62 *,a (0.36–1.38) |
| POOLRP | 49.66 (45.82–53.30) | 15.29 *,†,a (14.60–19.95) | 31.75 * (28.87–37.07) | 0.96 *,a (0.87–1.40) | 0.66 *,a(0.45–1.27) |
| POLWS | 47.58 *,† (39.72–52.87) | 16.70 *,†,a (14.73–18.42) | 32.80 * (29.31–38.77) | 0.94 *,a (0.79–1.15) | 0.70 *,a (0.37–1.37) |
| POLTPOMW | 50.26 (45.94–57.58) | 19.36 *,†,a (17.02–22.98) | 29.03 (19.73–31.08) | 1.32 *,† (1.09–1.56) | 0.99 *,a (0.43–1.64) |
| PEWS | 47.86 † (42.29–53.56) | 15.41 a (12.32–17.71) | 33.18 * (31.89–39.53) | 0.77 *,a (0.62–1.02) | 0.43 *,a (0.30–0.83) |
| PEWSGM | 46.42 † (44.03–58.02) | 15.90 *,†,a (12.83–17.61) | 34.43 * (27.07–41.08) | 0.78 *,a (0.60–1.24) | 0.53 *,a (0.33–0.77) |
| HEBS | 34.56 *,† (24.70–44.95) | 24.18 *,†,a (15.72–28.08) | 43.59 *,† (25.55–46.70) | 2.24 † (2.16–2.47) | 1.85 †,a (0.87–2.39) |
| HEOLRP | 36.93 *,†,a (25.38–44.23) | 18.14 *,†,a (12.87–23.03) | 44.56 *,†,a (34.01–53.36) | 1.15 *,†,a (0.91–1.78) | 1.40 *,†,a (0.94–2.03) |
| CC2WS | 50.00 (43.03–60.96) | 14.97 a (11.46–17.31) | 27.95 (26.08–34.19) | 0.86 *,a (0.79–1.19) | 0.34 *,a (0.31–0.76) |
| CC505WS | 47.93 † (44.30–55.33) | 14.61 a (12.39–18.38) | 32.37 * (26.51–34.42) | 0.89 *,a (0.69–1.49) | 0.36 *,a (0.25–0.66) |
| 24-h Fermentation (t = 24 h) | |||||
| Molar Rratio (%) | |||||
| Acetate | Propionate | Butyrate | BSCFAs | Other SCFAs | |
| NC | 49.74 (45.86–56.75) | 9.85 a (9.34–10.93) | 19.85 a (18.89–23.29) | 8.38 †,a (7.92–9.59) | 7.34 †,b (6.41–8.71) |
| INU2 | 60.56 a,b (53.61–68.09) | 8.74 (7.58–15.56) | 23.16 b (18.02–33.35) | 0.63 *,a (0.55–1.51) | 0.79 *,a (0.42–1.13) |
| POWS | 43.42 *,†,b (38.20–47.77) | 13.54 *,a (12.36–18.99) | 40.67 *,†,b (34.42–45.00) | 0.91 *,a (0.64–1.37) | 0.83 *,a (0.41–1.27) |
| POOLRP | 44.23 †,b (40.94–50.11) | 15.76 *,†,a (13.62–17.83) | 36.57 *,†,b (31.91–40.41) | 0.90 *,a (0.61–1.29) | 0.93 *,a (0.55–1.55) |
| POLWS | 40.96 *,†,b (34.42–44.62) | 15.40 *,†,b (13.70–20.78) | 42.43 *,†,b (34.62–46.90) | 1.34 *,a (0.70–1.49) | 0.77 *,a (0.45–1.10) |
| POLTPOMW | 44.69 †,a,b (41.51–46.43) | 19.00 *,†,a (16.01–21.62) | 34.31 b (30.58–36.42) | 1.85 † (1.39–2.21) | 1.34 *,a (0.48–2.16) |
| PEWS | 36.54 *,†,a,b (30.43–46.46) | 14.80 *,a (11.35–17.74) | 45.21 *,†,a,b (37.14–56.21) | 0.60 *,a,b (0.51–0.93) | 0.34 *,a (0.24–0.65) |
| PEWSGM | 38.92 *,†,a,b (33.40–46.16) | 15.56 *,†,a (11.71–18.44) | 45.15 *,†,a,b (34.44–52.52) | 0.76 *,a (0.48–1.00) | 0.46 *,a (0.34–0.60) |
| HEBS | 35.84 *,†,a (26.89–41.64) | 24.78 *,†,a,b (20.15–27.92) | 35.10 * (25.41–40.94) | 3.91 † (3.08–5.10) | 3.17 †,b (1.66–4.41) |
| HEOLRP | 20.49 *,†,a,b (19.59–34.32) | 14.48 *,†,a (13.28–23.42) | 60.51 *,†,a,b (39.30–63.66) | 1.52 *,† (1.06–2.47) | 1.74 *,†,a (0.96–1.91) |
| CC2WS | 42.52 *,†,a,b (29.66–43.38) | 16.49 *,†,a (13.31–16.65) | 43.00 *,†,a,b (35.65–47.00) | 0.92 *,a (0.66–1.52) | 0.37 *,a (0.22–0.65) |
| CC505WS | 40.39 *,†,a,b (34.37–42.31) | 16.18 *,†,a (13.18–20.97) | 43.16 *,†,a,b (37.21–47.54) | 1.14 *,a (0.65–1.45) | 0.26 *,†,a (0.21–0.50) |
| p overall | <0.001 | 0.272 | 0.004 | <0.001 | 0.010 |
| Target | Primer | Primer Sequence (5′-3′) | Annealing Temperature | Product Size | Reference Strains | References |
|---|---|---|---|---|---|---|
| Total Bacteria (Universal) | Forward Reverse | TCCTACGGGAGGCAGCAGT GGACTACCAGGGTATCTAATCCTGTT | 60 °C | 466 bp | Bacteroides fragilis MM44 (ATCC 25285) | [71] |
| Lactobacillus group | Forward Reverse | AGCAGTAGGGAATCTTCCA CACCGCTACACATGGAG | 58 °C | 341 bp | Lactobacillus gasseri DSM 20243 | [72] |
| Bifidobacterium spp. | Forward Reverse | TCGCGTCYGGTGTGAAAG CCACATCCAGCRTCCAC | 58 °C | 243 bp | Bifidobacterium bifidum DSM 20456 | [72] |
| Bacteroides spp. | Bac303F Bfr-Fmrev | GAAGGTCCCCCACATTG CGCKACTTGGCTGGTTCAG | 60 °C | 103 bp | Bacteroides fragilis MM44 (ATCC 25285) | [73] |
| Clostridium perfringens group | CPF CPR | ATGCAAGTCGAGCGATG TATGCGGTATTAATCTCCCTTT | 55 °C | 120 bp | Clostridium perfringens ATCC 13124 | [74] |
| Roseburia spp.- Eubacterium rectale | RrecF Rrec630mR | GCGGTRCGGCAAGTCTGA CCTCCGACACTCTAGTMCGAC | 60 °C | 81 bp | Roseburia intestinalis DSM 14610 | [75] |
| Faecalibacterium prausnitzii | FPR-2FFprau645R | GGAGGAAGAAGGTCTTCGG AATTCCGCCTACCTCTGCACT | 60 °C | 248 bp | Faecalibacterium prausnitzii DSM 17677 | [76,77,78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules 2020, 25, 2806. https://doi.org/10.3390/molecules25122806
Mitsou EK, Saxami G, Stamoulou E, Kerezoudi E, Terzi E, Koutrotsios G, Bekiaris G, Zervakis GI, Mountzouris KC, Pletsa V, et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules. 2020; 25(12):2806. https://doi.org/10.3390/molecules25122806
Chicago/Turabian StyleMitsou, Evdokia K., Georgia Saxami, Emmanuela Stamoulou, Evangelia Kerezoudi, Eirini Terzi, Georgios Koutrotsios, Georgios Bekiaris, Georgios I. Zervakis, Konstantinos C. Mountzouris, Vasiliki Pletsa, and et al. 2020. "Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study" Molecules 25, no. 12: 2806. https://doi.org/10.3390/molecules25122806
APA StyleMitsou, E. K., Saxami, G., Stamoulou, E., Kerezoudi, E., Terzi, E., Koutrotsios, G., Bekiaris, G., Zervakis, G. I., Mountzouris, K. C., Pletsa, V., & Kyriacou, A. (2020). Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules, 25(12), 2806. https://doi.org/10.3390/molecules25122806







