Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations
Abstract
1. Introduction
2. Results and Discussion
2.1. Medical Cannabis-Based Oil Extraction Procedure
2.2. Fatty Acid Composition of Lipid Sources
2.3. Targeted Cannabinoids in Pharmacists’ Oil Preparations
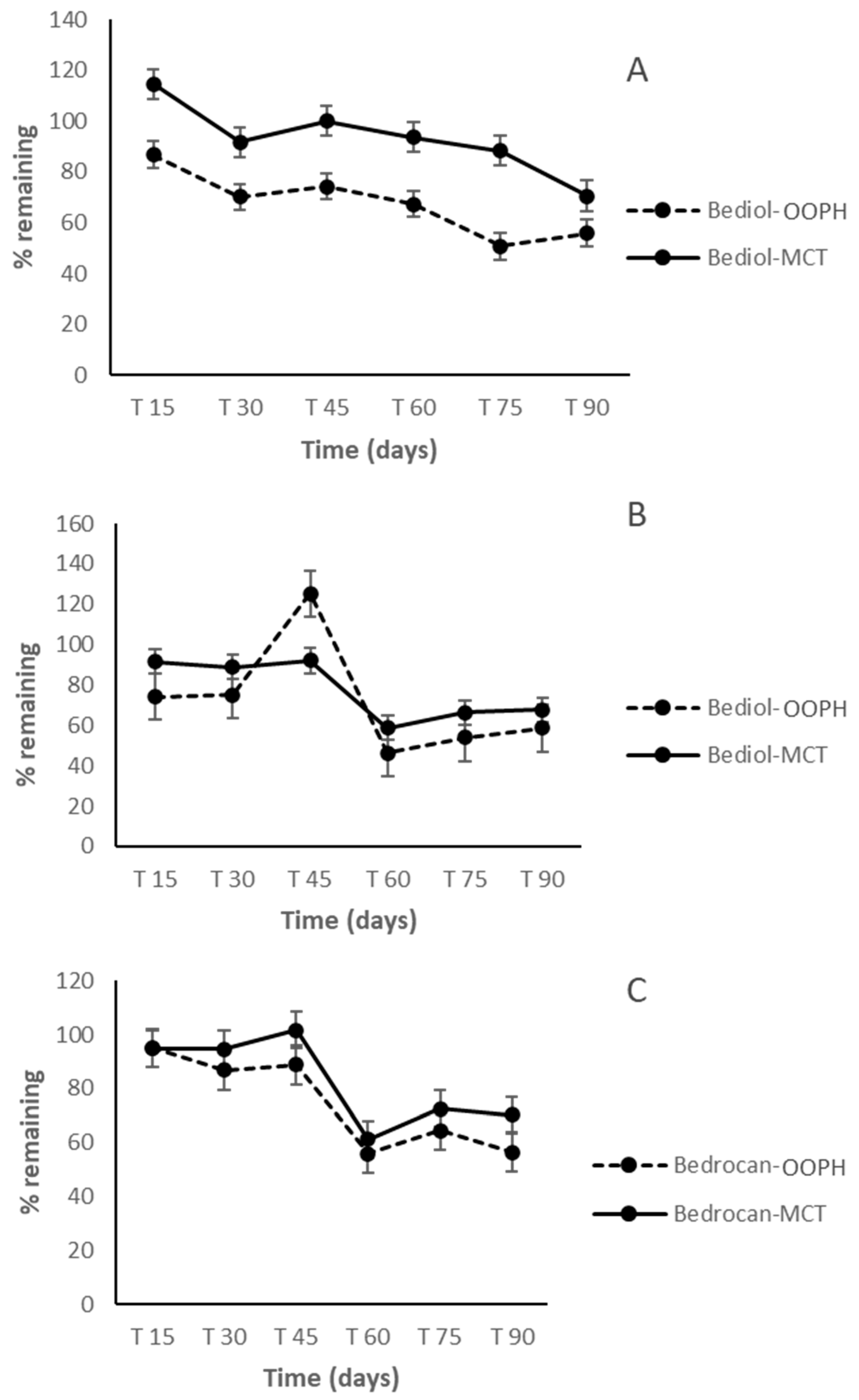
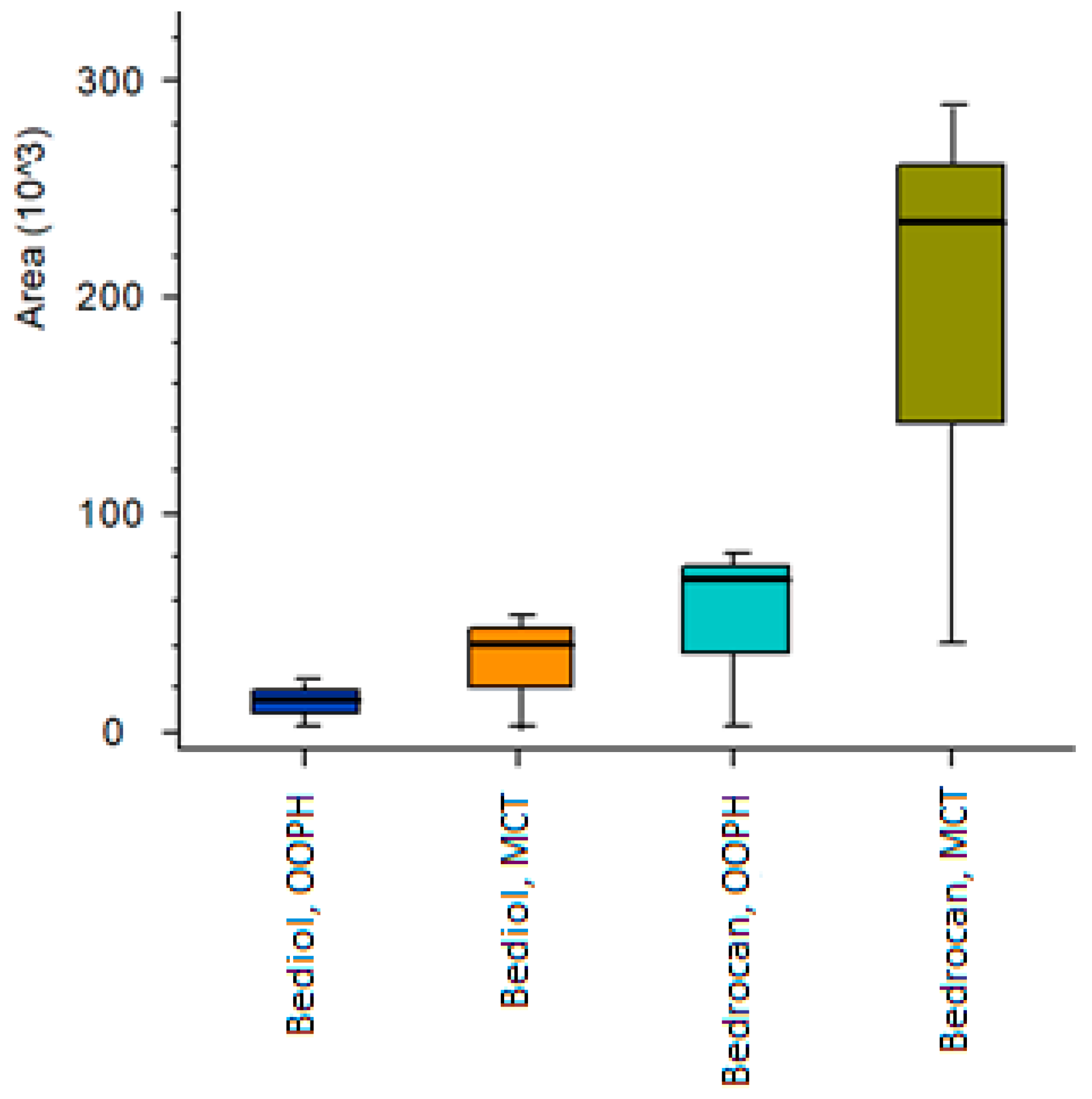
2.4. Untargeted Cannabinoids in Pharmacists’ Oil Preparations
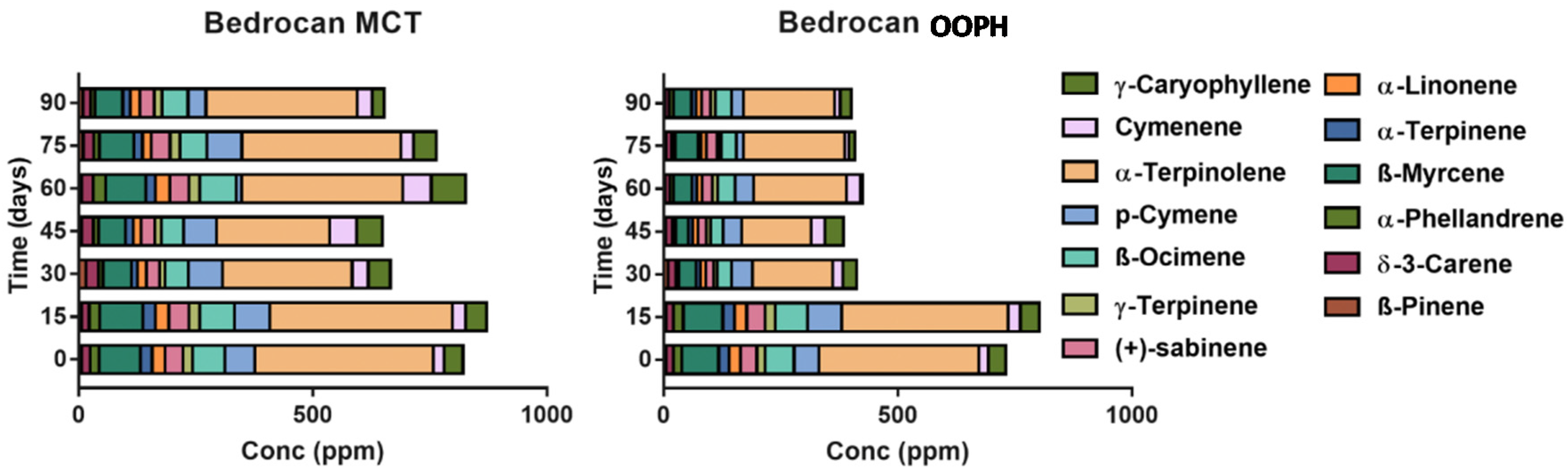
2.5. Terpene Profile in Pharmacists’ Oil Preparations
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Medical Cannabis-Based Oil Preparation Procedure
3.3. Cannabinoid HPLC-Q-Exactive-Orbitrap-MS Analysis
3.3.1. Targeted Approach by Quantitative Analysis
3.3.2. Untargeted Approach by Investigating for Novel Cannabinoids
3.4. HS-SPME and GC-MS Analysis for Terpene Analysis
3.5. Fatty Acid Composition of Lipid Sources
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Russo, E.B.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypoth. 2006, 66, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M. Management of chemotherapy-induced nausea and vomiting: Focus on newer agents and new uses for older agents. Drugs 2013, 73, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; Mcnicol, E.; Baron, R.; Dworkin, R.H.; Gilron, L.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Leussink, V.I.; Husseini, L.; Warnke, C.; Broussalis, E.; Hartung, H.P.; Kieseier, B.C. Symptomatic therapy in multiple sclerosis: The role of cannabinoids in treating spasticity. Adv. Neurol. Disord. 2012, 5, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J. Acquir. Immune Defic. Syndr. 2007, 5, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharm. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Calvi, L.; Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Giorgi, A. Quality traits of Medical Cannabis sativa L. inflorescences and derived products based on comprehensive analytical investigation. In Recent Advances in Cannabinoid Research; IntechOpen: London, UK, 2018. [Google Scholar]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef]
- Website: Decreto 9 Novembre 2015: Funzioni di Organismo Statale per la Cannabis Previsto Dagli Articoli 23 e 28 Della Convenzione Unica Sugli Stupefacenti del 1961, Come Modificata nel 1972. Available online: http://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg;jsessionid=p1rnwNujUKlqQ5azhA%20Q95A__.ntc-as3-guri2a (accessed on 1 May 2020).
- Carcieri, C.; Tomasello, C.; Simiele, M.; De Nicolò, A.; Avataneo, V.; Canzoneri, L.; Cusato, J.; Di Perri, G.; D’Avolio, A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm. Pharmacol. 2018, 70, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 28, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass pectrometry method. J Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- DAC/NRF. 2015/2 Cannabidiol. Available online: http://dacnrf.pharmazeutische-zeitung.de/index.php?id=557 (accessed on 23 April 2020).
- Gruschow, A. A Comparison of Antioxidant Potential, Total Phenolic Content, and Cannabidiol (CBD) Content of Cannabis Infused Hemp, MCT, and Olive Oils. Theses Diss. Culminating Proj. Montclair State Univ. 2020, 472, 1. [Google Scholar]
- Neha, D.; Shah, N.; Limketkai, N. The Use of Medium-Chain Triglycerides in Gastrointestinal Disorders. Nutr. Issues Gastroenterol. 2017. Available online: https://www.essentialnutrition.com.br/media/artigos/mctlift/1.pdf (accessed on 23 April 2020).
- Yamahira, Y.; Noguchi, T.; Takenaka, H.; Maeda, T. Biopharmaceutical studies of lipid-containing oral dosage forms: Relationship between drug absorption rate and digestibility of vehicles. Int. J. Pharm. 1979, 3, 23–31. [Google Scholar]
- Yamahira, Y.; Noghuchi, T.; Noghuchi, T.; Takenaka, H.; Maeda, T. Absorption of Diazepam from a Lipid-Containing Oral Dosage Form. Chem. Pharm. Bulletin. 1979, 27, 1190–1198. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.M.; Vandelli, A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 566–579. [Google Scholar] [CrossRef]
- Ternelli, M.; Brighenti, V.; Anceschi, L.; Poto, M.; Bertelli, D.; Licata, M.; Pellati, F. Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. Anal. 2020, 186, 113296. [Google Scholar] [CrossRef] [PubMed]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a lc method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Hazekamp, A. Cannabis Oil: Chemical evaluation of an upcoming cannabis based medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Bettiol, A.; Lombardi, N.; Crescioli, G.; Maggini, V.; Gallo, W.; Mugelli, V.; Firenzuoli, F.; Baronti, R.; Vannacci, A. Galenic preparations of therapeutic cannabis sativa differ in cannabinoids concentration: A quantitative analysis of variability and possible clinical implications. Front. Pharm. 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Trofin, I.G.; Dabija, G.; Vaireanu, D.; Filipescu, L. Long - term Storage and Cannabis Oil Stability. Rev. Chim. 2012, 63, 293–297. [Google Scholar]
- European Pharmacopoeia (Ph. Eur.) 9th Edition”. European Directorate for the Quality of Medicines & HealthCare (EDQM). Available online: www.EDQM.eu (accessed on 20 April 2020).
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Rial, S.A.; Karelis, A.D.; Bergeron, K.-F.; Mounier, C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients 2016, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Torres, H.E.; Ludorf, J.; Brin, M. The effect of medium-chain triglycerides on the bioavailability of vitamin E. Int. J. Vitam. Nutr. Res. 1978, 48, 240–241. [Google Scholar] [PubMed]
- Lesser, S.; Cermak, R.; Wolffram, S. The fatty acid pattern of dietary fat influences the oral bioavailability of the flavonol quercetin in pigs. Brit. J. Nutr. 2006, 96, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of Altitude on Phytochemical Composition of Hemp Inflorescence: A Metabolomic Approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Wardle, M.C.; Marcus, B.A.; de Wit, H. A Preliminary investigation of individual differences in subjective responses to D-amphetamine, alcohol, and delta-9-Tetrahydrocannabinol using a within-subjects randomized trial. PLoS ONE 2015, 10, e0140501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Booth, J.; Bohlmann, J. Terpenes in Cannabis sativa – From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A Headspace Solid-Phase Microextraction Gas-Chromatographic Mass-Spectrometric Method (Hs-Spme-Gc/Ms) to Quantify Hexanal in Butter during Storage as Marker of Lipid Oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; De Marinis, P.; Granelli, G.; Chiesa, L.M.; Panseri, S. Secondary metabolite profile, antioxidant capacity, and mosquito repellent activity of Bixa orellana from Brazilian Amazon region. J. Chem. 2013. [Google Scholar] [CrossRef]
- Giorgi, A.; Panseri, S.; Mattara, M.S.; Andreis, C.; Chiesa, L.M. Secondary metabolites and antioxidant capacities of waldheimia glabra (decne.) regel from Nepal. J. Sci. Food Agric. 2013, 93, 1026–1034. [Google Scholar] [CrossRef]
- Giorgi, A.; Panseri, S.; Nanayakkara, N.N.M.C.; Chiesa, L.M. HS-SPME-GC/MS analysis of the volatile compounds of Achillea collina: Evaluation of the emissions fingerprint induced by Myzus persicae infestation. J. Plant. Biol. 2012, 55, 251–260. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, L.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Animal Feeding Stuffs. Determination of the Content of Fatty Acids—Part 2: Gas Chromatographic Method; Czech Standards Institute: Prague, Czech Republic, 2007; pp. 1–24, ČSN CEN ISO/TS 17764–2. [Google Scholar]
Sample Availability: Sample requests can be made to the authors. |





| Author | Year | Cannabis Oil Typology | Determination | Stability Tests | Analytical Method |
|---|---|---|---|---|---|
| Pavlovic et al. [9] | 2018 | Cannabidiol Oils, European Commercially Available Preparations, Bedrolite® oil extract | Cannabinoids Content, Terpene Fingerprint, Secondary Lipid’s Oxidation Products | No | SPME-GC-MS and HPLC-Q-Exactive-Orbitrap-MS |
| Pacifici et al. [14] | 2017 | Standardized preparations of cannabis oil FM2 | Cannabinoids concentration | Yes, short-term (up to 14 days) stability and after 1 year of storage in darkness at 4 °C | UHPLC-MS/MS |
| Pacifici et al. [15] | 2018 | Standardized preparations of cannabis oil FM2 | Cannabinoids concentration, Cannabinoids extraction efficiency | Yes, samples stored at room temperature (25 °C) and in the refrigerator (4 °C) at the following timeintervals: 1, 3, 7, and 14 days. | UHPLC-MS/MS |
| Citti et al. [16] | 2016 | Cannabis-based extracts–different solvent (Olive oil and ethyl alcohol) | Cannabinoids concentration and their stability, Terpenes | Short-term stability in olive oil and ethyl alcohol for 24 h at room temperature and at 10 °C. Stability of CBDA, CBD, CBN, Δ9-THC and THCA at room temperature (25 °C) and in a refrigerator (8 °C) for 3, 5, 6, and 10 days. | HPLC-UV, HPLC-ESI-QTOF, GC–MS |
| Calvi et al. [17] | 2017 | Macerated oils, Bedrocan®, Bediol® | In-depth fingerprint of volatile compounds, investigation of targeted and untargeted cannabinoids | Yes, storage at 4 and 25 °C for 6 weeks, the analyses were performed at 0, 7, 14, 21, 28, 35, and 42 days of storage. | HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) |
| Deidda et al. [25] | 2019 | Cannabis olive oil extracts | Cannabinoids (CBD and Δ9-THC) | No | RP-HPLC/UV |
| Romano et al. [26] | 2013 | Concentrated cannabis extracts, Rick Simpson oil, Bedrocan® | Cannabinoids, terpenes | No | GC/FID, HPLC/PDA |
| Bettiol et al. [27] | 2019 | magistral oil preparations, Bediol®, Bedrobinol®, Bedrolite® or FM-2 70 or 100 mg/mL | Cannabidiol (CBD), Cannabinol (CBN), Tetrahydrocannabinol (Δ9-THC), and Tetrahydrocannabinolic acid (THCA) | No | HPLC-DAD |
| Carcieri et al. [13] | 2017 | Bediol®; Bedrocan®; Bedrolite®; cannabis olive oil | Cannabinoids (Δ9-THC, CBD, THCA, CBDA and CBN) | No | LC-MS |
| Trofin et al. [28] | 2012 | Cannabis Oil | Cannabinoids (Δ9-THC, CBN and CBD) | Long term storage in different conditions; four years in darkness at 4 °C and in laboratory light at 22 °C. | GC–FID, HPLC |
| Casiraghi et al. [11] | 2017 | Cannabis Olive Oil Preparations | Cannabinoids | No | GC/FID, GC/MS |
| Preparation Step | Bedrocan® Medical Cannabis Based Oil | Bediol® Medical Cannabis Based Oil | ||
|---|---|---|---|---|
| Extraction solvent | PhEur grade olive oil (OOPH) * | Medium Chain Triglycerides (MCT) * | PhEur grade olive oil (OOPH) * | Medium Chain Triglycerides (MCT) * |
| Oil weight | 50 mL OOPH (d 0.916 = 45.8 g) | 50 mL MCT oil (d 0.950 = 47.5 g) | 50 mL OOPH (d 0.916 = 45.8 g) | 50 mL MCT oil (d 0.950 = 47.5 g) |
| Cannabis inflorescence | 5.01 g ± 0.01 Ratio plant/oil (1:10) | 5.01 g ± 0.01 Ratio plant/oil (1:10) | 5.01 g ± 0.01 Ratio plant/oil (1:10) | 5.01 g ± 0.01 Ratio plant/oil (1:10) |
| Inflorescence weight after decarboxylation step | 4.63 g ± 0.02 0.37 g ± 0.01 weight loss | 4.63 g ± 0.01 0.37 g ± 0.01 weight loss | 4.72 g ± 0.02 0.28 g ± 0.01 weight loss | 4.75 g± 0.01 0.25 ± 0.01 g weight loss |
| Inflorescence and oil weight | 50.43 g ± 0.03 | 52.12 g ± 0.04 | 50.52 g ± 0.02 | 52.24 g ± 0.05 |
| Macerated oil weight after extraction process | 50.32 g ± 0.02 0.11 g ± 0.01 weight loss | 52.00 g ± 0.02 0.12 g ± 0.01 weight loss | 50.31 g ± 0.01 0.21 g ± 0.01 weight loss | 52.12 g ± 0.01 0.12 g ± 0.01 weight loss |
| Macerated oil weight after filtration process | 44.64 g ± 0.02 | 46.23 g ± 0.03 | 43.91 g ± 0.02 | 45.78 g ± 0.02 |
| Inflorescence weight and oil after filtration process | 5.17 g ± 0.06 inflorescences and oil 0.54 g ± 0.06 oil retained by plant material | 5.15 g ± 0.05 inflorescences and oil 0.52 g ± 0.04 oil retained by plant material | 5.99 g ± 0.05 inflorescences and oil 1.27 g ± 0.05 oil retained by plant material | 5.62 g ± 0.07 inflorescences and oil 0.87 g ± 0.06 oil retained by plant material |
| Extraction efficiency | 97.46 % | 97.32 % | 95.87 % | 96.37 % |
| Bediol® | Bedrocan® | |||
|---|---|---|---|---|
| mean ± SD | CV% | mean ± SD | CV% | |
| CBD | 862.5 ± 25.2 | 2.9 | 37.5 ± 0.3 | 1.0 |
| THC | 720.2 ± 67.8 | 9.4 | 2008.9 ± 102.0 | 5.1 |
| CBN | 5.3 ± 0.04 | 0.9 | 8.4 ± 0.7 | 8.6 |
| CBD-A | 96.5 ± 15.5 | 15.1 | 30.2 ± 0.1 | 0.4 |
| THC-A | 30.3 ± 0.9 | 3.0 | 35.6 ± 2.1 | 6.0 |
| Preparation step | Weight/Duration | Details/Description |
|---|---|---|
| Inflorescence weighing | 5 g Bedrocan® or Bediol® medical cannabis varieties | Analytical balance—1:10 ratio plant/oil |
| Inflorescence grinding | 2 min | Grinder—Plant material homogenization |
| Decarboxylation | 125 °C; 30 min * * (decarboxylation time is considered when oven reaches the temperature setting) | Laboratory oven without air convection with automatic thermostat - conversion of acid cannabinoids into neutral forms especially for Δ9-THC and CBD active compounds |
| Inflorescence Cooling | 10 min; room temperature (25 °C, 60% RH) | |
| SHAKING | 10 min; room temperature (25 °C; 60% RH) | Mechanical rod stirrer—Homogenization of decarboxylated plant material and oil (MCT and olive) |
| Macerated oil extraction | 100 °C; 30 min * * (maceration time is considered when oil reaches the temperature setting) | Magnetic stirrer with heating plate |
| Oil filtration | 5 min | Mechanical press with filter system - separation of plant residues and oil |
| Magistral oil labelling and storage | Refrigerated storage (4 °C ± 1) | Storage in amber glass containers to prevent photo-oxidation phenomena |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramella, A.; Roda, G.; Pavlovic, R.; Dei Cas, M.; Casagni, E.; Mosconi, G.; Cecati, F.; Minghetti, P.; Grizzetti, C. Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations. Molecules 2020, 25, 2986. https://doi.org/10.3390/molecules25132986
Ramella A, Roda G, Pavlovic R, Dei Cas M, Casagni E, Mosconi G, Cecati F, Minghetti P, Grizzetti C. Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations. Molecules. 2020; 25(13):2986. https://doi.org/10.3390/molecules25132986
Chicago/Turabian StyleRamella, Alberto, Gabriella Roda, Radmila Pavlovic, Michele Dei Cas, Eleonora Casagni, Giacomo Mosconi, Francisco Cecati, Paola Minghetti, and Carlo Grizzetti. 2020. "Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations" Molecules 25, no. 13: 2986. https://doi.org/10.3390/molecules25132986
APA StyleRamella, A., Roda, G., Pavlovic, R., Dei Cas, M., Casagni, E., Mosconi, G., Cecati, F., Minghetti, P., & Grizzetti, C. (2020). Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations. Molecules, 25(13), 2986. https://doi.org/10.3390/molecules25132986







