Sustainable Palm Oil—The Role of Screening and Advanced Analytical Techniques for Geographical Traceability and Authenticity Verification
Abstract
:1. Introduction
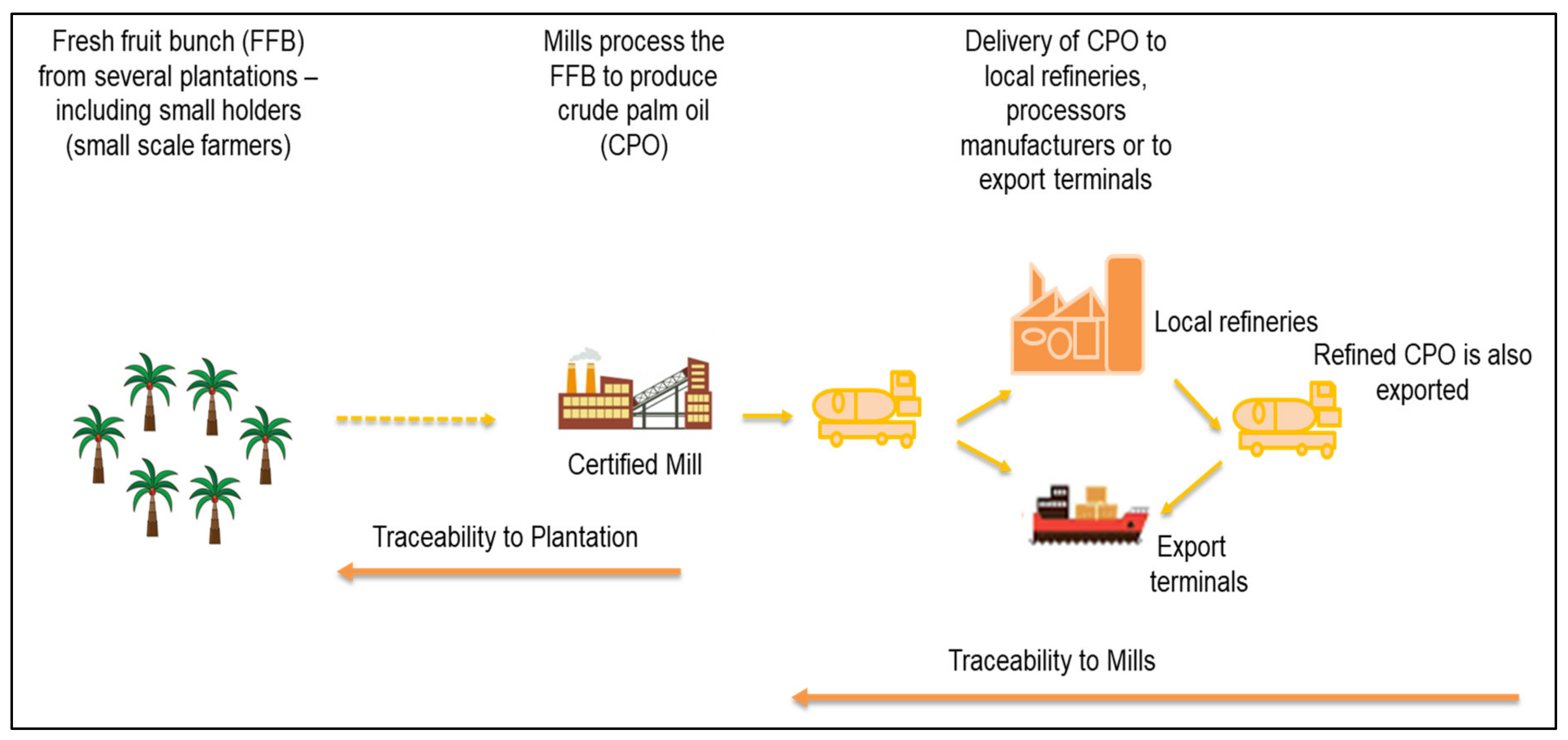
2. Traceability as Part of the Legislative Requirement for Regulating the Industry
3. Analytical Methods for Traceability of Palm Oil Based on Geographical Origin
3.1. Traceability of Palm Oil Using Chromatographic Techniques
3.2. Analysis of a Stable Isotope as a Traceability System for Palm Oil
4. Analytical Methods for Detecting Adulterations in Palm Oil
5. DNA Analysis as a Tool for Traceability and Authenticity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Patent
References
- Kushairi, A.; Ong-Abdullah, M.; Nambiappan, B.; Hishamuddin, E.; Bidin, M.N.I.Z.; Ghazali, R.; Subramaniam, V.; Sundram, S.; Parveez, G.K.A. Oil palm economic performance in Malaysia and R&D Progress in 2018. J. Oil Palm Res. 2019, 31, 165–194. [Google Scholar]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- May, C.Y.; Nesaretnam, K. Research advancements in palm oil nutrition. Eur. J. Lipid Sc. Tech. 2014, 116, 301–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustamam, A.F.K.; Beng, Y.C.; Sulaiman, N. Revision of the Malaysian Standard for Palm Oil—Specification MS 814:2007, (Second Revision) Amendment 1:2018—What’s New? Palm Oil Developments 2019, 71, 18–32. [Google Scholar]
- Navia, E.A.; Ávila, R.A.; Daza, E.E.; Restrepo, E.F.; Romero, H.M. Assessment of tolerance to bud rot in oil palm under field conditions. Eur. J. Plant Path. 2014, 140, 711–720. [Google Scholar] [CrossRef]
- Rajanaidu, N.; Tan, B.K.; Rao, V. Analysis of fatty acid composition (FAC) in Elaeis guineensis, Elaeis oleifera, their hybrids and its implications in breeding. PORIM Bull. 1983, 7, 9–20. [Google Scholar]
- Kushairi, A.; Loh, S.K.; Azman, I.; Hishamuddin, E.; Ong-Abdullah, M.; Izuddin, Z.; Razmah, G.; Sundram, S.; Parveez, G.K.A. Oil palm economic performance in Malaysia and R&D progress in 2017. J. Oil Palm Res. 2018, 30, 163–195. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.; Wahid, M.B. World Palm Oil Supply, Demand, Price and Prospects: Focus on Malaysian and Indonesian Palm Oil Industry; Malaysian Palm Oil Board Press: Petaling Jaya, Malaysia, 2010. [Google Scholar]
- Mukherjee, I.; Sovacool, B.K. Palm oil-based biofuels and sustainability in Southeast Asia: A review of Indonesia, Malaysia, and Thailand. Renew. Sustain. Ener. Rev. 2014, 37, 1–12. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Azhar, B.; Saadun, N.; Prideaux, M.; Lindenmayer, D.B. The global palm oil sector must change to save biodiversity and improve food security in the tropics. J. Environ. Manag. 2017, 203, 457–466. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Available online: http:/www.fao.org/economic/esa (accessed on 7 May 2020).
- Corley, R.H.V. How much palm oil do we need? Environ. Sci. Policy 2009, 12, 134–139. [Google Scholar] [CrossRef]
- Nambiappan, B.; Ismail, A.; Hashim, N.; Ismail, N.; Nazrima, S.; Idris, N.A.N.; Omar, N.; Saleh, K.; Hassan, N.A.M.; Kushairi, A. Malaysia: 100 years of resilient palm oil economic performance. J. Oil Palm Res. 2018, 30, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Malaysian Oil Palm Statistics 2019, 39th ed.; MPOB: Bangi, Malaysia, 2020.
- Parveez, G.K.A.; Ong-Abdullah, M.; Hasan, Z.A.A.; Hishamuddin, E.; Loh, S.K.; Zanal Bidin, M.N.I.; Salleh, K.M.; Sundram, S.; Idris, Z. Oil Palm Economic Performance in Malaysia and R&D Progress in 2019. J. Oil Palm Res. 2020, in press. [Google Scholar] [CrossRef]
- Palm oil and deforestation of rainforests. 2017. Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2017-0098_EN.html (accessed on 7 May 2020).
- Meijaard, E.; Garcia-Ulloa, J.; Sheil, D.; Wich, S.A.; Carlson, K.M.; Juffe-Bignoli, D.; Brooks, T.M. Oil palm and biodiversity—A situation analysis; IUCN Oil Palm Task Force: Gland, Switzerland, 2018. [Google Scholar]
- Goggin, K.A.; Murphy, D.J. Monitoring the traceability, safety and authenticity of imported palm oils in Europe. OCL 2018, 25, A603. [Google Scholar] [CrossRef]
- Kuntom, A.; Kushairi, A.; Choo, Y.M. Malaysian Sustainable Palm Oil. Oil Palm Bull. 2003, 71, 15–27. [Google Scholar]
- Malaysian Palm Oil Website. Available online: http://sustainability.mpob.gov.my/ (accessed on 7 May 2020).
- Roundtable on Sustainable Palm Oil. Available online: https://rspo.org/ (accessed on 7 May 2020).
- Von Geibler, J. Market-based governance for sustainability in value chains: Conditions for successful standard setting in the palm oil sector. J. Clean. Prod. 2013, 56, 39–53. [Google Scholar] [CrossRef]
- Hobbs, J.E.; Bailey, D.; Dickinson, D.L.; Haghiri, M. Traceability in the Canadian red meat sector: Do consumers care? Can. J. Agr. Econ. 2005, 53, 47–65. [Google Scholar] [CrossRef]
- Pouliot, S.; Sumner, D.A. Traceability, liability, and incentives for food safety and quality. Am. J. Agr. Econ. 2008, 90, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Tres, A.; Ruiz-Samblás, C.; Van derVeer, G.; Van Ruth, S.M. Geographical provenance of palm oil by fatty acid and volatile compound fingerprinting techniques. Food Chem. 2013, 137, 142–150. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Seow, E.K.; Omar, A.M.; Rodhi, A.M.; Hassan, H.M.; Lalung, J.; Lee, S.C.; Ibrahim, B. Variation of δ2H, δ18O & δ13C in crude palm oil from different regions in Malaysia: Potential of stable isotope signatures as a key traceability parameter. Sci. Jus. 2018, 58, 59–66. [Google Scholar] [CrossRef]
- Janin, M.; Medini, S.; Techer, I. Methods for PDO olive oils traceability: State of art and discussion about the possible contribution of strontium isotopic tool. Eur. Food Res. Technol. 2014, 239, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Aranda, F.; Gomez-Alonso, S.; Rivera del Alamo, R.M.; Salvador, M.D.; Fregapane, G. Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with other Spanish cultivars. Food Chem. 2004, 4, 485–492. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guerere, M. Differentiation of French virgin olive oil RDOs by sensory characteristics, fatty acid and triacylglycerol compositions and chemometrics. Food Chem. 2006, 97, 382–393. [Google Scholar] [CrossRef]
- Bikrani, S.; Jiménez-Carvelo, A.M.; Nechar, M.; Bagur-González, M.G.; Souhail, B.; Cuadros-Rodrìguez, L. Authentication of the geographical origin of margarines and fat-spread products from liquid chromatographic UV-absorption fingerprints and chemometrics. Food 2019, 8, 588. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef]
- Matos, L.C.; Cunha, S.C.; Amaral, J.S.; Pereira, J.A.; Andrade, P.B.; Seabra, R.M.; Oliveira, B.P. Chemometric characterization of three varietal olive oils (Cvs. Cobrançosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chem. 2007, 102, 406–414. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Armenta, S.; De La Guardia, M. Trace-element composition and stable-isotope ratio for discrimination of foods with Protected Designation of Origin. TrAC Trend Anal. Chem. 2009, 28, 1295–1311. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TrAC Trend Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Da Ros, A.; Masuero, D.; Riccadonna, S.; Brkić Bubola, K.; Mulinacci, N.; Mattivi, F.; Lukić, I.; Vrhovsek, U. Complementary untargeted and targeted metabolomics for differentiation of extra virgin olive oils of different origin of purchase based on volatile and phenolic composition and sensory quality. Molecules 2019, 24, 2896. [Google Scholar] [CrossRef] [Green Version]
- Charlebois, S.; Sterling, B.; Haratifar, S.; Naing, S.K. Comparison of global food traceability regulations and requirements. Compr. Rev. Food Sci. F. 2014, 13, 1104–1123. [Google Scholar] [CrossRef]
- Van Duijn, G. Traceability of the palm oil supply chain. Lipid Tech. 2013, 25, 15–18. [Google Scholar] [CrossRef]
- Ayat, K.A.R.; Abdullah, R.; Simeh, M.A.; Shariff, F.M. Management of the Malaysian oil palm supply chain: The role of FFB dealers. Oil Palm Ind. Eco. J. 2009, 9, 20–28. [Google Scholar]
- Malaysian Palm Oil Board (Licensing) Regulations. 2005. Available online: http://led.mpob.gov.my/wp-content/uploads/2016/12/MALAYSIAN-PALM-OIL-BOARD-LICENSING-REGULATIONS-2005.pdf (accessed on 7 May 2020).
- Rival, A.; Montet, D.; Pioch, D. Certification, labelling and traceability of palm oil: Can we build confidence from trustworthy standards? OCL Oilseeds Fats Crops Lipids 2016, 23, D609. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Rabiei, Z.; Enferadi, S.T. Traceability of origin and authenticity of olive oil. In Olive Oil Constituents, Quality, Health Properties and Bioconversions; Boskou, D., Ed.; Intech Open Access: Rijeka, Croitia, 2012; pp. 163–184. [Google Scholar]
- Araghipour, N.; Colineau, J.; Koot, A.; Akkermans, W.; Rojas, J.M.M.; Beauchamp, J.; Wisthaler, A.; Märk, T.D.; Downey, G.; Guillou, C.; et al. Geographical origin classification of olive oils by PTR-MS. Food Chem. 2008, 108, 374–383. [Google Scholar] [CrossRef]
- Dowell, L.; Rosenbarger, A.; Lake, S. Palm oil mill data: A step towards transparency. Available online: https://www.wri.org/blog/2015/12/palm-oil-mill-data-step-towards-transparency (accessed on 7 May 2020).
- Tres, A.; Van der Veer, G.; Alewijn, M.; Kok, E.; Van Ruth, S.M. Palm oil authentication: Classical methodology and state-of-the-art techniques. In Oil Palm: Cultivation, Production and Dietary Components; Penna, S.A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–44. [Google Scholar]
- Report of the Fourteenth Session of the Codex Committee on Fats and Oils; Codex Alimentarius Commission: Rome, Italy, 1995.
- Aparicio, R.; Morales, M.T.; Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. Authenticity of olive oil: Mapping and comparing official methods and promising alternatives. Food Res. Int. 2013, 54, 2025–2038. [Google Scholar] [CrossRef]
- Benincasa, C.; Russo, A.; Romano, E.; Perri, E. Traceability of olive oil by carbon stable isotopes ratio and fatty acids composition. Presented at the VII International Symposium on Olive Growing, San Juan, Argentina, 25–29 September 2012. [Google Scholar]
- Vichi, S. Extraction techniques for the analysis of virgin olive oil aroma. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier Inc.: New York, NY, USA, 2010. [Google Scholar]
- Temime, S.B.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Volatile compounds from Chétoui olive oil and variations induced by growing area. Food Chem. 2006, 99, 315–325. [Google Scholar] [CrossRef]
- Ortíz, C.L.; Moya, M.P.; Navarro, V.B. A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J. Food Compos. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- Cañabate-Díaz, B.; Carretero, A.S.; Fernández-Gutiérrez, A.; Vega, A.B.; Frenich, A.G.; Vidal, J.M.; Martos, J.D. Separation and determination of sterols in olive oil by HPLC-MS. Food Chem. 2007, 102, 593–598. [Google Scholar] [CrossRef]
- Cavaliere, B.; De Nino, A.; Hayet, F.; Lazez, A.; Macchione, B.; Moncef, C.; Perri, E.; Sindona, G.; Tagarelli, A. A metabolomic approach to the evaluation of the origin of extra virgin olive oil: A conventional statistical treatment of mass spectrometric analytical data. J. Agric. Food Chem. 2007, 55, 1454–1462. [Google Scholar] [CrossRef]
- Haddada, F.M.; Manai, H.; Daoud, D.; Fernandez, X.; Lizzani-Cuvelier, L.; Zarrouk, M. Profiles of volatile compounds from some monovarietal Tunisian virgin olive oils. Comparison with French PDO. Food Chem. 2007, 103, 467–476. [Google Scholar] [CrossRef]
- Lazzez, A.; Perri, E.; Caravita, M.A.; Khlif, M.; Cossentini, M. Influence of olive maturity stage and geographical origin on some minor components in virgin olive oil of the Chemlali variety. J. Agric. Food Chem. 2008, 56, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Jolayemi, O.S.; Ajatta, M.A.; Adegeye, A.A. Geographical discrimination of palm oils (Elaeis guineesis) using quality characteristics and UV-visible spectroscopy. Food Sci. Nutr. 2018, 6, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obisesan, K.A.; Jiménez-Carvelo, A.M.; Cuadros-Rodrìguez, L.; Ruisánchez, I.; Callao, M.P. HPLC-UV and HPLC-CAD chromatographic data fusion for the authentication of the geographical origin of palm oil. Talanta 2017, 170, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castaño, E.; Ruiz-Samblás, C.; Medina-Rodríguez, S.; Quirós-Rodríguez, V.; Jiménez-Carvelo, A.M.; Valverde-Som, L.; González-Casado, A.; Cuadros-Rodríguez, L. Comparison of different analytical classification scenarios: Application for the geographical origin of edible palm oil by sterolic (NP) HPLC fingerprinting. Anal. Meth. 2015, 7, 4192–4201. [Google Scholar] [CrossRef]
- Ruiz-Samblás, C.; Arrebola-Pascual, C.; Tres, A.; van Ruth, S.; Cuadros-Rodrìguez, L. Authentication of geographical origin of palm oil by chromatographic fingerprinting of triacylglycerols and partial least square-discriminant analysis. Talanta 2013, 116, 788–793. [Google Scholar] [CrossRef]
- Ramli, U.S.; Othman, A.; Muhammad, N.H.; Tahir, N.I.; Rozali, N.L.; Singh, R.; Sambanthamurthi, R.; Muhammad, S.A.; Parveez, G.K.A. Detecting chemical fingerprints of crude palm oil for traceability in the palm oil supply chain. Presented at the MPOB International Palm Oil Congress (PIPOC 2019), Kuala Lumpur, Malaysia, 19–21 November 2019; p. 116. [Google Scholar]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Sielemann, S.; Wolfgang, D.B.; Lourdes, A.; Miguel, V. Direct classification of olive oils by using two types of ion mobility spectrometers. Anal. Chim. Acta. 2011, 696, 108–115. [Google Scholar] [CrossRef]
- Perri, E.; Benincasa, C.; Muzzalupo, I. Olive oil traceability. In Olive germplasm—The olive cultivation, table olive and olive oil industry in Italy; Intech Open: London, UK, 2012; pp. 265–286. [Google Scholar]
- Breas, O.; Guillou, C.; Reniero, F.; Sada, E.; Angerosa, F. Oxygen-18 measurement by continuous flow pyrolysis/isotope ratio mass spectrometry of vegetable oils. Rapid Commun. Mass Spectro. 1998, 12, 188–192. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Nicolini, G.; Bontempo, L.; Bertoldi, D.; Perini, M.; Schlicht, C.; Schellenberg, A.; Thomas, F.; Heinrich, K.; et al. Isotopic and elemental data for tracing the origin of European olive oils. J. Agric. Food Chem. 2010, 58, 570–577. [Google Scholar] [CrossRef]
- Bat, K.B.; Eler, K.; Mazej, D.; Vodopivec, B.M.; Mulič, I.; Kump, P.; Ogrinc, N. Isotopic and elemental characterisation of Slovenian apple juice according to geographical origin: Preliminary results. Food Chem. 2016, 203, 86–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.; Yang, S.; Ye, Z. Recent developments in application of stable isotope analysis on agro product authenticity and traceability. Food Chem. 2014, 145, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Portarena, S.; Gavrichkova, O.; Lauteri, M.; Brugnoli, E. Authentication and traceability of Italian extra-virgin olive oils by means of stable isotopes techniques. Food Chem. 2014, 164, 12–16. [Google Scholar] [CrossRef] [PubMed]
- De Rijke, E.; Schoorl, J.C.; Cerli, C.; Vonhof, H.B.; Verdegaal, S.J.A.; Vivó-Truyols, G.; Lopatka, M.; Dekter, R.; Bakker, D.; Sjerps, M.J.; et al. The use of δ2H and δ18O isotopic analyses combined with chemometrics as a traceability tool for the geographical origin of bell peppers. Food Chem. 2016, 204, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Wadood, S.A.; Boli, G.; Xiaowen, Z.; Hussain, I.; Yimin, W. Recent development in the application of analytical techniques for the traceability and authenticity of food of plant origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Kumar, P.P.; Krishna, A.G. Physico-chemical characteristics and nutraceutical distribution of crude palm oil and its fractions. Grasasy Aceites 2014, 65, e018. [Google Scholar] [CrossRef] [Green Version]
- Azlan, A.; Prasad, K.N.; Khoo, H.E.; Abdul-Aziz, N.; Mohamad, A.; Ismail, A.; Amom, Z. Comparison of fatty acids, vitamin E and physicochemical properties of Canarium odontophyllum Miq. (dabai), olive and palm oils. J. Food Compos. Anal. 2010, 23, 772–776. [Google Scholar] [CrossRef]
- Berger, K.G. Understanding oils and fats. Global Oil Fat Bus. Mag. 2006, 3, 50–53. [Google Scholar]
- Tan, Y.A. Analytical techniques in palm oil and palm kernel oil specifications. In Selected Readings on Palm Oil and Its Uses; Palm Oil Research Institute Malaysia: Bandar Baru Bangi, Malaysia, 1994; pp. 45–77. [Google Scholar]
- Park, Y.W.; Chang, P.S.; Lee, J. Application of triacylglycerol and fatty acid analyses to discriminate blended sesame oil with soybean oil. Food Chem. 2010, 123, 377–383. [Google Scholar] [CrossRef]
- Yadav, S. Edible oil adulterations: Current issues, detection techniques, and health hazards. IJCS 2018, 6, 1393–1397. [Google Scholar]
- Lerma-García, M.J.; Herrero-Martínez, J.M.; Ramis-Ramos, G.; Simó-Alfonso, E.F. Evaluation of the quality of olive oil using fatty acid profiles by direct infusion electrospray ionization mass spectrometry. Food Chem. 2008, 107, 1307–1313. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Sun, X.; Wang, X.; Xu, B.; Wang, X.; Ma, F.; Zhang, Q.; Ding, X. Classification and adulteration detection of vegetable oils based on fatty acid profiles. J. Agric. Food Chem. 2014, 62, 8745–8751. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, K.; Aziz, S.A.; Yahya, A.; Rokhani, F.Z.; Steward, B.L. Detection of sludge contamination in crude palm oil using dielectric spectroscopy. Trans. ASABE 2015, 227–232. [Google Scholar]
- Majid, R.A.; Mohamad, A.W.; May, C.Y. Properties of residual palm pressed fibre oil. J. Oil Palm Res. 2012, 24, 1310–1317. [Google Scholar]
- Gee, P.T. Analytical characteristics of crude and refined palm oil and fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 373–379. [Google Scholar] [CrossRef]
- Ibrahim, N.; Menon, N.R. Mitigation for 3-MCPD esters at palm oil mills. Palm Oil Eng. Bull. No. 2017, 124, 11–15. [Google Scholar]
- Othman, A.; Goggin, K.A.; Tahir, N.I.; Brodrick, E.; Singh, R.; Sambanthamurthi, R.; Parveez, G.K.A.; Davies, A.N.; Murad, A.J.; Muhammad, N.H.; et al. Use of head-space-gas chromatography-ion mobility spectrometry to detect volatile fingerprints of palm fibre oil and sludge palm oil in samples of crude palm oil. BMC Res. Notes 2019, 12, 229. [Google Scholar] [CrossRef]
- Schwolow, S.; Gerhardt, N.; Rohn, S.; Weller, P. Data fusion of GC-IMS data and FT-MIR spectra for the authentication of olive oils and honeys—Is it worth to go the extra mile? Anal. Bioanal. Chem. 2019, 411, 6005–6019. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Lu, D.; Chen, B. Detection of adulteration in canola oil by using GC-IMS and chemometric analysis. Int. J. Anal. Chem. 2018. [Google Scholar] [CrossRef]
- Liedtke, L.; Seifert, L.; Ahlmann, N.; Hariharan, C.; Franzke, J.; Vautz, W. Coupling laser desorption with gas chromatography and ion mobility spectrometry for improved olive oil characterisation. Food Chem. 2018, 255, 323–331. [Google Scholar] [CrossRef]
- Jafari, M.T.; Khayamian, T.; Shaer, V.; Zarei, N. Determination of veterinary drug residues in chicken meat using corona discharge ion mobility spectrometry. Anal. Chim. Acta 2007, 581, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Abdul Mutalib, M.S.; Khaza’ai, H.; Chang, S.K. Detection of fresh palm oil adulteration with recycled cooking oil using fatty acid composition and FTIR spectral analysis. Int. J. Food Prop. 2018, 21, 2428–2451. [Google Scholar] [CrossRef] [Green Version]
- Sheng, N.J.; Fadhullah, W.; Kadir, M.O.A.; Mohd Rodhi, A.; Abu Bakar, N.H.; Muhammad, S.A. An assessment of FT-IR and FT-NIR capability in screening crude palm oil authenticity and quality combined with chemometrics. Malay. J. Anal. Sci. 2019, 23, 870–879. [Google Scholar]
- Inthiram, A.K.; Mirhosseini, H.; Tan, C.P.; Mohamad, R.; Lai, O.M. Application of multivariate analysis for detection of crude palm oil adulteration through fatty acid composition and triacylglycerol profile. Trop. Agric. Sci. 2015, 38, 389–398. [Google Scholar]
- Ghazali, H.H.; Tukiram, N.A. Analysis of pork adulteration in recycled frying oils using Raman spectroscopy. In Proceedings of the International Conference of Islamic Civilization and Technology Management, Kuala Terengganu, Malaysia, 23–24 November 2019. [Google Scholar]
- Che Man, Y.B.; Marina, A.M.; Rohman, A.; Al-Khatani, H.A.; Norazura, O. A fourier transform infrared spectroscopy method for analysis of palm oil adulterated with lard in pre-fried French fries. Int. J. Food Prop. 2014, 17, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Che Man, Y.B.; Gan, H.L.; NorAini, I.; Nazimah, H.; Tan, C.P. Detection of lard adulteration in RBD palm olein using an electric nose. Food Chem. 2005, 90, 828–835. [Google Scholar] [CrossRef]
- Marikkar, J.M.N.; Lai, O.M.; Ghazali, H.M.; Man, Y.C. Compositional and thermal analysis of RBD palm oil adulterated with lipase-catalyzed interesterified lard. Food Chem. 2002, 76, 249–258. [Google Scholar] [CrossRef]
- Flor, R.V. Establishing adulteration of olive oil by triglyceride analysis. J. Am. Oil Chem. Soc. 1989, 66, 431. [Google Scholar]
- Gordon, M.H.; Griffith, R.E. Steryl ester analysis as an aid to the identification of oils in blends. Food Chem. 1992, 43, 71–78. [Google Scholar] [CrossRef]
- Woodbury, S.E.; Evershed, R.P.; Rossell, J.B.; Griffith, R.E.; Farnell, P. Detection of vegetable oil adulteration using gas chromatography combustion/isotope ratio mass spectrometry. Anal. Chem. 1995, 67, 2685–2690. [Google Scholar] [CrossRef]
- Giménez, M.J.; Pistón, F.; Martín, A.; Atienza, S.G. Application of real-time PCR on the development of molecular markers and to evaluate critical aspects for olive oil authentication. Food Chem. 2010, 118, 482–487. [Google Scholar] [CrossRef]
- Ayed, R.B.; Grati-Kamoun, N.; Moreau, F.; Rebaï, A. Comparative study of microsatellite profiles of DNA from oil and leaves of two Tunisian olive cultivars. Eur. Food Res. Technol. 2009, 229, 757–762. [Google Scholar] [CrossRef]
- Perez-Jimenez, M.; Besnard, G.; Dorado, G.; Hernandez, P. Varietal tracing of virgin olive oils based on plastid DNA variation profiling. PLoS ONE 2013, 8, e70507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uncu, A.T.; Uncu, A.O.; Frary, A.; Doganlar, S. Barcode DNA length polymorphisms vs fatty acid profiling for adulteration detection in olive oil. Food Chem. 2017, 221, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, L.M.; Janovick, J.L.; Carrasquilla-Garcia, N.; Hatzakis, E.; Wang, S.C. Comparison of DNA analysis, targeted metabolite profiling, and non-targeted NMR fingerprinting for differentiating cultivars of processed olives. Food Control 2020, 114, 107264. [Google Scholar] [CrossRef]
- Piarulli, L.; Savoia, M.A.; Taranto, F.; D’Agostino, N.; Sardaro, R.; Girone, S.; Gadaleta, S.; Fucili, V.; De Giovanni, C.; Montemurro, C.; et al. A Robust DNA Isolation Protocol from Filtered Commercial Olive Oil for PCR-Based Fingerprinting. Foods 2019, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yang, T.; Wang, Y.; Zhou, B.; Yan, L.; Teng, L.; Wang, F.; Chen, L.; He, Y.; Guo, K.; et al. New method for effective identification of adulterated Camellia oil basing on Camellia oleifera-specific DNA. Arab. J. Chem. 2018, 11, 815–826. [Google Scholar] [CrossRef]
- Ooi, L.C.L.; Singh, R.; Cheah, S.C. Detection of DNA in crude palm oil, Project Completion (Viva) Report; Malaysian Palm Oil Board: Bandar Baru Bangi, Malaysia, 2006.
- Zhang, L.; Wu, G.; Wu, Y.; Cao, Y.; Xiao, L.; Lu, C. The gene MT3-B can differentiate palm oil from other oil samples. J. Agric. Food Chem. 2009, 57, 7227–7232. [Google Scholar] [CrossRef]
- Lucchetti, S.; Pastore, G.; Leoni, G.; Arima, S.; Merendino, N.; Baima, S.; Ambra, R. A simple microsatellite-based method for hazelnut oil DNA analysis. Food Chem. 2018, 245, 812–819. [Google Scholar] [CrossRef]
- Li, Y.; Shao, L.; Fang, X.; Wan, D.; Wu, Y.; Li, J.; Li, J.; Zhu, L. Evaluation of five DNA extraction methods for commercial vegetable oils. Oil Crop Sci. 2018, 3, 122. [Google Scholar] [CrossRef]
- Francois, G.; Fabrice, V.; Didier, M. Traceability of fruits and vegetables. Phytochemistry 2020, 173, 112291. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Identity Characteristics | Range | Mean |
|---|---|---|
| Apparent density, kg litre−1, at 50 °C | 0.8890 to 0.8950 | 0.89050 ± 0.0002 |
| Refractive Index, nD 50 °C | 1.454 to 1.456 | 1.4543 ± 0.0002 |
| Saponification value, mg KOH g−1 oil | 194 to 205 | 199 ± 2.4 |
| Unsaponifiable matter, % | 0.19 to 0.44 | 0.32 ± 0.066 |
| Fatty acid composition, (wt % as methyl esters) | ||
| C12:0 | 0.0 to 0.5 | 0.2 ± 0.10 |
| C14:0 | 0.9 to 1.5 | 1.1 ± 0.08 |
| C16:0 | 39.2 to 45.8 | 43.5 ± 0.95 |
| C16:1 | 0.0 to 0.4 | 0.2 ± 0.05 |
| C18:0 | 3.7 to 5.1 | 4.3 ± 0.18 |
| C18:1 | 37.4 to 44.1 | 39.8 ± 0.94 |
| C18:2 | 8.7 to 12.5 | 10.3 ± 0.56 |
| C18:3 | 0.0 to 0.6 | 0.3 ± 0.07 |
| C20:0 | 0.0 to 0.5 | 0.2 ± 0.16 |
| Iodine value (Wijs) | 50.4 to 53.7 | 52.0 ± 0.66 |
| Slip melting point, °C | 33.8 to 39.2 | 36.7 ± 0.84 |
| Total carotenoids (as β-carotene), mg kg−1 | 474 to 689 | 581 ± 45.5 |
| Quality Characteristics | Special Quality CPO | Standard Quality CPO |
| Free fatty acid (as palmitic), % max | 2.5 | 5.0 |
| Moisture and impurities, % max | 0.25 | 0.25 |
| Peroxide value, meq O2 kg−1 max | 1.0 | 2.0 |
| Anisidine value, max | 4.0 | 5.0 |
| DOBI, min | 2.8 | 2.3 |
| Performance Indicators | 2019 | 2018 | Difference | |
|---|---|---|---|---|
| Vol./Value | % | |||
| Opening stocks (mil tonnes) | 3.22 | 2.73 | 0.48 | 17.7 |
| CPO production (mil tonnes) | 19.86 | 19.52 | 0.34 | 1.8 |
| FFB yield (t ha−1) | 17.19 | 17.16 | 0.03 | 0.2 |
| Oil extraction rate (%) | 20.21 | 19.95 | 0.26 | 1.3 |
| PO exports (mil tonnes) | 18.47 | 16.49 | 1.98 | 12.0 |
| PO imports (mil tonnes) | 0.98 | 0.84 | 0.14 | 16.1 |
| Closing stocks (mil tonnes) | 2.01 | 3.22 | (1.21) | (37.5) |
| CPO price (RM t−1) | 2,079.00 | 2,232.50 | (153.50) | (6.9) |
| Export revenue (RM billion) | 64.84 | 67.52 | (2.68) | (4.0) |
| Parameters Measured | Analytical Methods | Data Interpretation | Summary and Main Findings | Advantages | Limitations | Reference |
|---|---|---|---|---|---|---|
| Chromatographic Approaches | ||||||
| TAG | HPLC-DAD | SIMCA and PLS-DA | Margarines and fat spread products were successfully differentiated based on their region of origin (Spain and Morocco) using UV-absorption fingerprints of unsaturated fatty acid proportion of TAGs and multivariate chemometric tools. | Pre-treatment of samples prior to chromatographic analysis is not required. Samples only need to be dissolved in n-hexane prior to analysis. | Samples from Europe are similar to those from Spain and were not differentiated by the model. | [31] |
| FAC and VOC | GC-FID and PTR-MS | PLS-DA | Analysis of FAs/VOCs by GC-FID/PTR-MS combined with chemometrics was a feasible approach to separate CPO by continent. Saturated FAs such as C12:0, C14:0, and C16:0 were higher in CPO from South East Asia while South American samples contained higher monounsaturated FAs from the n-9 series, such as C18:1n-9 and C20:1n-9. Polyene chain of some carotenoids and their degradation compounds, furfural, or heptanal were proposed as VOC markers for regional classification. | PTR-MS is sensitive with low fragmentation. VOC in the sample head-space are detected without prior chromatographic separation, and non-conformities that would be unnoticed using the traditional targeted approach are detected. GC-FID is a reliable standard method in FA analysis and can successfully discriminate samples based on the fatty acid profile. | PTR-MS is not very effective in identifying the VOC compounds as it is essentially a low-resolution one-dimensional technique. PTR-MS hardly measures less volatile VOC components such as carotenoids, which could also be degraded during palm oil extraction and storage. | [26] |
| K extinction values (K270, K232), colour, chlorophyll, carotenoids content | UV-visible spectroscopy | PCA and OPLS-DA | UV-visible spectroscopy coupled with quality characteristic assessment proved effective in discriminating 60 CPO samples from North, South, and Central Nigeria based on spectral (K270, K232, colour, chlorophyll, and carotenoids) and chemical (FFA, acidity, and PV) assessment. Central Nigeria CPO samples were distinguished by significantly high carotene content, free fatty acids, acid value, and peroxide value while North Nigeria samples were separated by K extinction values, colour density, and chlorophyll content. | UV-visible spectroscopy is cost-effective and one of the less complicated techniques in food analysis. It has shorter analysis time and small sample requirement. This approach could also be a valid tool to avoid misrepresentation of CPO quality. | Requirement for a large sample size to identify differences and distinguish samples based on the region of origin. | [57] |
| Chromatographic data fusion | HPLC-UV and HPLC-CAD | PLS-DA | Geographical authentication of 100 CPO samples obtained from diverse regions (South-East Asia, West Africa, and South America) was achieved by the analysis of FAME, phytosterols, and terpene alcohols, using two different analytical tools combined with the PLS-DA classification approach. | Improved classification and high accuracy of the model (ranging from 87% to 100%) were achieved by applying data fusion strategies to combine the data from HPLC-UV and HPLC-CAD. | Additional cost due to the use of multiple platforms to analyse the samples. | [58] |
| Sterolic chromatographic data | HPLC | SIMCA and PLS-DA | Sterolic chromatography was used to discern the geographical origin of 102 CPO samples from South-East Asia, West Africa, and South America. Sterolic chromatographic profiles for African CPO did not show any unique pattern where some samples were similar to those obtained from South America and South-East Asia. This is likely due to the common origin of the commercially planted oil palm in the three regions. | Application of HPLC with properly optimised chromatographic conditions and suitable detector can be used to generate fingerprint data for differentiating samples. | None of the tested chemometric tools were sensitive enough to distinguish samples based on their region of origin. There was still up to 15% inaccurate classification of samples, even when using the best system implemented in the study. | [59] |
| TAG | HPLC–CAD and HTGC–MS | PLS-DA | TAG fingerprinting measured by HPLC-CAD and HTGC-MS was applied to describe the origin of palm oil from three main palm oil producing regions, Southeast Asia, West Africa, and South America. A similar chemometric process employing PLS-DA was used on both types of chromatographic data and their results were compared. The authors concluded that the methods provide a rapid tool for palm oil classification to verify labelling compliance of geographical origin. | Both chromatographic methods (HPLC-CAD and HTGC-MS) provide a significant advantage over conventional LC and GC as they only involve sample dilution without additional sample treatment or derivatization procedures prior to injection. The use of a different detector such as CAD also improves acquisition time. | FA composition of TAGs was not elucidated. PLS-DA model from HTGC-MS data show low specificity of 75% for South East Asian samples. | [60] |
| Stable Isotope Approach | ||||||
| Stable carbon (δ13C), hydrogen (δ2H), and oxygen (δ18O) isotopic compositions | EA- and TC/EA-IRMS | HCA, PCA, and OPLS-DA. | The first report that used stable isotope profiles to study traceability of CPO samples to its origin. The study evaluated samples from different regions in Malaysia. | Possible to predict the origin of CPO samples at a narrower geographical area, i.e., based on regions in a specific country | Accessibility to instrument is limited since its availability is not as widespread as other hyphenated mass spectrometers | [27] |
| Stable δ13C | EA-IRMS | ANOVA and Multiple comparison tests (Tukey and Duncan tests) | The preliminary study revealed that specific chemical fingerprints related to the carbon isotope could be detected in the CPO samples, which reveals their potential for application in a traceability system. | Possible to differentiate the origin of CPO samples produced from different palm oil mills likely due to the different milling practices | Geographical origin discrimination may be difficult when the areas in question have similar climate. However, other factors such as agricultural regime and milling practice can come into play for a successful discrimination. As such, there is a need to analyse multiple stable isotopes instead of depending on a single element | [61] |
| VOC Fingerprinting | ||||||
| VOC fingerprints | GC-IMS | Chemometrics | The potential utility of GC-IMS as a fast monitoring technology of VOC fingerprints of palm oil sourced from various continents/sub-continents (Malaysia, Africa, and South America) was demonstrated. VOC fingerprints that are only present in palm oil produced in Colombia were reported, which could be used as rapid markers for visualizing similarities and differences in VOC composition of different samples. | The higher resolved 3D fingerprints obtained from the GC-IMS system provide superior resolving power for non-targeted profiling of VOC fractions from highly complex samples such as palm oil. IMS can also be coupled to other techniques such as GC or LC to increase sensitivity and selectivity. This facilitates its application for preliminary screening of oils in the field as well as in larger analytical laboratories. | GC-IMS is not suitable for identifying unknown compounds. Hyphenation with other powerful technologies is required to identify individual VOC components. | [19] |
| Sample ID | N | Subset for Alpha = 0.05 | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Tukey HSDa | M1Pn | 10 | −31.088110 | ||
| M2Pn | 10 | −30.890524 | |||
| M4Pn | 10 | −30.832998 | |||
| M3Pn | 10 | −30.692361 | |||
| Sig | 1.000 | 0.481 | 1.000 | ||
| Duncana | M1Pn | 10 | −31.088110 | ||
| M2Pn | 10 | −30.890524 | |||
| M4Pn | 10 | −30.832998 | |||
| M3Pn | 10 | −30.692361 | |||
| Sig | 1.000 | 0.157 | 1.000 | ||
| Study Case | Parameters Measured | Analytical Methods | Data Interpretation | Summary and Main Findings | Advantages | Limitations | Reference |
|---|---|---|---|---|---|---|---|
| Adulteration of crude palm oil with secondary and used cooking oils | |||||||
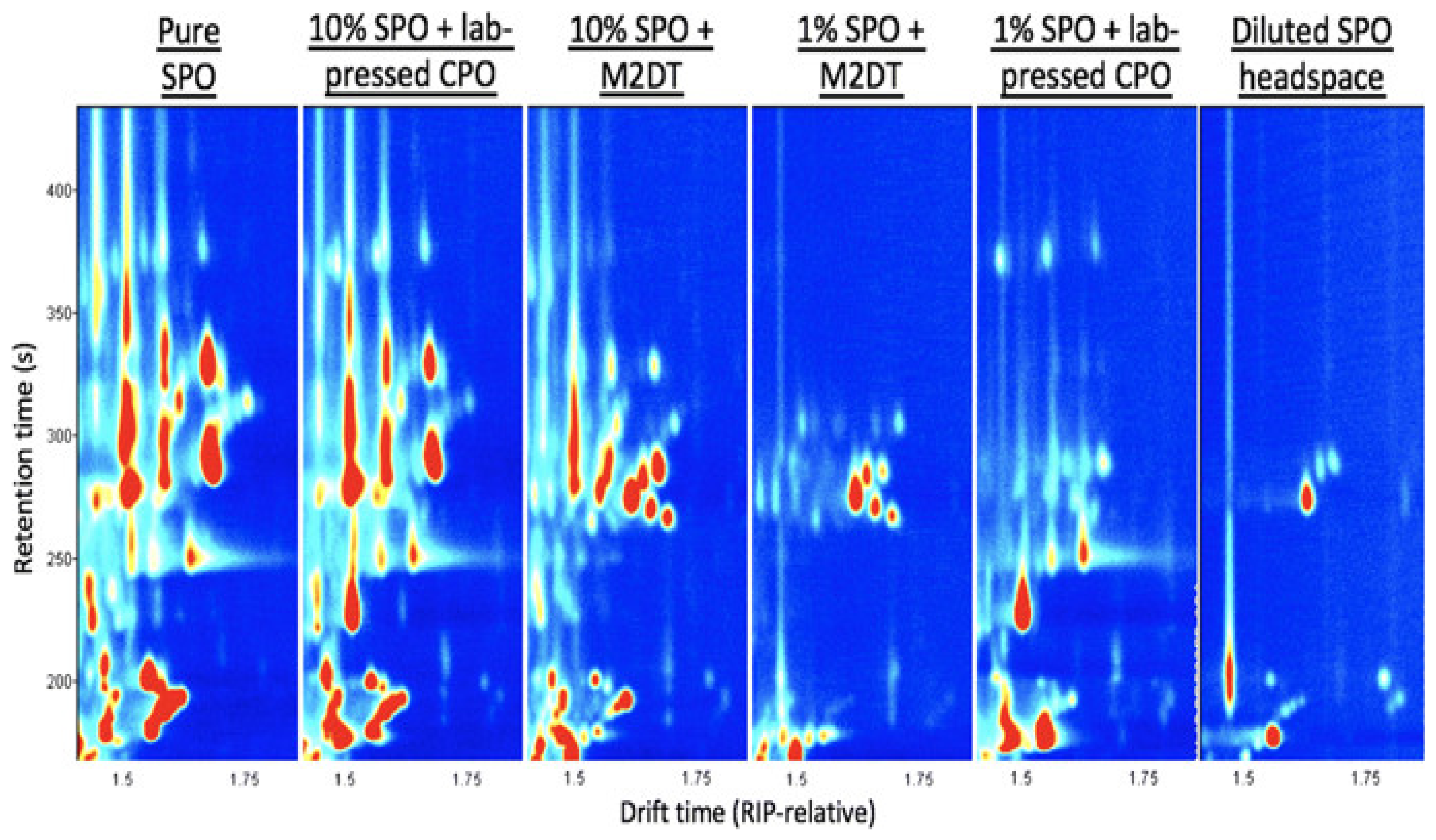
| Adulteration of CPO with PFO and SPO | VOC | GC-IMS | Topographical plot | The paper reports on the assessment of GC–IMS for rapid screening of VOC fingerprints of secondary oils such as PFO and SPO manually added into CPO. VOC fingerprints of SPO were distinguished at 1% to 10% concentration. Seven PFO and 21 SPO markers were effectively detected. | In comparison to classical techniques, IMS coupled to GC is potentially a more rapid, accurate, and cheaper analytical system to discriminate different types of edible oil samples. In addition, it may assist in developing appropriate references for grade identification for use by vegetable oil refineries. | Clear differences between samples could be observed. However, it was difficult to realize digital expression. Therefore, it was necessary to apply appropriate chemometric tools to establish a recognition model. | [83] |
| Adulteration of CPO with SPO and used UVO | FAC, TAG | GC-FID and HPLC-ELSD | PCA and CO | The combination of chemical properties and multivariate analysis was applied to detect differences between CPO and CPO blended with SPO or UVO. Detection of concentrations of 5% and 2% (v/v) was achieved for SPO and UVO based on FAC (GC-FID) and TAG (HPLC-ELSD) analysis | Potential to differentiate adulterated CPO with SPO and UVO. | Depending solely on FAC and TAG for distinguishing pure and adulterated CPO is not ideal due to the dominant effect and similar chemical properties of CPO and adulterant SPO and UVO. Detection level of the adulterants was relatively low and changes in the chromatograms were not very significant. | [90] |
| Adulteration of CPO with SPO | Storage and dissipation of electric and magnetic energy in oil | Dielectric spectroscopy | PCR and PLS | Significant differences between the dielectric constants of pure CPO and contaminated CPO (p < 0.01) were observed. The results clearly showed that the dielectric constant of contaminated CPO increased with the incorporation of sludge oil from 0.6% to 10%. This was likely due to the high moisture content in SPO, which further induced its polarity and coincided with the dielectric constant value. | Dielectric spectroscopy technique is a rapid analysis system. Furthermore, the non-destructive nature of the analysis makes this an interesting alternative tool to enhance the efficiency of monitoring palm oil quality. | Dielectric spectroscopy is considered as a relatively new technique. Its efficiency in effectively extracting valuable information for application in the field of food quality and authentication of palm oil has not been fully verified. | [79] |
| Adulteration of processed palm oil with used cooking oil | |||||||
| Adulteration of RFO | Raman spectroscopy | Raman microscope | PCA | Raman microscope and PCA were used to detect adulterated and unadulterated frying oils. Spiked samples were prepared by adding recycled cooking oils (heated-palm cooking oil, fried-pork oil, fried-chicken oil, fried-dory fish oil, and fried-banana oil). | Raman spectroscopy is cost-effective, non-destructive, fast, and convenient and requires little to no sample pre-treatment. | Raman spectroscopy is not able to quantify the actual percentage or level of adulteration in the spiked samples. It can only detect that adulteration has occurred | [91] |
| Adulteration of RCO | FAC and FTIR | GC-FID and FTIR spectroscopy | The use of the analytical systems could differentiate pure and adulterated PO at 100% accuracy, even at very low adulterant concentration (1%). The FTIR data combined with PLS-DA showed extremely good performance (coefficient of determination, R2 = 0.995). The level of polyunsaturated fatty acids (PUFAs) decreased with the increase in adulteration, which by itself is a good indicator for adulteration with RCO.The findings from this study provides useful information to regulatory authorities in constructing standard guidelines for the detection of cooking oil adulteration. | Both analytical techniques are rapid, reliable, and accurate. They are easy to use and require minimal or no sample preparation. | Both techniques applied needed refinement with regard to principles and concepts in order to achieve the conclusive outcome of detecting adulteration of PO with RCO. | [88] | |
| Adulteration of UCO | Mid-IR (MIR) and NIR spectrum | FTIR and FT-NIR | DA | The techniques were successful for authenticity screening with 96.7% correct classification on FT-IR and 83.3% correct classification on FT-NIR. Further improvements can be made for the spectroscopy techniques for routine application to authenticate and assure quality of edible palm oil in the future. | MIR and NIR spectroscopy is fast, sensitive, reliable, and non-destructive. In addition, it is relatively easy to operate and there is almost no sample preparation. It is cost-effective and environmentally-friendly. | Cannot separate individual components/adulterants but only identifies adulteration.Data analysis and interpretation is not simple, and requires chemometrics. | [89] |
| Adulteration of PPO with animal fats | |||||||
| Adulteration frying oil with lard | FTIR spectraand FAC | FTIR and GC | PLS | FTIR spectroscopy analysis in combination with multivariate calibration of PLS-DA was effectively used for detecting the presence of lard in pre-fried French fries. The result indicated that lard in a mixture of PO can be detected even at 0.5% level. PLS-DA model gave a linear regression with good coefficient of determination (R2 = 0.9791), which reflects good prediction of FTIR spectra against the actual level of lard. The FAC was used to verify the spectral band width. Unlike PO, lard contains two fold higher linolenic acyl groups than PO. | The simplicity, relatively fast analysis, and high accuracy of FTIR spectra method will facilitate its incorporation for routine quality control analysis. All PO samples adulterated with lard were clearly differentiated from non-adulterated PO. | Not applicable | [92] |
| Adulteration of PPO with lard | Electric nose | A zNose vapour analysis system | Analysis of variance | Surface acoustic wave sensing electronic nose enabled the detection of lard in refined, bleached, deodorized palm olein at levels as low as 1%. There was higher concentration of VOC for lard compared to olein. Results were compared to GC analysis of FAME, which found that C15:0, C17:0, and C17:1 fatty acids were unique to lard. | Electronic nose is regarded as a green technique in this field, which makes it ideal for quality control purposes. It is non-destructive, rapid, and relatively low cost. | Method may not be applicable for adulteration levels ≤1% | [93] |
| Adulteration of RBD PO with lipase-catalysed inter esterified lard | TAG, FAC, DSC | GLC, RP-HPLC, and DSC | Step-wise multiple linear regression | The presence of lipase-catalysed interesterified lard in RBD palm olein was examined using GLC, RP-HPLC, and DSC. Among the methods employed, DSC was most effective at detecting lard using its thermal characteristics, even at 1% levels. | DSC was more sensitive for both quantitative and qualitative determination of ERLD in palm oil. In addition to the accuracy and speed, the DSC method also provides a better means of identification of lard with a detection limit of 1%. | Applications of HPLC and GLC were less effective for qualitative and quantitative analysis of adulterants such as ERLD. Both analytical instruments were not able to distinguish between PO adulterated with lard and those adulterated with chicken fat. | [94] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, U.S.; Tahir, N.I.; Rozali, N.L.; Othman, A.; Muhammad, N.H.; Muhammad, S.A.; Tarmizi, A.H.A.; Hashim, N.; Sambanthamurthi, R.; Singh, R.; et al. Sustainable Palm Oil—The Role of Screening and Advanced Analytical Techniques for Geographical Traceability and Authenticity Verification. Molecules 2020, 25, 2927. https://doi.org/10.3390/molecules25122927
Ramli US, Tahir NI, Rozali NL, Othman A, Muhammad NH, Muhammad SA, Tarmizi AHA, Hashim N, Sambanthamurthi R, Singh R, et al. Sustainable Palm Oil—The Role of Screening and Advanced Analytical Techniques for Geographical Traceability and Authenticity Verification. Molecules. 2020; 25(12):2927. https://doi.org/10.3390/molecules25122927
Chicago/Turabian StyleRamli, Umi Salamah, Noor Idayu Tahir, Nurul Liyana Rozali, Abrizah Othman, Nor Hayati Muhammad, Syahidah Akmal Muhammad, Azmil Haizam Ahmad Tarmizi, Norfadilah Hashim, Ravigadevi Sambanthamurthi, Rajinder Singh, and et al. 2020. "Sustainable Palm Oil—The Role of Screening and Advanced Analytical Techniques for Geographical Traceability and Authenticity Verification" Molecules 25, no. 12: 2927. https://doi.org/10.3390/molecules25122927
APA StyleRamli, U. S., Tahir, N. I., Rozali, N. L., Othman, A., Muhammad, N. H., Muhammad, S. A., Tarmizi, A. H. A., Hashim, N., Sambanthamurthi, R., Singh, R., Manaf, M. A. A., & Parveez, G. K. A. (2020). Sustainable Palm Oil—The Role of Screening and Advanced Analytical Techniques for Geographical Traceability and Authenticity Verification. Molecules, 25(12), 2927. https://doi.org/10.3390/molecules25122927






