Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material, Extraction, and Isolation
2.3. Free Radical Scavenging Activity Assay
2.4. Assessment of ABTS Radical Scavenging Activity
2.5. Brine Shrimp Lethality Bioassay
2.6. Cell Culture and Sulforhodamine B Assay
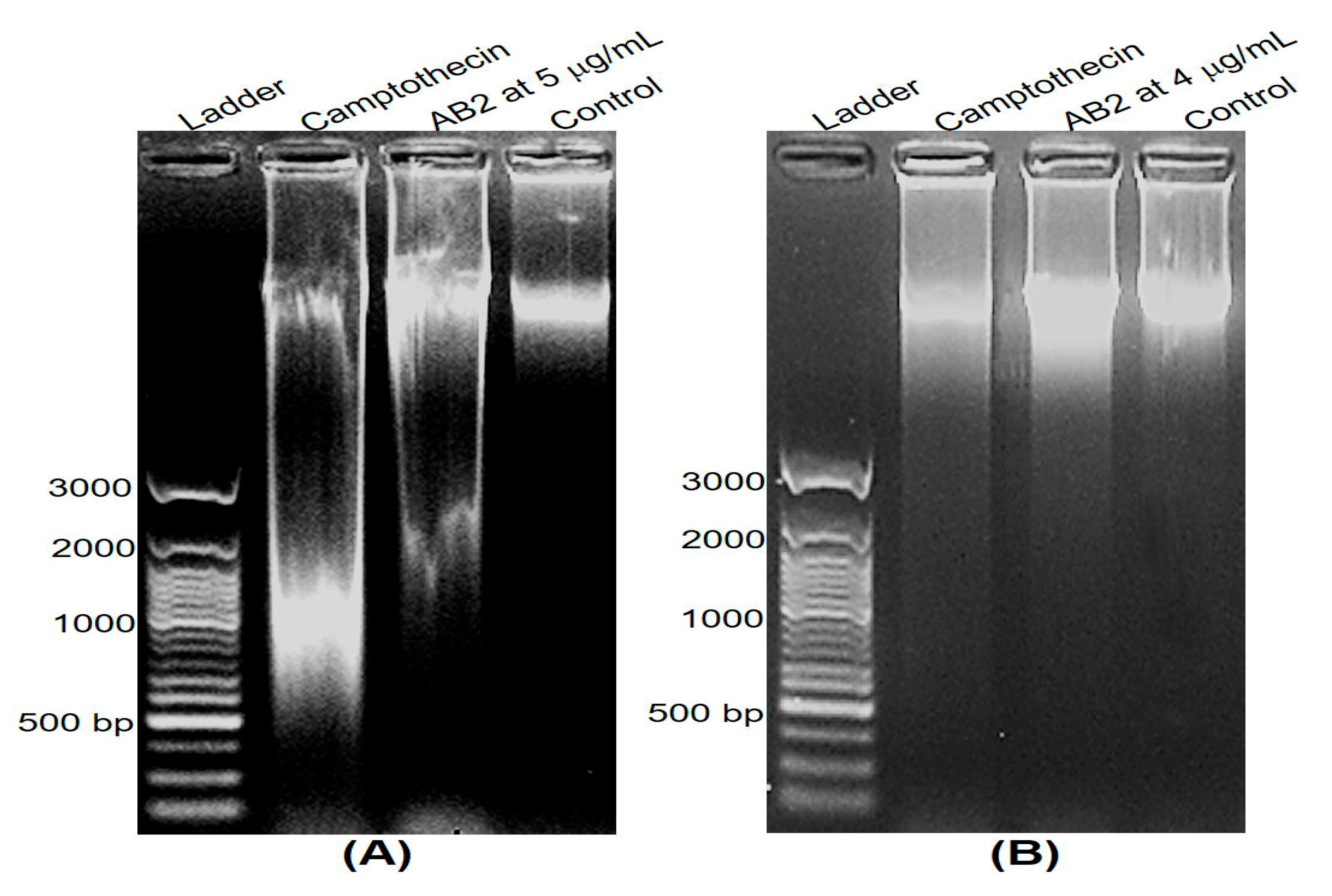
2.7. DNA Fragmentation and Apoptosis Induced by AB2
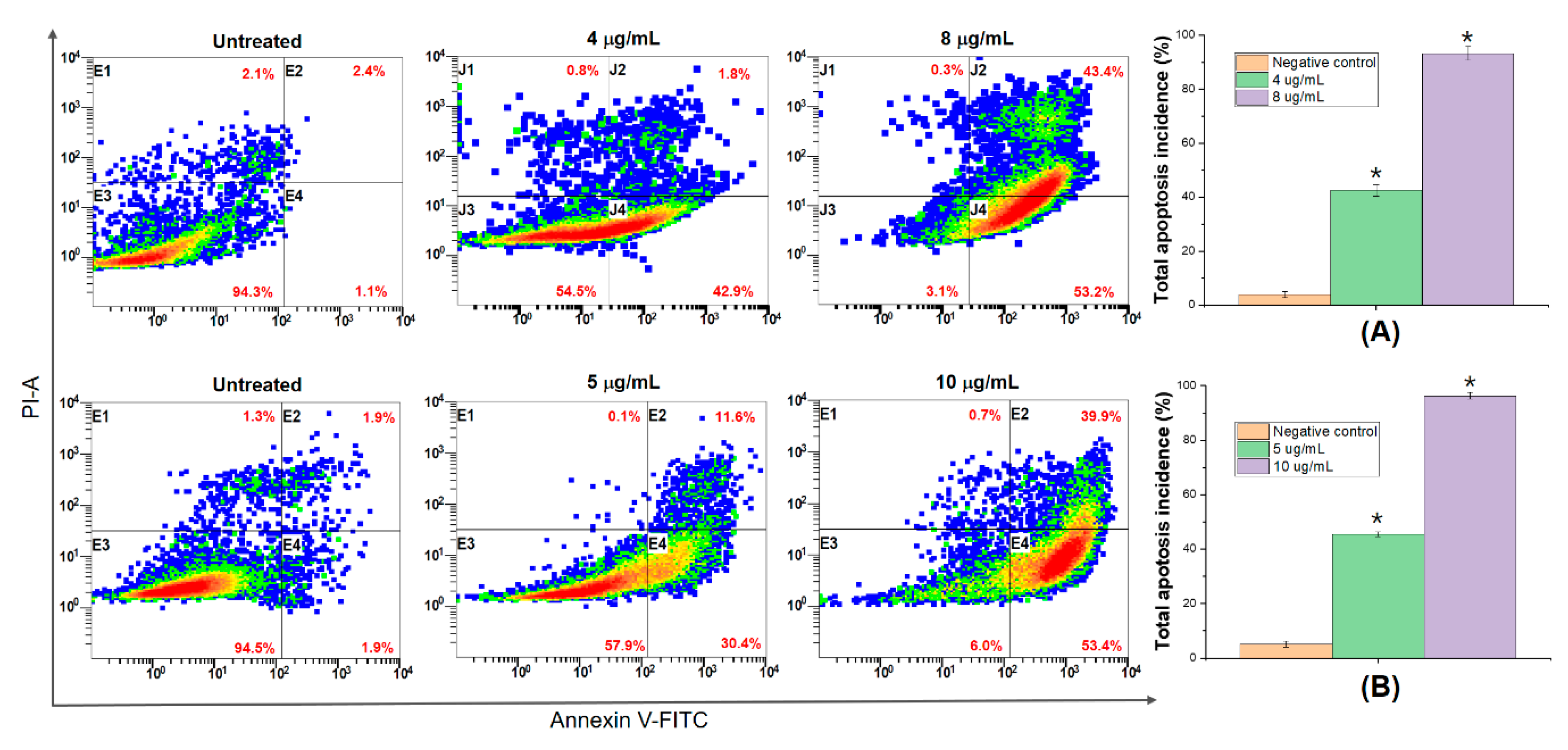
2.8. Detection of Mitochondrial Membrane Potential
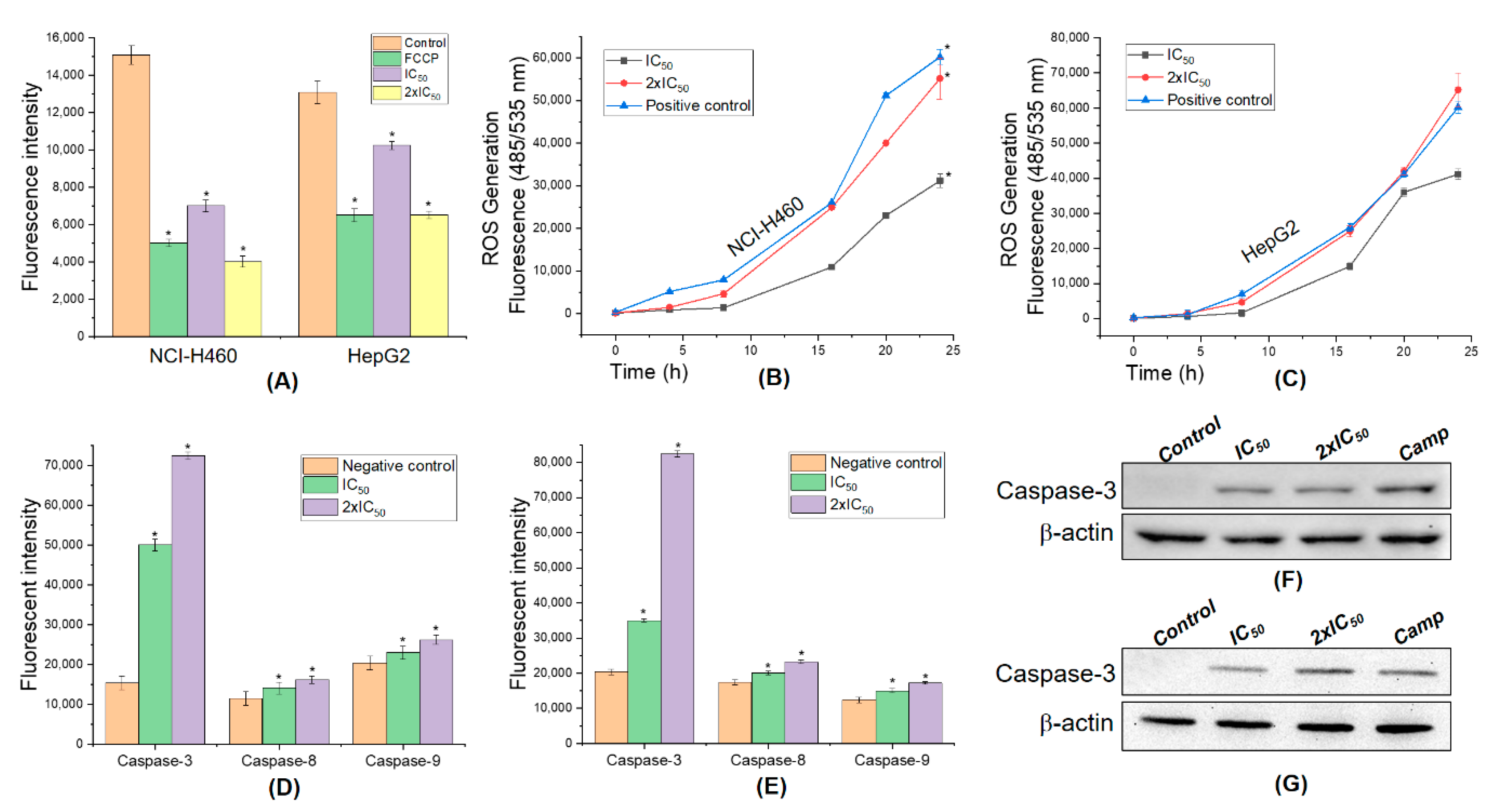
2.9. Fluorescent Assays for Measuring Caspase-3 Activity and Intracellular Reactive Oxygen Species (ROS) Generation
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Brine Shrimp Lethality Test
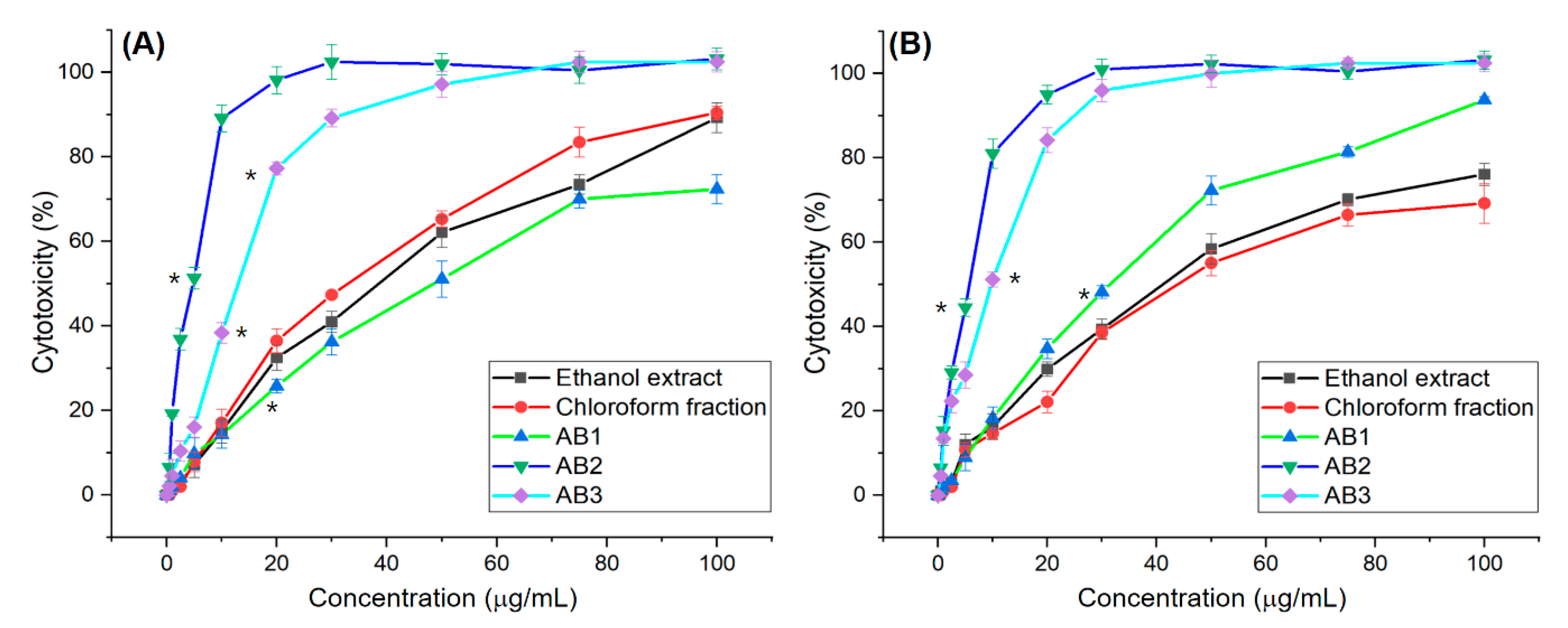
3.2. In Vitro Screening for Cytotoxic Activity of Extracts and Fractions
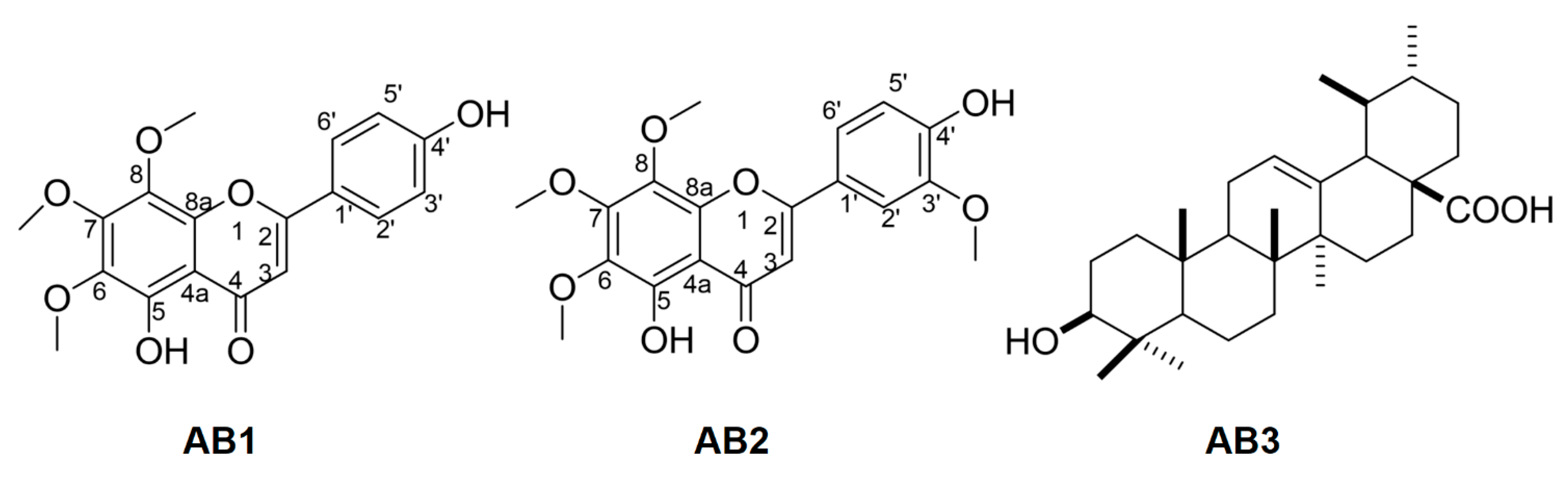
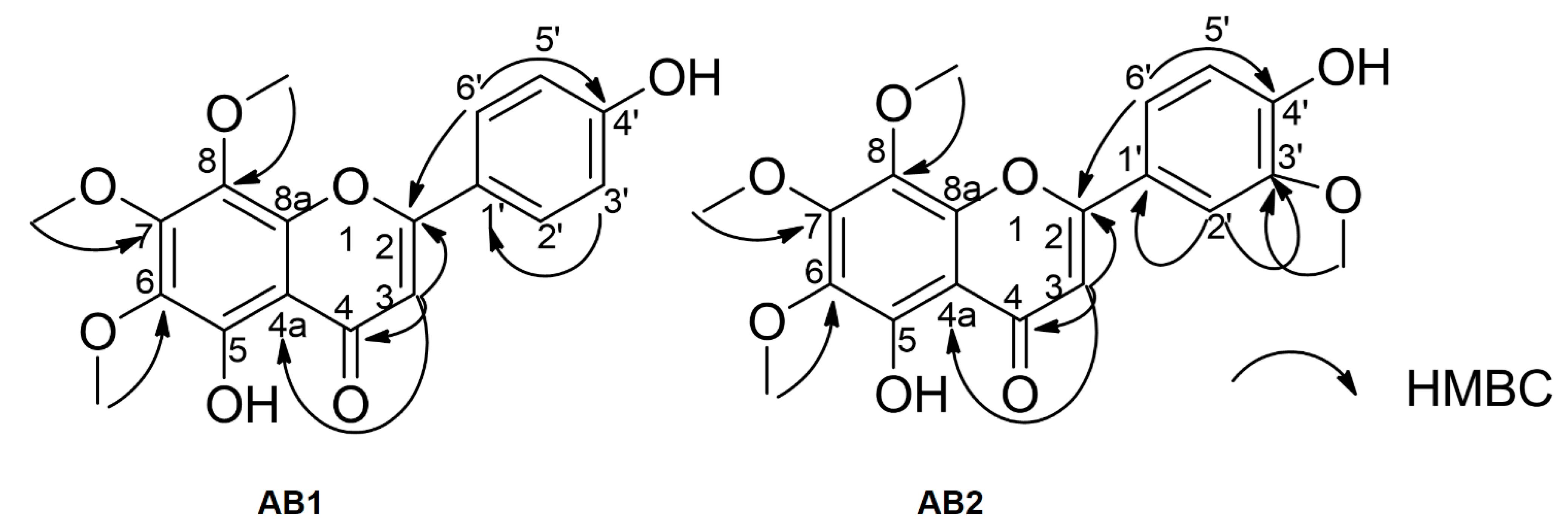
3.3. Spectroscopic Data of Isolated Compounds
3.4. Bioactivity of Isolated Compounds
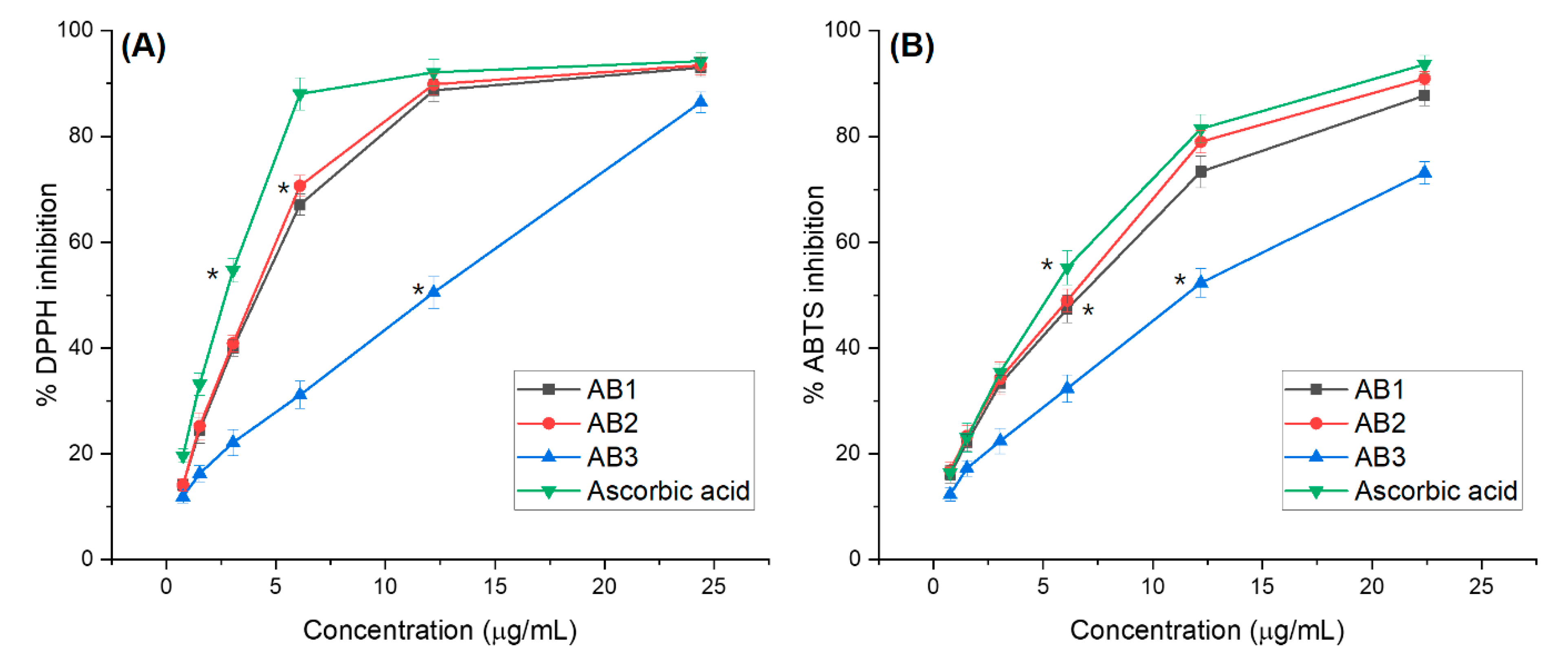
3.4.1. Antioxidant Activity Assessments
3.4.2. Brine Shrimp Bioassay
3.5. In Vitro Screening for Cytotoxic Activity of Compounds
3.6. DNA Fragmentation and Apoptosis Induced by 5,4’-Dihydroxy-6,7,8,3’-Tetramethoxyflavone
3.7. 5,4’-Dihydroxy-6,7,8,3’-Tetramethoxyflavone Triggers Apoptosis Sensitivity by ROS- Caspase-3 Mediated in Human Hepatocyte Carcinoma Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 8973. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.M.; Athwal, S.; Panahloo, A. The advantages and disadvantages of a ’herbal’ medicine in a patient with diabetes mellitus: A case report. Diabet. Med. 2004, 21, 625–627. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Wei, W.-C.; Jian, F.-Y.; Yang, N.-S. Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complement. Altern. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, T.T.; Ma, P.C.; Ta, Q.T.H.; Duong, T.-H.; Van Giau, V. Potential Antimicrobial and Anticancer Activities of an Ethanol Extract from Bouea macrophylla. Molecules 2020, 25, 1996. [Google Scholar] [CrossRef]
- Duong, T.H.; Beniddir, M.A.; Trung, N.T.; Phan, C.D.; Vo, V.G.; Nguyen, V.K.; Le, Q.L.; Nguyen, H.D.; Le Pogam, P. Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha. Molecules 2020, 25, 1830. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Agarwal, G.; Carcache, P.J.B.; Addo, E.M.; Kinghorn, A.D. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol. Adv. 2020, 38, 107337. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.H.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, A.; Ahmad, R. Potential phytocompounds for developing breast cancer therapeutics: Nature’s healing touch. Eur. J. Pharmacol. 2018, 827, 125–148. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol. Med. 2018, 24, 29. [Google Scholar] [CrossRef]
- Power, F.B.; Salway, A.H. Chemical Examination of Micromeria Chamissonis. J. Am. Chem. Soc. 1908, 30, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Jahaniani, F.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Mahmoudian, M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 2005, 66, 1581–1592. [Google Scholar] [CrossRef]
- Fattahi, M.; Cusido, R.M.; Khojasteh, A.; Bonfill, M.; Palazon, J. Xanthomicrol: A comprehensive review of its chemistry, distribution, biosynthesis and pharmacological activity. Mini-Rev. Med. Chem. 2014, 14, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Ebrahimi, S.A.; Akhavan, M.M. Antiangiogenic Activity of Xanthomicrol and Calycopterin, Two Polymethoxylated Hydroxyflavones in Both In Vitro and Ex Vivo Models. Phytother. Res. 2014, 28, 1661–1670. [Google Scholar] [CrossRef]
- Harmand, P.-O.; Delage, C.; Simon, A.; Duval, R.E. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Shih, Y.-L.; Yeh, M.-Y.; Liao, N.-C.; Chung, H.-Y.; Liu, K.-L.; Lee, M.-H.; Chou, P.-Y.; Hou, H.-Y.; Chou, J.-S.; et al. Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release Through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro. In Vivo 2019, 33, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [Green Version]
- Weng, H.; Tan, Z.-J.; Hu, Y.-P.; Shu, Y.; Bao, R.-F.; Jiang, L.; Wu, X.-S.; Li, M.; Ding, Q.; Wang, X.-A.; et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.-G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch Pharm. Res. 2011, 34, 1363–1372. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Zhou, Z.; Lai, C.-S.; Zhang, L.; Quan, Z.; Shao, X.; Pan, M.-H.; Ho, C.-T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010, 122, 831–835. [Google Scholar] [CrossRef]
- Tsankova, E.T.; Kuleva, L.V.; Thanh, L.T. Composition of the Essential Oil of Adenosma bracteosum Bonati. J. Essent. Oil Res. 1994, 6, 305–306. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Pham, Q.T.; Luong, T.N.H.; Le, H.K.; Van Giau, V. Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Naher, S.; Aziz, A.; Akter, M.I.; Rahman, S.M.M.; Sajon, S.R.; Mazumder, K. Anti-diarrheal activity and brine shrimp lethality bioassay of methanolic extract of Cordyline fruticosa (L.) A. Chev. leaves. Clin. Phytosci. 2019, 5, 15. [Google Scholar] [CrossRef]
- Syahmi, A.R.M.; Vijayarathna, S.; Sasidharan, S.; Latha, L.Y.; Kwan, Y.P.; Lau, Y.L.; Shin, L.N.; Chen, Y.; Vijayaratna, S. Acute Oral Toxicity and Brine Shrimp Lethality of Elaeis guineensis Jacq., (Oil Palm Leaf) Methanol Extract. Molecules 2010, 15, 8111–8121. [Google Scholar] [CrossRef] [Green Version]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-Protocol 2016, 6, 1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skehan, P.; Scudiero, M.; Vistica, D.; Bokesch, H.; Kenney, S.; Storeng, R.; Monks, A.; McMahon, J.; Warren, J.T.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E.; Goetz, C.M.; McLaughlin, J.L.; Suffness, M. A blind comparison of simple bench-top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991, 2, 107–111. [Google Scholar] [CrossRef]
- Arcanjo, D.; Albuquerque, A.; Melo-Neto, B.; Santana, L.; Medeiros, M.; Citó, A.; Arcanjo, D.D.R. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz. J. Boil. 2012, 72, 505–509. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Song, M.; Charoensinphon, N.; Wu, X.; Zheng, J.; Gao, Z.; Xu, F.; Wang, M.; Xiao, H. Inhibitory Effects of Metabolites of 5-Demethylnobiletin on Human Nonsmall Cell Lung Cancer Cells. J. Agric. Food Chem. 2016, 64, 4943–4949. [Google Scholar] [CrossRef]
- Song, M.; Wu, X.; Charoensinphon, N.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Li, Z.; Li, F.; Zhou, J.; et al. Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 2017, 8, 954–963. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kuršvietienė, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and Antioxidant Effects of Ursolic Acid in Isolated Rat Heart Mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Punia, S.; Sharma, A.K.; Sharma, A. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar] [PubMed]
- Sultana, N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzym. Inhib. Med. Chem. 2011, 26, 616–642. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian Cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, A.; Radhiga, T.; Pugalendi, K.V. Effect of ursolic acid and Rosiglitazone combination on hepatic lipid accumulation in high fat diet-fed C57BL/6J mice. Eur. J. Pharmacol. 2014, 741, 297–303. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panagiotidis, M. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Dobhal, M.P.; Joshi, Y.C.; Chahar, M.K. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ghasemzadeh, A.; Jaafar, H. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef] [Green Version]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Goldin, A.; Venditti, J.M.; Macdonald, J.S.; Muggia, F.M.; Henney, J.E.; Devita, V.T., Jr. Current results of the screening program at the Division of Cancer Treatment, National Cancer Institute. Eur. J. Cancer 1981, 17, 129–142. [Google Scholar] [CrossRef]
- Protocols for screening chemical agents and natural products against animal tumors and other biological systems: Drug Evaluation Branch. Nat. Cancer Inst. 1962, 3, 1–107.
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2017, 58, 568–574. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Noshita, T.; Kidachi, Y.; Umetsu, H.; Hayashi, M.; Komiyama, K.; Funayama, S.; Ryoyama, K. Isolation of Ursolic Acid from Apple Peels and Its Specific Efficacy as a Potent Antitumor Agent. J. Health Sci. 2008, 54, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Du, R.; Yu, X. Ursolic Acid Exhibits Potent Anticancer Effects in Human Metastatic Melanoma Cancer Cells (SK-MEL-24) via Apoptosis Induction, Inhibition of Cell Migration and Invasion, Cell Cycle Arrest, and Inhibition of Mitogen-Activated Protein Kinase (MAPK)/ERK Signaling Pathway. Med. Sci. Monit. 2019, 25, 1283–1290. [Google Scholar] [PubMed]
- Feng, X.; Su, X. Anticancer effect of ursolic acid via mitochondria-dependent pathways. Oncol. Lett. 2019, 17, 4761–4767. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 2011, e2597. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.G.; An, S.S.A.; Hulme, J.P. Mitochondrial therapeutic interventions in Alzheimer’s disease. J. Neurol. Sci. 2018, 395, 62–70. [Google Scholar]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mates, J.M.; Sanchez-Jimenez, F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef]
- Suski, J.M.; Lebiedzińska-Arciszewska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation Between Mitochondrial Membrane Potential and ROS Formation. Methods Mol. Biol. 2011, 810, 183–205. [Google Scholar]
- Waterhouse, N.J.; Goldstein, J.C.; Von Ahsen, O.; Schuler, M.; Newmeyer, D.D.; Green, D.R. Cytochrome C Maintains Mitochondrial Transmembrane Potential and Atp Generation after Outer Mitochondrial Membrane Permeabilization during the Apoptotic Process. J. Cell Biol. 2001, 153, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.E.; Gottlieb, R.A.; Green, D.R. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 2003, 160, 65–75. [Google Scholar] [CrossRef]
- Xie, L.-L.; Shi, F.; Tan, Z.; Li, Y.; Bode, A.M.; Cao, Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018, 109, 3686–3694. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, X.; Zhang, L.; Su, B.; Tian, D.; Li, Y.; Zhao, J.; Wang, Y.-M.; Peng, S. Overexpression of HO-1 assisted PM2.5-induced apoptosis failure and autophagy-related cell necrosis. Ecotoxicol. Environ. Saf. 2017, 145, 605–614. [Google Scholar] [CrossRef]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.-U.; Kimchi, A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef]
- Peixoto, M.S.; Galvão, M.F.D.O.; De Medeiros, S.R.B. Cell death pathways of particulate matter toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Santos, U.P.; Campos, J.F.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; Souza, K.D.P.; Dos Santos, U.P. Antioxidant, Antimicrobial and Cytotoxic Properties as Well as the Phenolic Content of the Extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11, e0167531. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, J.C.; Macorini, L.F.B.; Antunes, K.Á.; Dos Santos, U.P.; Campos, J.F.; Dias-Júnior, N.M.; Sangalli, A.; Cardoso, C.A.L.; Vieira, M.D.C.; Rabelo, L.A.; et al. Antioxidant and Cytotoxic Activity of Hydroethanolic Extract from Jacaranda decurrens Leaves. PLoS ONE 2014, 9, e112748. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Espindola, P.P.D.T.; Torquato, H.F.V.; Vital, W.; Justo, G.Z.; Silva, D.B.; Carollo, C.A.; Souza, K.D.P.; Paredes-Gamero, E.J.; Dos Santos, E.L. Leaf and Root Extracts from Campomanesia adamantium (Myrtaceae) Promote Apoptotic Death of Leukemic Cells via Activation of Intracellular Calcium and Caspase-3. Front. Pharmacol. 2017, 8, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldivia, D.S.; Leite, D.F.; De Castro, D.T.H.; Campos, J.F.; Dos Santos, U.P.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; Souza, K.D.P.; Dos Santos, E.L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |
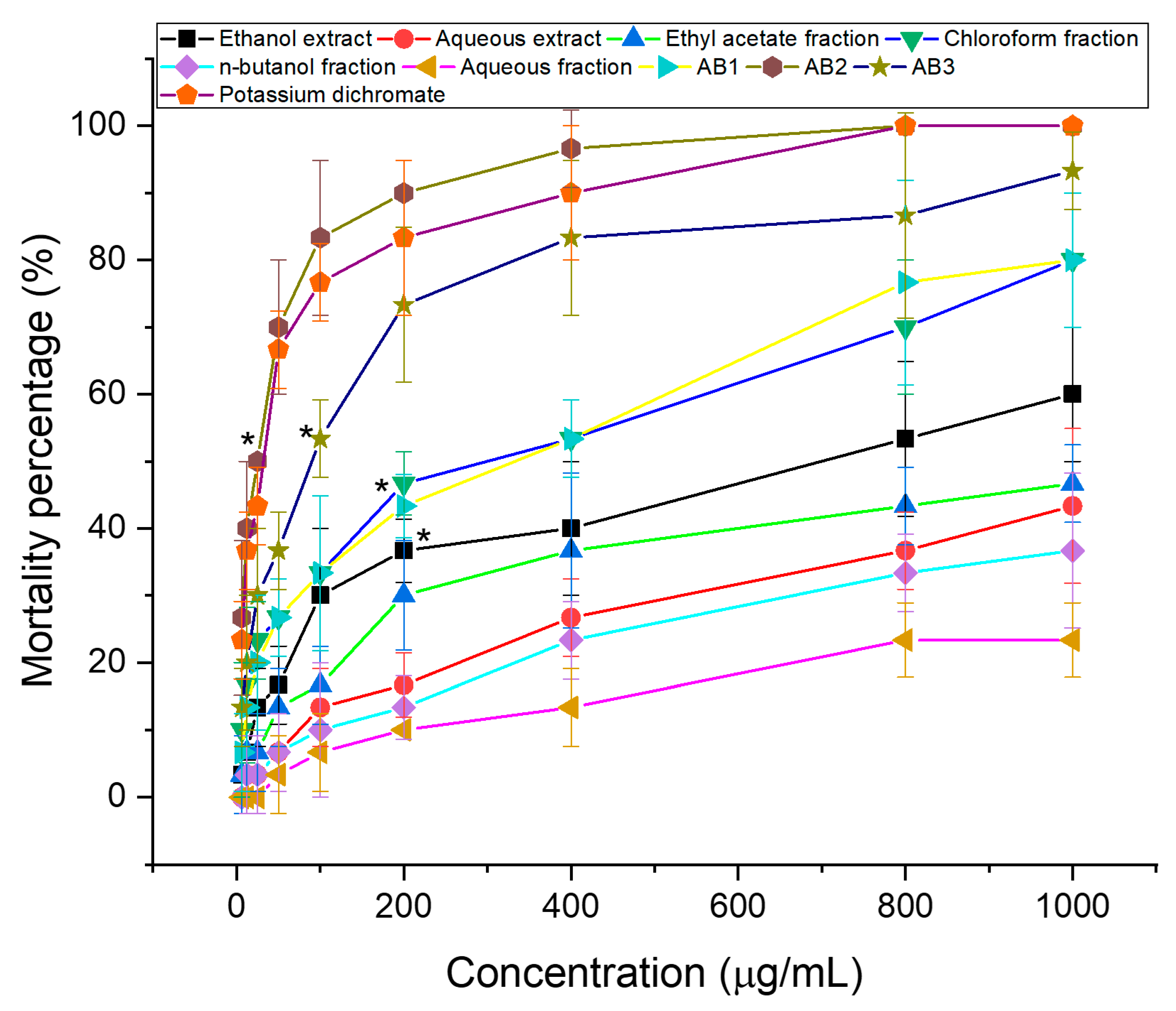
| Sample | Ethanol Extract | Aqueous Extract | Chloroform Fraction | Ethyl Acetate Fraction | n-Butanol Fraction | Aqueous Fraction | Compound | Potassium Dichromate | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AB1 | AB2 | AB3 | ||||||||
| LC50 (µg/mL) | 647.64 | >1000 | 205.58 | >1000 | >1000 | >1000 | 202.8 | 20.34 | 65.71 | 27.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.H.; Ta, Q.T.H.; Pham, Q.T.; Luong, T.N.H.; Phung, V.T.; Duong, T.-H.; Vo, V.G. Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules 2020, 25, 2912. https://doi.org/10.3390/molecules25122912
Nguyen NH, Ta QTH, Pham QT, Luong TNH, Phung VT, Duong T-H, Vo VG. Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules. 2020; 25(12):2912. https://doi.org/10.3390/molecules25122912
Chicago/Turabian StyleNguyen, Ngoc Hong, Qui Thanh Hoai Ta, Quang Thang Pham, Thi Ngoc Han Luong, Van Trung Phung, Thuc-Huy Duong, and Van Giau Vo. 2020. "Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells" Molecules 25, no. 12: 2912. https://doi.org/10.3390/molecules25122912
APA StyleNguyen, N. H., Ta, Q. T. H., Pham, Q. T., Luong, T. N. H., Phung, V. T., Duong, T.-H., & Vo, V. G. (2020). Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules, 25(12), 2912. https://doi.org/10.3390/molecules25122912






