Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic, Flavonoid and Condensed Tannins Contents
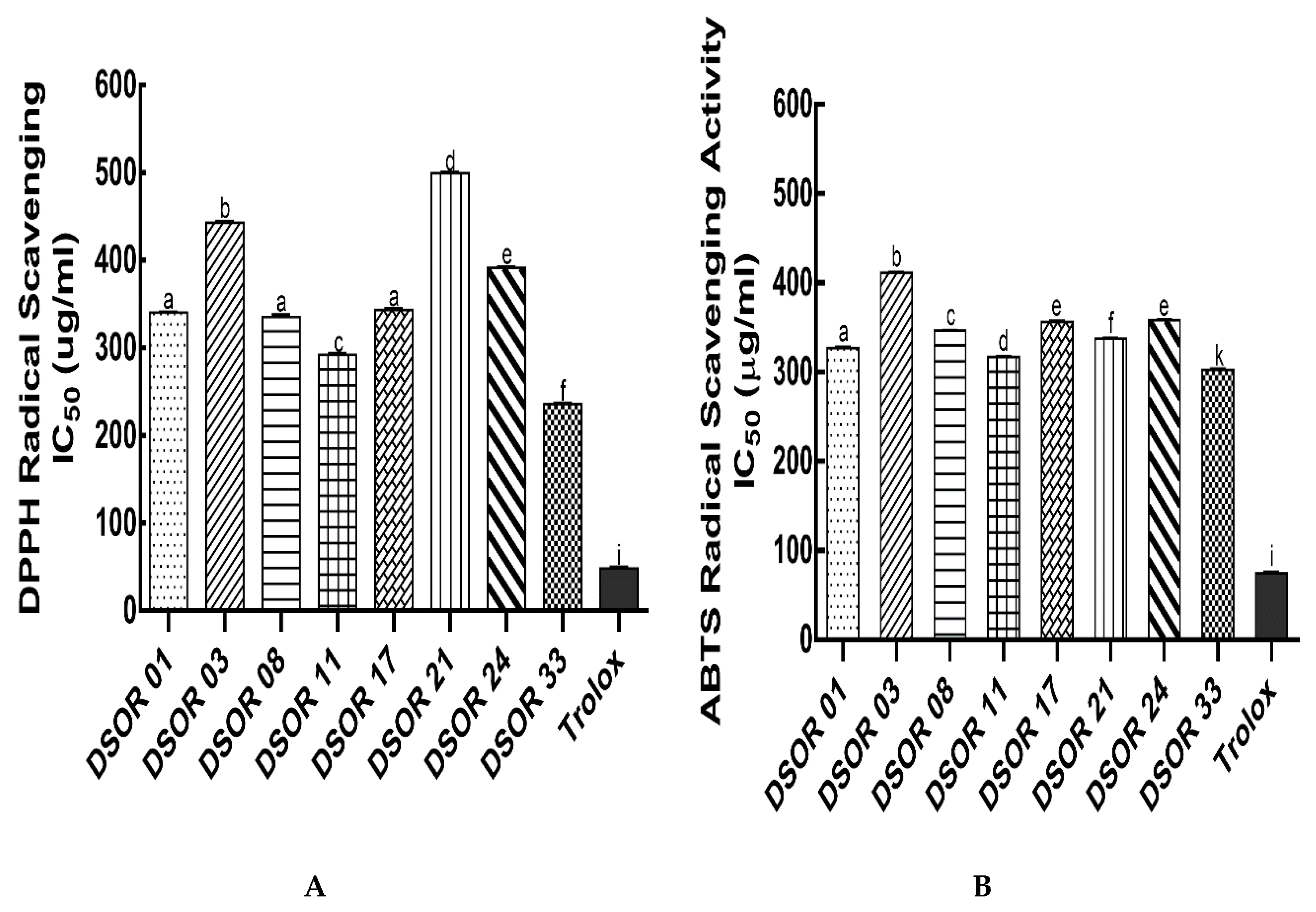
2.2. Antioxidant Properties
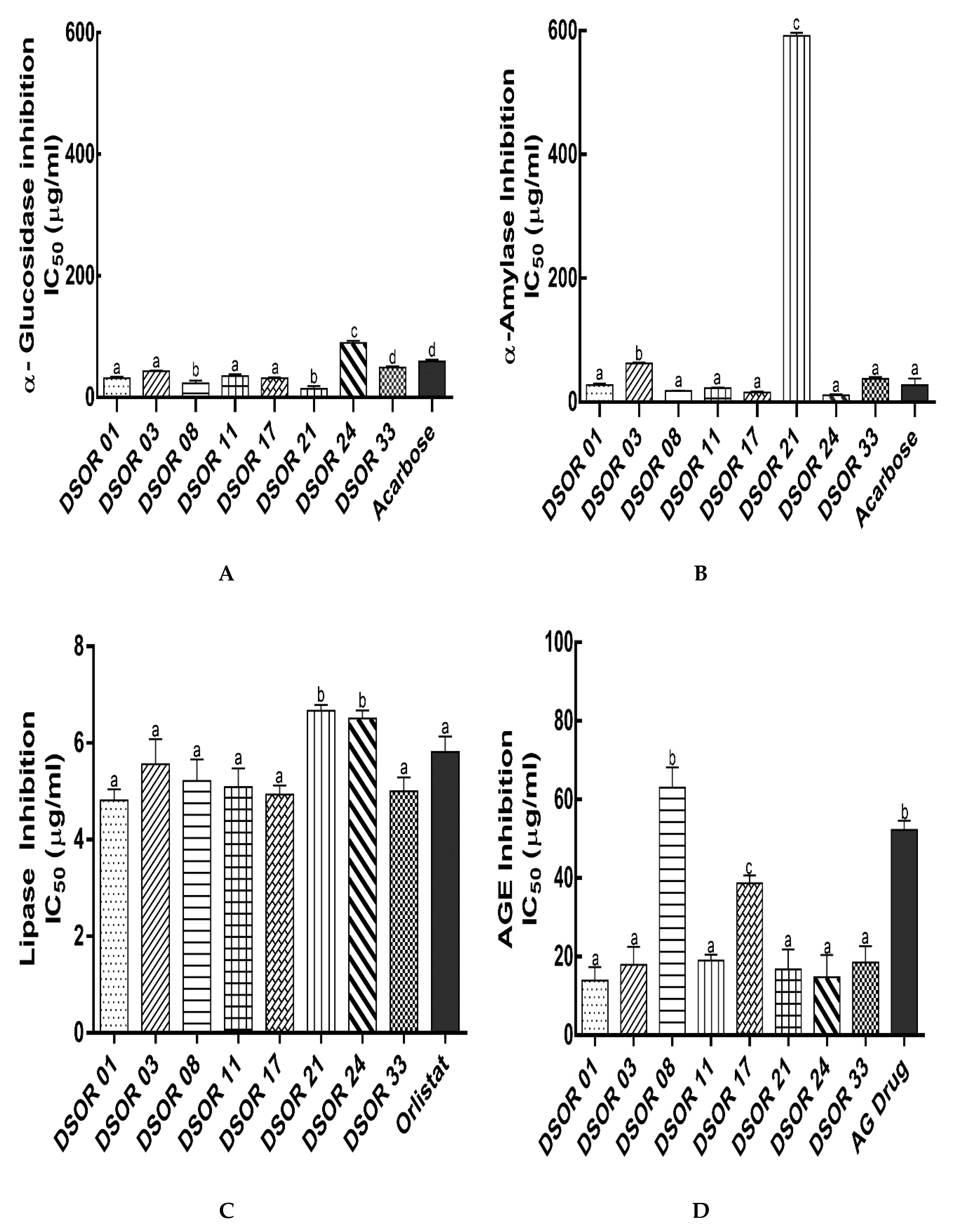
2.3. Digestive Enzymes and Glycation Formation Inhibition
2.4. Correlation Analysis of Total Phenolics, Antioxidant Capacity and Digestive Enzymes Activities
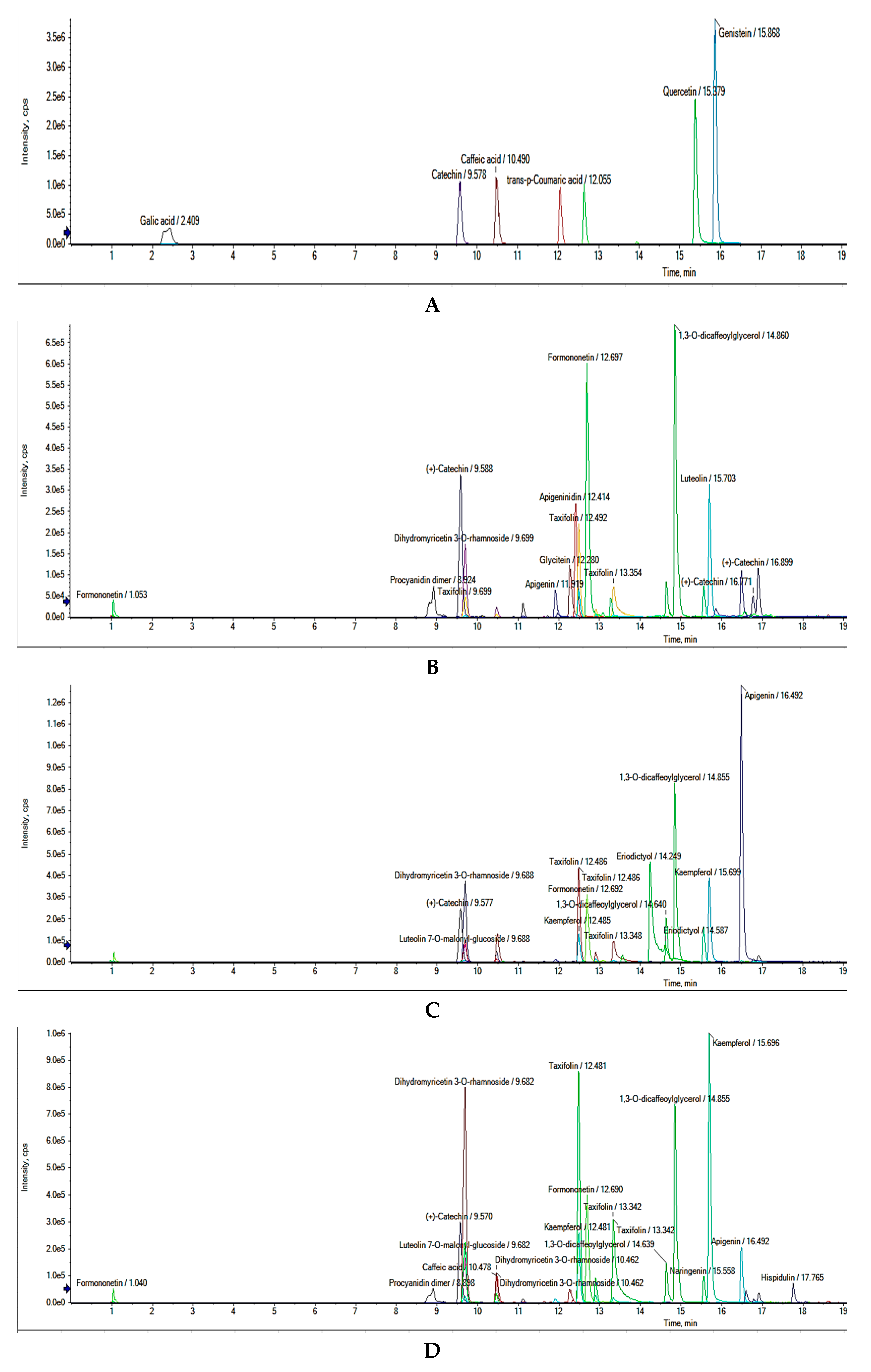
2.5. Untargeted UHPLC-Q-TOF-MS2 Phenolic Compounds Identification
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.2.1. Extraction of Polyphenols
3.2.2. Total Phenolic Content (TPC)
3.2.3. Total Flavonoid Content (TFC)
3.2.4. Total Condensed Tannin Content (CTC)
3.2.5. DPPH Radical Scavenging Activity
3.2.6. ABTS Radical Scavenging Activity
3.2.7. α-Amylase Inhibitory Assay
3.2.8. α-Glucosidase Inhibitory Assay
3.2.9. Pancreatic Lipase Inhibition Assay
3.2.10. Inhibition of Protein Glycation
3.2.11. UHPLC-Q-TOF-MS/MS Phenolic Compounds Characterization
3.2.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Awika, J.M. Sorghum: Its unique nutritional and health-promoting attributes. In Gluten-Free Ancient Grains; Elsevier: Duxford, UK, 2017; pp. 21–54. [Google Scholar]
- Althwab, S.; Carr, T.P.; Weller, C.L.; Dweikat, I.M.; Schlegel, V. Advances in grain sorghum and its co-products as a human health promoting dietary system. Food Res. Int. 2015, 77, 349–359. [Google Scholar] [CrossRef]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awika, J.M.; McDonough, C.M.; Rooney, L.W. Decorticating sorghum to concentrate healthy phytochemicals. J. Agric. Food Chem. 2005, 53, 6230–6234. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2020, 21, 140. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai J. Med. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Migdal, C.; Serres, M. Reactive oxygen species and oxidative stress. MS Med. Sci. 2011, 27, 405–412. [Google Scholar]
- Chang, A.M.; Smith, M.J.; Bloem, C.J.; Galecki, A.T.; Halter, J.B. Effect of lowering postprandial hyperglycemia on insulin secretion in older people with impaired glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E906–E911. [Google Scholar] [CrossRef]
- Vinson, J.A.; Howard III, T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutri. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
- Bagheri, M.; Farzadfar, F.; Qi, L.; Yekaninejad, M.S.; Chamari, M.; Zeleznik, O.A.; Kalantar, Z.; Ebrahimi, Z.; Sheidaie, A.; Koletzko, B. Obesity-related metabolomic profiles and discrimination of metabolically unhealthy obesity. J. Proteome Res. 2018, 17, 1452–1462. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus–screening for pancreatic lipase and α-Amylase inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; González-Aguilar, G.A.; Rouzaud-Sández, O.; Loarca-Piña, G.; Gorinstein, S.; Robles-Sánchez, M. Sorghum bran supplementation ameliorates dyslipidemia, glucose dysregulation, inflammation and stress oxidative induced by a high-fat diet in rats. CyTA-J Food. 2020, 18, 20–30. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, A.R.; de Castro Moreira, M.E.; Grancieri, M.; Toledo, R.C.L.; de Oliveira Araújo, F.; Mantovani, H.C.; Queiroz, V.A.V.; Martino, H.S.D. Extruded sorghum (Sorghum bicolor L.) improves gut microbiota, reduces inflammation, and oxidative stress in obese rats fed a high-fat diet. J. Funct. Foods 2019, 58, 282–291. [Google Scholar] [CrossRef]
- Moraes, É.A.; da Silva Marineli, R.; Lenquiste, S.A.; Queiroz, V.A.V.; Camargo, R.L.; Borck, P.C.; Carneiro, E.M.; Júnior, M.R.M. Whole sorghum flour improves glucose tolerance, insulin resistance and preserved pancreatic islets function in obesity diet-induced rats. J. Funct. Foods 2018, 45, 530–540. [Google Scholar] [CrossRef]
- Moraes, É.A.; Marineli, R.d.S.; Lenquiste, S.A.; Steel, C.J.; Menezes, C.B.D.; Queiroz, V.A.V.; Maróstica Júnior, M.R. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef]
- Dia, V.P.; Pangloli, P.; Jones, L.; McClure, A.; Patel, A. Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 2016, 7, 3410–3420. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Busconi, M.; Marocco, A.; Trevisan, M.; Lucini, L. Pigmented sorghum polyphenols as potential inhibitors of starch digestibility: An in vitro study combining starch digestion and untargeted metabolomics. Food Chem. 2020, 312, 126077. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Batterham, M.J.; Grant, R.; Ashton, J.; Tapsell, L.C. A diet enriched with red sorghum flaked biscuits, compared to a diet containing white wheat flaked biscuits, does not enhance the effectiveness of an energy-restricted meal plan in overweight and mildly obese adults. J. Am. Coll. Nutri. 2017, 36, 184–192. [Google Scholar] [CrossRef]
- Kim, J.-S.; Hyun, T.K.; Kim, M.-J. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011, 124, 1647–1651. [Google Scholar] [CrossRef]
- Irondi, E.A.; Adegoke, B.M.; Effion, E.S.; Oyewo, S.O.; Alamu, E.O.; Boligon, A.A. Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Hum. Wellness 2019, 8, 142–148. [Google Scholar] [CrossRef]
- Lim, J.; Zhang, X.; Ferruzzi, M.G.; Hamaker, B.R. Starch digested product analysis by HPAEC reveals structural specificity of flavonoids in the inhibition of mammalian α-amylase and α-glucosidases. Food Chem. 2019, 288, 413–421. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.-Y.; Chu, G.-X.; Xie, Z.-W.; Bao, G.-H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic composition, antioxidant properties, and inhibition toward digestive enzymes with molecular docking analysis of different fractions from Prinsepia utilis royle fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef] [Green Version]
- Farrar, J.L.; Hartle, D.K.; Hargrove, J.L.; Greenspan, P. A novel nutraceutical property of select sorghum (Sorghum bicolor) brans: Inhibition of protein glycation. Phytother. Res. 2008, 22, 1052–1056. [Google Scholar] [CrossRef]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Food Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, C.R.; Junior, M.R.M. Health Benefits of Flavonoids. In Bioactive Compounds; Elsevier: Duxford, UK, 2019; pp. 185–201. [Google Scholar]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Allison, D.B.; Gadbury, G.; Schwartz, L.G.; Murugesan, R.; Kraker, J.L.; Heshka, S.; Fontaine, K.R.; Heymsfield, S.B. A novel soy-based meal replacement formula for weight loss among obese individuals: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2003, 57, 514–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutri. 2020, 60, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Sekhon-Loodu, S.; Rupasinghe, H. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Phenol-Explorer: Comprehensive Database on Polyphenol Content in Foods. Available online: https://phenol-explorer.eu/ (accessed on 16 March 2020).
- Wu, G.C.; Johnson, S.K.; Bornman, J.F.; Bennette, S.J.; Clarke, M.W.; Singh, V.; Fang, Z.H. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 6, 21835. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Allred, K.F.; Dykes, L.; Allred, C.D.; Awika, J.M. Enhanced action of apigenin and naringenin combination on estrogen receptor activation in non-malignant colonocytes: Implications on sorghum-derived phytoestrogens. Food Funct. 2015, 6, 749–755. [Google Scholar] [CrossRef]
- Ravisankar, S.; Abegaz, K.; Awika, J.M. Structural profile of soluble and bound phenolic compounds in teff (Eragrostis tef) reveals abundance of distinctly different flavones in white and brown varieties. Food Chem. 2018, 263, 265–274. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 2017, 15, 1559325817691158. [Google Scholar] [CrossRef]
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Nagayasu, H.; Yamashita, Y.; Ashida, H. Green tea ameliorates hyperglycemia by promoting the translocation of glucose transporter 4 in the skeletal muscle of diabetic rodents. Int. J. Mol. Sci. 2019, 20, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, D.; Faubion, J.; Rooney, L. Sorghum phenolic acids, their high performance liquid chromatography separation and their relation to fungal resistance. Cereal Chem. 1983, 60, 255–259. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Pradeep, P.; Sreerama, Y.N. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2018, 247, 46–55. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Apea-Bah, F.B.; Minnaar, A.; Bester, M.J.; Duodu, K.G. Sorghum–cowpea composite porridge as a functional food, Part II: Antioxidant properties as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2016, 197, 307–315. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Chelliah, R.; Ham, H.J.; Kim, J.-H.; Han, S.-I.; Hur, J.H.; Oh, D.-H. Phenolic Profile, Antioxidant, and Antidiabetic Potential Exerted by Millet Grain Varieties. Antioxidants 2020, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-F.; Chang, Y.-Q.; Deng, J.; Li, W.-X.; Jian, J.; Gao, J.-S.; Wan, X.; Gao, H.; Kurihara, H.; Sun, P.-H. Prediction and evaluation of the lipase inhibitory activities of tea polyphenols with 3D-QSAR models. Sci. Rep. 2016, 6, 34387. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the decorticated sorghum grain soluble extracts are available from the authors. |

| Decorticated Varieties | TPC (mg Ferulic Acid Equivalent/100 g, DW) | TFC (mg Catechin Equivalent/100 g, DW) | CTC (mg Catechin Equivalent/100 g, DW) |
|---|---|---|---|
| DSOR 01 | 129.7 ± 17.06 a | 113.5 ± 9.10 a | 128.87 ± 4.27 a |
| DSOR 03 | 101.2 ± 16.49 b | 111.8 ± 8.51 a | 67.49 ± 3.70 b |
| DSOR 08 | 118.2 ± 5.05 b | 126.5 ± 10.42 a | 81.48 ± 4.02 c |
| DSOR 11 | 120.5 ± 12.33 b | 115.2 ± 10.11 a | 92.95 ± 4.26 c |
| DSOR 17 | 84.14 ± 5.02 b | 111.5 ± 11.34 a | 132.50 ± 3.92 a |
| DSOR 21 | 74.79 ± 5.90 b | 110.3 ± 9.30 a | 116.40 ± 4.22 a |
| DSOR 24 | 112.9 ± 7.05 b | 118.2 ± 10.46 a | 98.56 ± 3.65 c |
| DSOR 33 | 137.2 ± 12.45 a | 118.0 ± 9.13 a | 56.65 ± 4.71 b |
| Peak No. | RT (min) | Molecular Formula | Molecular Weight | [M−H]− (m/z) | MS/MS (% Abundance) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 8.86 | C30H26O12 | 578.1428 | 577.1354 | 289 (92), 407 (62), 425 (55) | Procyanidin dimer |
| 2 | 9.58 | C15H14O6 | 290.0797 | 289.0719 | 109 (78), 123 (100), 203 (34) | (+)-Catechin |
| 3 | 9.69 | C21H22O12 | 466.1115 | 465.1042 | 275 (48), 285 (100), 303 (22) | Dihydromyricetin 3-O-rhamnoside |
| 4 | 11.89 | C15H10O5 | 270.0531 | 269.0459 | 117 (100), 149 (33), 227 (9) | Apigenin |
| 5 | 12.26 | C16H12O5 | 284.0690 | 283.0612 | 196 (100), 240 (88), 268 (32) | Glycitein |
| 6 | 12.39 | C15H10O4 | 254.0585 | 253.0508 | 117 (91), 210 (100), 225 (17) | Apigeninidin |
| 7 | 12.49 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 8 | 12.69 | C16H12O4 | 268.0743 | 267.0666 | 180 (100), 224 (91), 252 (34) | Formononetin |
| 9 | 14.86 | C21H20O9 | 416.1112 | 415.1037 | 135 (88), 161 (100), 253 (94) | 1,3-O-dicaffeoylglycerol |
| 10 | 15.56 | C15H12O5 | 272.0686 | 271.0614 | 107 (38), 119 (100), 151 (30) | Naringenin |
| 11 | 15.62 | C15H10O6 | 286.0481 | 285.0406 | 133 (100), 151 (43), 217 (9) | Luteolin |
| 12 | 16.49 | C15H10O5 | 270.0531 | 269.0459 | 117 (100), 149 (33), 227 (9) | Apigenin |
| Peak No. | RT (min) | Molecular Formula | Molecular Weight | [M−H]− (m/z) | MS/MS (% Abundance) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 9.58 | C15H14O6 | 290.0797 | 289.0721 | 109 (78), 123 (100), 203 (34) | (+)-Catechin |
| 2 | 9.64 | C21H22O12 | 466.1115 | 465.1042 | 275 (48), 285 (100), 303 (22) | Dihydromyricetin 3-O-rhamnoside |
| 3 | 9.67 | C24H22O14 | 534.0988 | 533.0918 | 200 (20), 325 (100), 447 (3) | Luteolin 7-O-malonyl-glucoside |
| 4 | 9.69 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 5 | 10.49 | C9H8O4 | 180.0424 | 179.0350 | 117 (7), 135 (100) | Caffeic acid |
| 6 | 12.41 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 7 | 12.45 | C15H10O6 | 286.0481 | 285.0406 | 199 (100), 175 (65), 217 (26) | Kaempferol |
| 8 | 12.69 | C16H12O4 | 268.0743 | 267.0664 | 180 (100), 224 (91), 252 (34) | Formononetin |
| 9 | 14.62 | C15H12O6 | 288.0636 | 287.0562 | 125 (100), 151 (33), 193 (27) | Eriodictyol |
| 10 | 14.86 | C21H20O9 | 416.1112 | 415.1037 | 135 (88), 161 (100), 253 (94) | 1,3-O-dicaffeoylglycerol |
| 11 | 15.56 | C15H12O5 | 272.0686 | 271.0614 | 107 (38), 119 (100), 151 (30) | Naringenin |
| 12 | 15.63 | C15H10O6 | 286.0481 | 285.0406 | 133 (100), 151 (43), 217 (9) | Luteolin |
| 13 | 16.49 | C15H10O5 | 270.0531 | 269.0457 | 117 (100), 149 (33), 227 (9) | Apigenin |
| Peak No. | RT (min) | Molecular Formula | Molecular Weight | [M−H]− (m/z) | MS/MS (% Abundance) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 8.83 | C30H26O12 | 578.1428 | 577.1355 | 289 (92), 407 (62), 425 (55) | Procyanidin dimer |
| 2 | 9.58 | C15H14O6 | 290.0797 | 289.0721 | 109 (78), 123 (100), 203 (34) | (+)-Catechin |
| 3 | 9.64 | C21H22O12 | 466.1115 | 465.1044 | 275 (48), 285 (100), 303 (22) | Dihydromyricetin 3-O-rhamnoside |
| 4 | 9.67 | C24H22O14 | 534.0988 | 533.0918 | 200 (20), 325 (100), 447 (3) | Luteolin 7-O-malonyl-glucoside |
| 5 | 9.69 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 6 | 10.49 | C9H8O4 | 180.0424 | 179.0350 | 117 (7), 135 (100) | Caffeic acid |
| 7 | 10.45 | C21H22O12 | 466.1115 | 465.1044 | 275 (48), 285 (100), 303 (22) | Dihydromyricetin 3-O-rhamnoside |
| 8 | 12.24 | C16H12O5 | 284.0690 | 283.0613 | 196 (100), 240 (88), 268 (32) | Glycitein |
| 9 | 12.41 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 10 | 12.45 | C15H10O6 | 286.0481 | 285.0406 | 199 (100), 175 (65), 217 (26) | Kaempferol |
| 11 | 12.69 | C16H12O4 | 268.0743 | 267.0664 | 180 (100), 224 (91), 252 (34) | Formononetin |
| 12 | 13.34 | C15H12O7 | 304.0587 | 303.0513 | 125 (100), 175 (24), 217 (8) | Taxifolin |
| 13 | 14.86 | C21H20O9 | 416.1112 | 415.1037 | 135 (88), 161 (100), 253 (94) | 1,3-O-dicaffeoylglycerol |
| 14 | 15.56 | C15H12O5 | 272.0686 | 271.0615 | 107 (38), 119 (100), 151 (30) | Naringenin |
| 15 | 15.69 | C15H10O6 | 286.0481 | 285.0408 | 133 (100), 151 (43), 217 (9) | Luteolin |
| 16 | 16.49 | C15H10O5 | 270.0531 | 269.0459 | 117 (100), 149 (33), 227 (9) | Apigenin |
| 17 | 16.55 | C16H12O6 | 300.0638 | 299.0563 | 136 (16), 256 (33), 284 (100) | Hispidulin |
| 18 | 17.76 | C16H12O6 | 300.0638 | 299.0563 | 136 (16), 256 (33), 284 (100) | Hispidulin |
| Compound | RT (min) | DSOR 11 (µg/100 g) | DSOR 17 (µg/100 g) | DSOR 33 (µg/100 g) | Polyphenol Class |
|---|---|---|---|---|---|
| Gallic acid | 2.40 | ND | ND | ND | Phenolic acid |
| Catechin | 9.58 | 103.03 | 83.55 | 140.99 | Flavonoid |
| Caffeic acid | 10.49 | ND | 44.93 | 49.82 | Phenolic acid |
| p-Coumaric acid | 12.06 | ND | ND | ND | Phenolic acid |
| Ferulic acid | 12.58 | ND | ND | ND | Phenolic acid |
| Quercetin | 15.38 | ND | ND | ND | Flavonoid |
| Genistein | 15.87 | ND | ND | ND | Flavonoid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Yeon, S.-J.; Ham, H.J.; Kim, J.-H.; Han, S.-I.; Oh, D.-H. Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules 2020, 25, 2854. https://doi.org/10.3390/molecules25122854
Ofosu FK, Elahi F, Daliri EB-M, Yeon S-J, Ham HJ, Kim J-H, Han S-I, Oh D-H. Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules. 2020; 25(12):2854. https://doi.org/10.3390/molecules25122854
Chicago/Turabian StyleOfosu, Fred Kwame, Fazle Elahi, Eric Banan-Mwine Daliri, Su-Jung Yeon, Hun Ju Ham, Joong-Hark Kim, Sang-Ik Han, and Deog-Hwan Oh. 2020. "Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities" Molecules 25, no. 12: 2854. https://doi.org/10.3390/molecules25122854
APA StyleOfosu, F. K., Elahi, F., Daliri, E. B.-M., Yeon, S.-J., Ham, H. J., Kim, J.-H., Han, S.-I., & Oh, D.-H. (2020). Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules, 25(12), 2854. https://doi.org/10.3390/molecules25122854








