LC/MS Analysis of Saponin Fraction from the Leaves of Elaeagnus rhamnoides (L.) A. Nelson and Its Biological Properties in Different In Vitro Models
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Plant Material
2.3. Preparation of the Saponin Fraction of Sea Buckthorn Leaves
2.4. LC-MS
2.5. Stock Solution
2.6. Blood Platelets and Plasma Isolation
2.7. PBMCs Isolation
2.8. Markers of Oxidative Stress
2.8.1. Lipid Peroxidation Measurement
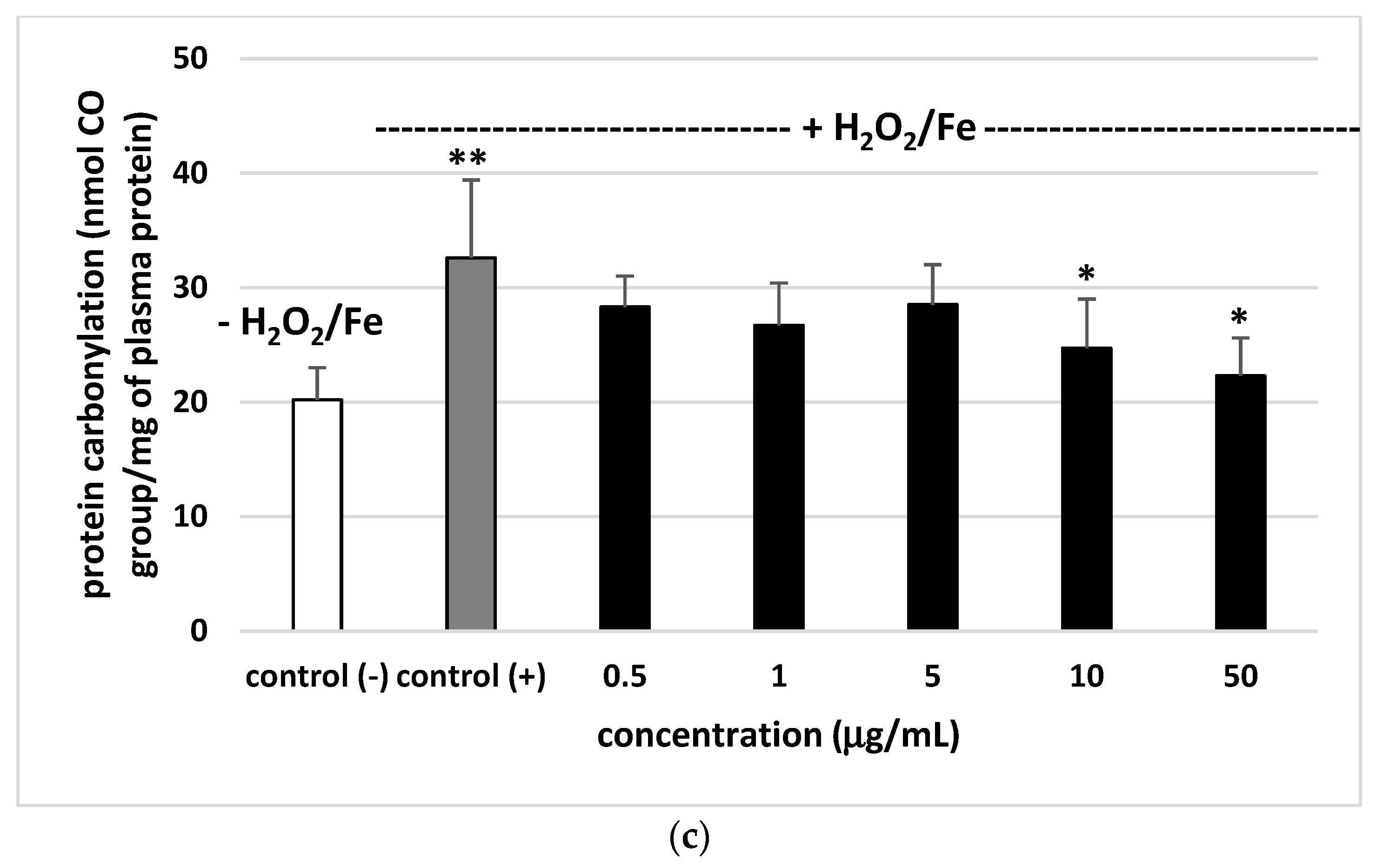
2.8.2. Carbonyl Group Measurement
2.8.3. Thiol Group Determination
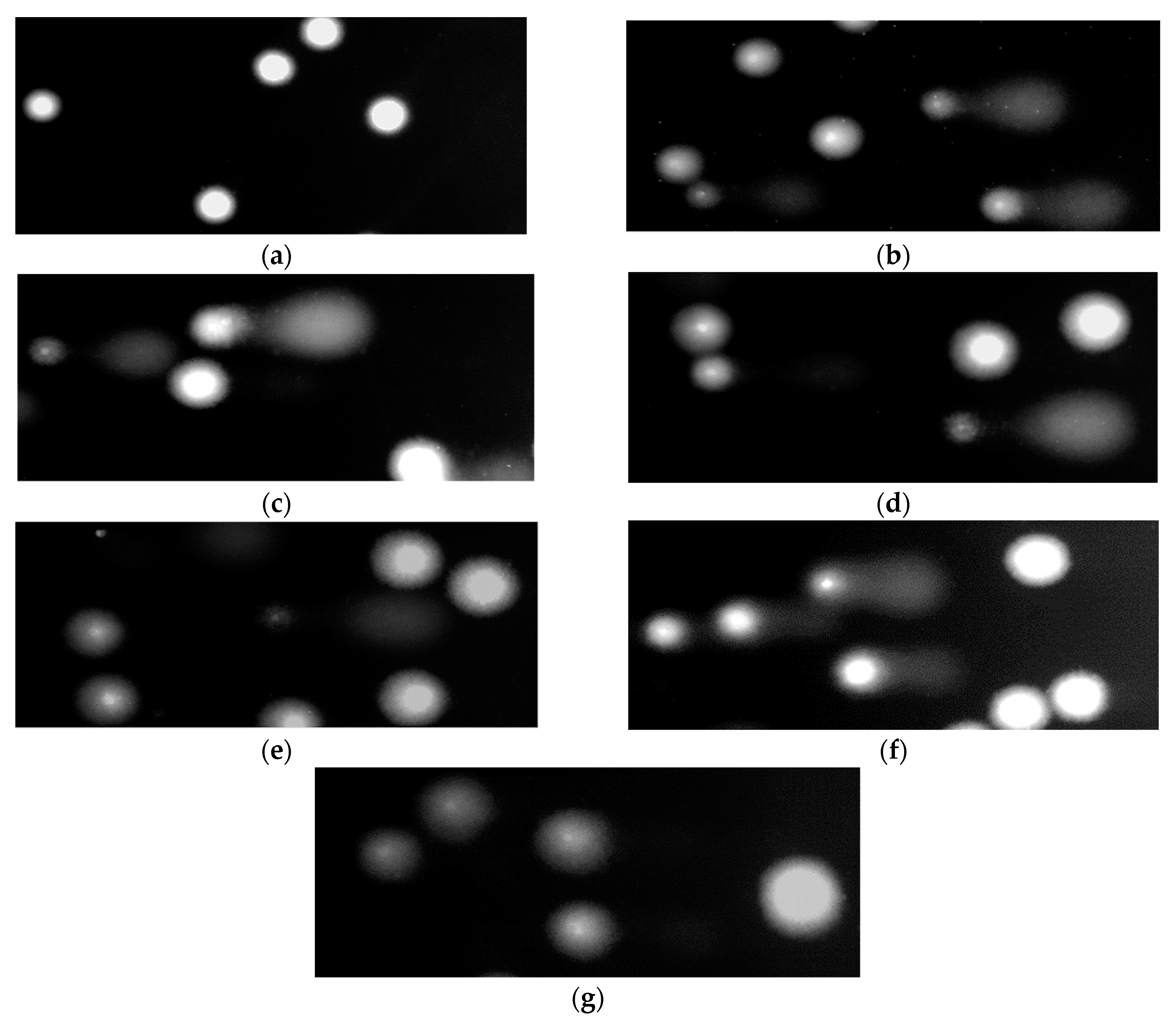
2.8.4. DNA Damage by the Comet Assay
2.8.5. Comets Analysis
2.9. Parameters of Hemostasis
2.9.1. The measurement of PT
2.9.2. The Measurement of APTT
2.9.3. The Measurement of TT
2.10. Parameters of Toxicity
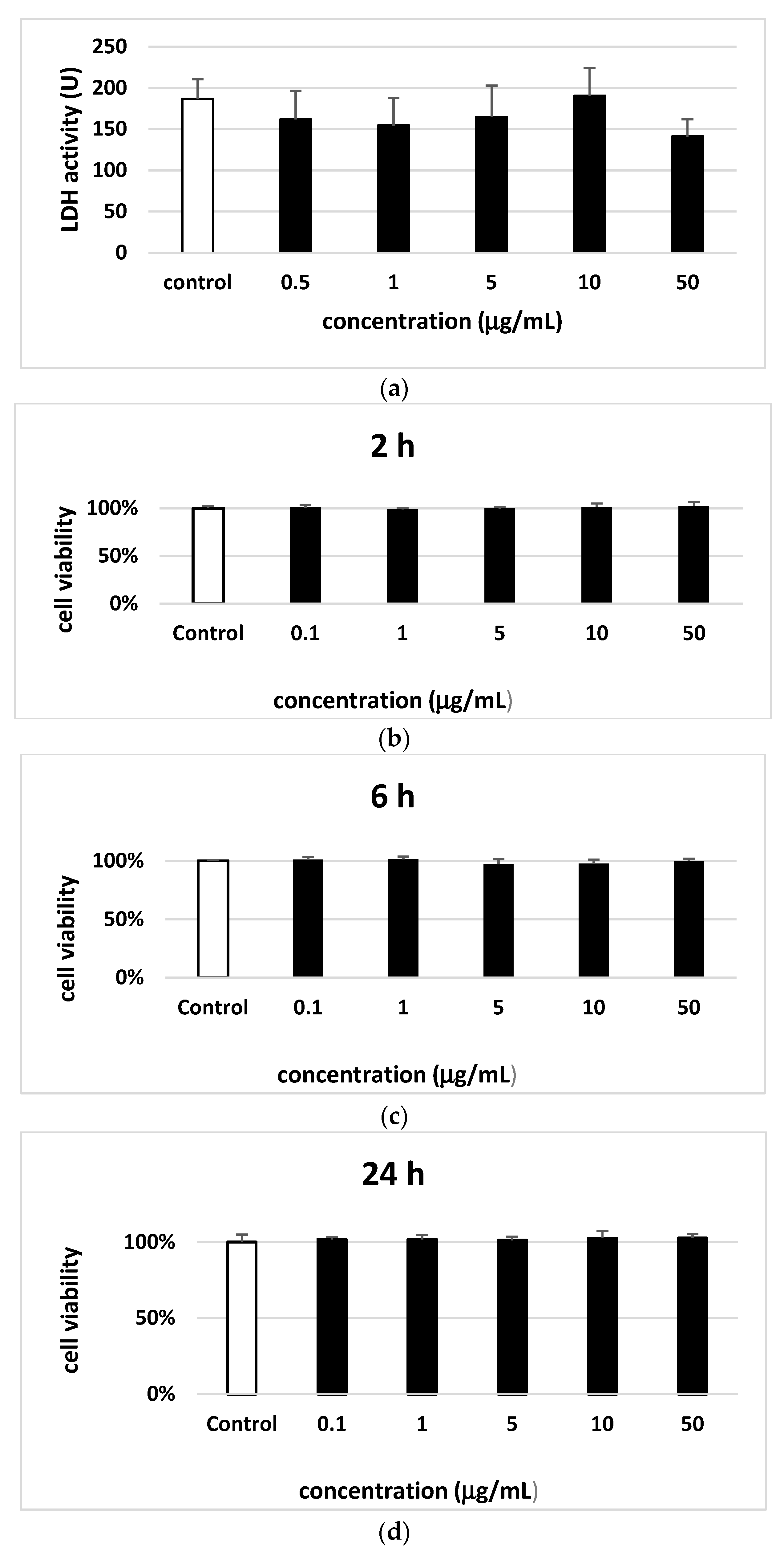
2.10.1. LDH Measurement
2.10.2. Cell Viability
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Malinowska, P.; Olas, B. Sea buckthorn—Valuable plant for health. Kosmos 2016, 2, 288–292. [Google Scholar]
- Yang, B.; Kallio, H. Supercritical CO2-extracted sea buckthorn (Hippophae rhamnoides) oils as new food ingredients for cardiovascular health. Proc. Health Ingred. Eur. 2002, 17, 17–19. [Google Scholar]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Skalski, B.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Phenolic fraction and nonpolar fraction from sea buckthorn leaves and twigs: Chemical profile and biological activity. Fut. Med. Chem. 2018, 10, 2381–2394. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Lis, B.; Olas, B.; Grabarczyk, Ł.; Stochmal, A.; Żuchowski, J. Biological properties of Elaeagnus rhamnoides (L.) A. Nelson twig and leaf extracts. BMC Complem. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, M.S.; Lee, K.M.; Park, S.K.; Sea, K.I.; Kim, H.J.; Kim, M.-J.; Choi, M.-S.; Lee, M.-K. Anti-visceral obesity and antioxidant effects of powdered sea buckthorn (Hippophae rhamnoides L.) leaf tea in diet-induced obese mice. Food Chem. Toxicol. 2011, 49, 2370–2376. [Google Scholar] [CrossRef]
- Pichiah, P.B.; Moon, H.J.; Park, J.E.; Moon, Y.J.; Cha, Y.S. Ethanolic extract of seabuckthorn (Hippophae rhamnoides L.) prevents high-fat diet-induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutr. Res. 2012, 32, 856–864. [Google Scholar] [CrossRef]
- Walkowiak, B.; Michalak, E.; Koziołkiewicz, W.; Cierniewski, C.S. Rapid photometric method for estimation of platelet count in blood plasma or platelet suspension. Thromb. Res. 1989, 56, 763–766. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef]
- Bartosz, G. Druga Twarz Tlenu; Warszawa PWN: Warszawa, Poland, 2008. [Google Scholar]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of sulphydryl groups. Biochim. Biophys. Acta 1973, 311, 38–44. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of disulphide groups. Biochim. Biophys. Acta 1973, 311, 26–37. [Google Scholar] [CrossRef]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Błasiak, J. All-trans retinoic acid modulates DNA damage response and the expression of the VEGF-A and MKI67 genes in ARPE-19 cells subjected to oxidative stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, J.; Kołodziejczyk-Czepas, J.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A.; Olas, B. Phenolic fractions from Trifolium pallidum and Trifolium scabrum aerial parts in human plasma protect against changes induced by hyperhomocysteinemia. Food Chem. Toxicol. 2012, 50, 4023–4027. [Google Scholar] [CrossRef]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–192. [Google Scholar] [CrossRef]
- Gupta, D.; Kaul, V. Qualitative analysis of bioactive compounds in leaves of Hippophae rhamnoides L. Nat. Acad. Sci. Lett. 2013, 36, 477–481. [Google Scholar] [CrossRef]
- Skalski, B.; Stochmal, A.; Żuchowski, J.; Grabarczyk, Ł.; Olas, B. Response of blood platelets to phenolic fraction and non-polar fraction from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson in vitro. Biomed. Pharmacother. 2020, 124, 109897. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Wen, X.-F.; Li, Y.-H.; Matsuzaki, K.; Kitanaka, S. Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW264. 7 cells. Chem. Pharm. Bull. 2013, 61, 279–285. [Google Scholar] [CrossRef]
- Zheng, W.-H.; Bai, H.-Y.; Han, S.; Bao, F.; Zhang, K.-X.; Sun, L.-L.; Du, H.; Yang, Z.-G. Analysis on the constituents of branches, berries, and leaves of Hippophae rhamnoides L. by UHPLC-ESI-QTOF-MS and their anti-inflammatory activities. Nat. Prod. Commun. 2019, 14, 1934578X1987140. [Google Scholar] [CrossRef]
- Chen, C.; Gao, W.; Cheng, L.; Shao, Y.; Kong, D.-Y. Four new triterpenoid glycosides from the seed residue of Hippophae rhamnoides subsp. sinensis. J. Asian Nat. Prod. Res. 2014, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, C.; Zhang, J.; Cheng, L.; Kong, D.-Y. Two new triterpene saponins from the seed residue of Hippophae rhamnoides L. Helv. Chim. Acta 2015, 98, 60–66. [Google Scholar] [CrossRef]
- Zheng, R.X.; Xu, X.D.; Tian, Z.; Yang, J.S. Chemical constituents from the fruits of Hippophae rhamnoides. Nat. Prod. Res. 2009, 23, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Kitanaka, S.; Kawata, K.; Goto, K. Anti-tumor promoters phenolics and triterpenoid from Hippophae rhamnoides. Fitoterapia 2009, 80, 164–167. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Li, H.-R.; Wang, L.-Y.; Li, Y.-H.; Lu, S.-G.; Wen, X.-F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef]
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A.Nelson fruits. Food Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef]
- Liu, C.; Feng, R.; Zou, J.; Xia, F.; Wan, J.B. 20(S)-protopanaxadiol saponins mainly contribute to the anti-atherogenic effects of Panax notoginseng in ApoE deficient mice. Molecules 2019, 24, 3723. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Zhao, J.; He, T.; Chen, H.; Wang, J.; Zhang, W.; Ma, W.; Fan, Y.; Song, X. Phospholipase Cγ2 signalling contributes to the haemostatic effect of Notoginsenoside Ft1. J. Pharm. Pharmacol. 2019, 71, 878–886. [Google Scholar] [CrossRef]
- Ouyang, X.L.; Mao, W.H.; Wang, C.G.; Pan, Y.M.; Liang, D.; Wang, H.S. Five 11α, 12α-epoxy pentacyclic triterpenoid saponins with antithrombus activities from Glechoma longituba. Fitoterapia 2019, 138, 104345. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Man, J.; Hu, Y.; Cui, X. Chemical and bioactive comparison of Panax notoginseng root and rhizome in raw and steamed forms. J. Ginseng Res. 2019, 43, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Su, H.; Wang, Y.T.; Wan, J.B. Therapeutic potential of ginsenosides in management of atherosclerosis. In Phytotherapies: Efficacy, Safety, and Regulation; Ramzon, I., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Dong, Y.; Duan, L.; Chen, H.W.; Liu, Y.M.; Zhang, Y.; Wang, J. Network pharmacology-based prediction and verification of the targets and mechanism for panax notoginseng saponins against coronary heart disease. Evid. Based Complement. Altern. Med. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Hou, J.G.; Liu, Y.; Zhang, R.B.; Jiang, S.; Ren, S.; Wang, Y.P.; Shen, Q.; Li, W.; Li, X.D.; et al. Supplementation of saponins from leaves of Panax quinquefoliu mitigates cisplatin-evoked cardiotoxicity via inhibiting oxidative stress-associated inflammation and apoptosis in mice. Antioxidants 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Miyamoto, A.; Zhao, S.; Liu, C.; Zheng, W.; Wang, H. Effects of steroidal saponins extract from ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response. Pharm. Biol. 2019, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, T.; Wu, Y.; Yang, W.; Hu, S.; Sun, Z.; Li, P.; Du, S. Panax notoginseng saponins suppress lipololysaccharide-induced barier disruption and monocyte adhesion on bEnd.3 cells via the opposite modulation of Nrf2 antioxidant and NF-κB inflammatory pathways. Phytother. Res. 2019, 30, 1–12. [Google Scholar]
- Kang, J.S.; Kim, S.O.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Chang, Y.-C.; Kim, W.-J.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.-R.; Park, S.K.; Shin, H.S.; Chae, S. Protective effects of timosaponin AIII against UVB-radiation induced inflammation and DNA injury in human epidermal keratinocytes. Biol. Pharm. Bull. 2019, 42, 1524–1531. [Google Scholar] [CrossRef]
- Alam, F.; Saqib, Q.N.; Waheed, A. Cytotoxic activity of extract and crude saponin from Zanthoxylum armatum DC. Against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. Evid.-Based Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Ji, C.-C.; Tang, H.-F.; Hu, Y.-Y.; Zhang, Y.; Zheng, M.-H.; Qin, H.-Y.; Li, S.-Z.; Wang, X.-Y.; Fei, Z.; Cheng, G. Saponin 6 derived from Anemone taipaiensis induces U87 human malignant glioblastoma cell apoptosis via regulation of Fas and Bcl-2 family proteins. Mol. Med. Rep. 2016, 14, 380–386. [Google Scholar] [CrossRef][Green Version]


| tR | Peak Area Fraction (%) | Parent Ion [M-H]− | MM/MS Fragments | Formula | Error (ppm) | mΣ | Tentative Identification | |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.84 | 0.37 | 317.0547 | 225.0081 (8) | unidentified | |||
| 245.0430 | 171.0050 (9), 152.9962 (10) | C6H15O8P | 0.7 | 3.5 | glycerophosphoglycerol | |||
| 2 | 5.02 | 0.13 | 433.0420 | 300.9992 (100) | C19H14O12 | −1.8 | 13.1 | ellagic acid-Pen |
| 3 | 5.38 | 0.7 | 300.9998 | C14H6O8 | −2.8 | 4.3 | ellagic acid | |
| 4 | 13.21 | 0.13 | 789.2613 | 626.2026 (11), 477.1050 (42), 313.0371 (100), 300.0283 (63) | C38H46O18 | −0.2 | 12.1 | I-dHex-Lin-Hex |
| 489.2714 | 327.2213 (15), 309.2073 (100), 171.1044 (21) | unidentified | ||||||
| 5 | 13.33 | 0.20 | 789.2613 | 626.2017 (10), 477.1049 (22), 313.0362 (100), 300.0280 (33) | C38H46O18 | −0.2 | 8.8 | I-dHex-Lin-Hex |
| 6 | 13.67 | 0.47 | 1119.5593 * | 1073.5551 (34),911.5017 (5), 749.4499 (9), 603.3913 (30), 585.3799 (24), 471.3485 (100) | C53H86O22 | −0.0 | 20.8 | C30H48O4-Pen-dHex-Hex-Hex |
| 7 | 13.79 | 0.14 | 927.4971 | 765.4453 (19), 603.3931 (68), 585.3795 (16), 471.3465 (36) | C47H76O18 | −1.3 | 26.4 | C30H48O4-Pen-Hex-Hex |
| 8 | 14.11 | 0.29 | 511.2406 * | 465.2338 (13), 293.0889 (100), 171.1405 (49) | C21H38O11 | −1.9 | 3.7 | |
| 927.4959 | 765.4440 (22), 603.3922 (100), 585.3794 (23), 471.3491 (55) | C47H76O18 | −0.0 | 25.0 | C30H48O4-Pen-Hex-Hex | |||
| 9 | 14.39 | 1.51 | 695.4018 * | 649.3976 (2), 487.3441 (100) | C36H58O10 | −0.8 | 10.7 | C30H48O5-Hex |
| 10 | 14.51 | 0.67 | 1427.6703 * | 1219.6117 (29), 1057.5600 (2), 733.4544 (20), 587.3969 (27), 569.3862 (24), 455.3540 (100) | C65H106O31 | −0.2 | 15.5 | C30H48O3-Pen-dHex-Hex-Hex-Hex-Hex |
| 11 | 14.95 | 4.80 | 713.3323 ** | 733.4534 (85), 587.3959 (74), 569.3854 (34), 455.3544 (100) | C65H106O31 | −1.4 | 8.8 | C30H48O3-Pen-dHex-Hex-Hex-Hex-Hex |
| 12 | 15.38 | 1.01 | 1235.6069 | 749.4480 (4), 587.3962 (100), 569.3869 (13), 455.3533 (20) | C59H96O27 | −0.3 | 35.5 | C30H48O3-Pen-Hex-Hex-Hex-Hex |
| 13 | 15.59 | 0.30 | 503.3385 | C30H48O6 | −1.3 | 3.3 | triterpenoid | |
| 14 | 15.78 | 0.16 | 489.2140 | 327.1618 (100), 312.1378 (78), 297.1141 (45), 255.0678 (32) | C26H34O9 | −2.1 | 10.5 | unidentified |
| 15 | 16.14 | 0.15 | 491.2301 | 329.1773 (100), 314.1542 (38) | C26H36O9 ? | −2.9 | 15.4 | unidentified |
| 16 | 16.64 | 4.99 | 1265.6167 * | 895.5050 (3), 733.4528 (43), 587.3972 (39), 569.3850 (36), 455.3538 (100) | C59H96O26 | 0.4 | 17.0 | C30H48O3-Pen-dHex-Hex-Hex-Hex |
| 17 | 16.86 | 8.97 | 1459.7114 | 1297.6621 (8), 973.5561 (15), 807.4554 (14), 661.3975 (22), 619.3858 (8), 601.3741 (25), 529.3548 (70), 487.3443 (12), 469.3341 (60), 183.1039 (32) | C71H112O31 | 0.0 | 17.6 | C30H48O5-Ac-Pen-dHex-Lin-Hex-Hex-Hex |
| 18 | 17.12 | 3.13 | 1313.6537 | 1151.5990 (5), 827.4988 (6), 809.4857 (6), 661.3968 (9), 619.3840 (5), 601.3760 (36), 487.3448 (2), 469.3329 (10), 183.1039 (14) | C65H102O27 | −0.1 | 15.8 | C30H48O5-Ac-Pen-Lin-Hex-Hex-Hex |
| 19 | 17.32 | 6.89 | 1313.6528 | 985.4993 (8), 823.4497 (11), 661.3964 (100), 619.3864 (9), 601.3751 (47), 529.3559 (7), 487.3426 (3), 469.3329 (10), 183.1031 (26) | C65H102O27 | 0.6 | 30.9 | C30H48O5-Ac-Pen-Hex-Lin-Hex-Hex |
| 20 | 17.91 | 0.26 | 329.1399 | 311.1294 (72), 275.0936 (1), 163.0768 (100), 149.0609 (87) | C19H22O5 | −1.3 | 3.5 | unidentified |
| 21 | 18.13 | 11.34 | 1401.7034 | 1239.6516 (4), 749.4478 (27), 603.3893 (39), 585.3804 (30), 471.3486 (100), 183.1032 (12) | C69H110O29 | 1.9 | 18.8 | C30H48O4-Pen-dHex-Lin-Hex-Hex-Hex |
| 22 | 18.26 | 7.07 | 1343.6633 * | 1297.6574 (100), 1135.6026 (17), 973.5552 (11), 807.4559 (10), 661.3972 (17), 619.3882 (10), 601.3747 (18), 529.3530 (57), 487.3442 (5), 469.3333 (41), 183.1027 (27) | C65H102O26 | 0.6 | 39.4 | C30H48O5-Ac-Pen-dHex-Lin-Hex-Hex |
| 551.3442 * | 505.3386 (100), 343.2872 (19), 179.0563 (18) | C26H50O9 | −0.9 | 8.6 | unidentified glycoside | |||
| 23 | 18.46 | 1.82 | 1151.5998 | 989.5454 (12), 985.5001 (16), 823.4575 (6), 661.3974 (7), 619.3875 (5), 601.3728 (25), 487.3423 (2), 469.3318 (19), 183.1045 (9) | C59H92O22 | 0.8 | 16.7 | C30H48O5-Ac-Pen-Hex-Lin-Hex |
| 315.1606 | C19H24O4 | −1.0 | 11.2 | unidentified | ||||
| 551.3433 * | 505.3389 (100), 343.2860 (9), 291.1442 (5) | unidentified | ||||||
| 24 | 18.67 | 2.30 | 1151.5995 | 989.5474 (14), 985.5027 (15), 823.4501 (7), 661.3946 (13), 619.3864 (4), 601.3744 (9), 501.3213 (17), 487.3432 (1), 469.3317 (7), 183.1030 (11) | C59H92O22 | 1.1 | 19.3 | C30H48O5-Ac-Pen-Hex-Lin-Hex |
| 25 | 18.80 | 7.40 | 1255.6457 | 1093.5980 (7), 769.4888 (5), 603.3899 (100), 585.3797 (23), 471.3473 (27), 183.1032 (12) | C63H100O25 | 1.9 | 17.2 | C30H48O4-Pen-Lin-Hex-Hex-Hex |
| 26 | 19.24 | 20.33 | 487.3431 | 409.3115 (5) | C30H48O5 | −0.4 | 2.7 | triterpenoid |
| 27 | 19.97 | 4.11 | 1239.6514 | 1077.5965 (8),915.5455 (4), 749.4484 (14), 603.3897 (37), 585.3785 (30), 471.3486 (100), 183.1027 (12) | C63H100O24 | 1.4 | 36.0 | C30H48O4-Pen-dHex-Lin-Hex-Hex |
| 28 | 20.20 | 0.15 | 649.3741 | 163.0394 (5), 145.0293 (31), 117.0340 (10) | C39H54O8 | 0.7 | 8.6 | C30H48O6-CouA |
| 29 | 20.32 | 0.30 | 487.3429 | C30H48O5 | 0.0 | 2.9 | triterpenoid | |
| 30 | 20.53 | 0.80 | 1093.5944 | 931.5389 (13), 765.4373 (6), 603.3888 (100), 585.3797 (25), 471.3474 (45), 183.1034 (10) | C57H90O20 | 0.8 | 15.7 | C30H48O4-Pen-Hex-Lin-Hex |
| 31 | 20.67 | 0.39 | 1057.5570 | 895.5035 (6), 733.4526 (7), 587.3952 (24), 569.3857 (14), 455.3534 (100) | C53H86O21 | 1.8 | 13.6 | C30H48O3-Pen-dHex-Hex-Hex |
| 32 | 21.19 | 2.98 | 329.1760 | 314.1528 (45) | C20H26O4 ? | −0.5 | 3.8 | unidentified |
| 473.3641 | 413.3427 (17) | C30H50O4 | −1.0 | 3.1 | triterpenoid | |||
| 33 | 21.98 | 0.32 | 293.2125 | 275.2018 (100), 171.1034 (17) | C18H30O3 | −1.1 | 5.0 | unidentified |
| 34 | 22.61 | 0.63 | 633.3798 | 455.3163 (8), 163.0404 (1), 145.0300 (26), 117.0352 (6) | C39H54O7 | −0.1 | 3.2 | C30H48O5-CouA |
| 571.2894 | 315.0504 (25), 255.2336 (100), 241.0129 (29), 223.0020 (9), 152.9971 (10) | C25H49O12P | −0.8 | 10.1 | palmitoyl-glycerophosphoinositol | |||
| 35 | 22.95 | 0.21 | 633.3794 | 145.0295 (27), 117.0343 (8) | C39H54O7 | 0.4 | 7.6 | C30H48O5-CouA |
| 36 | 23.21 | 0.39 | 571.2889 | 391.2265 (5), 315.0491 (8), 255.2332 (53), 241.0122 (18), 152.9957 (5) | C25H49O12P | −0.1 | 7.5 | palmitoyl-glycerophosphoinositol |
| 481.2575 | 253.2177 (82), 245.0438 (100), 171.0073 (8) | C22H43O9P | −0.5 | 9.9 | hexadecenoyl-glycerophosphoglycerol | |||
| 37 | 23.42 | 0.20 | 507.2734 | 279.2336 (100), 152.9956 | C24H45O9P | −1.0 | 11.1 | octadecadienoyl-glycerophosphoglycerol |
| 471.3483 | C30H48O4 | −0.7 | 8.0 | triterpenoid | ||||
| 38 | 23.72 | 1.19 | 555.2854 | 225.0077 | unidentified | |||
| 481.2577 | 253.2178 (22), 152.9958 (7) | C22H43O9P | −1.1 | 11.4 | hexadecenoyl-glycerophosphoglycerol | |||
| 39 | 24.33 | 0.55 | 433.2363 | 279.2326 (11), 152.9963 (28) | C21H39O7P | −0.4 | 10.3 | octadecadienoyl-glycerolphosphate |
| 40 | 25.25 | 0.19 | 409.2358 | 25.2339 (12), 152.9956 (30) | C19H39O7P | 0.6 | 10.7 | palmitoyl-glycerolphosphate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żuchowski, J.; Skalski, B.; Juszczak, M.; Woźniak, K.; Stochmal, A.; Olas, B. LC/MS Analysis of Saponin Fraction from the Leaves of Elaeagnus rhamnoides (L.) A. Nelson and Its Biological Properties in Different In Vitro Models. Molecules 2020, 25, 3004. https://doi.org/10.3390/molecules25133004
Żuchowski J, Skalski B, Juszczak M, Woźniak K, Stochmal A, Olas B. LC/MS Analysis of Saponin Fraction from the Leaves of Elaeagnus rhamnoides (L.) A. Nelson and Its Biological Properties in Different In Vitro Models. Molecules. 2020; 25(13):3004. https://doi.org/10.3390/molecules25133004
Chicago/Turabian StyleŻuchowski, Jerzy, Bartosz Skalski, Michał Juszczak, Katarzyna Woźniak, Anna Stochmal, and Beata Olas. 2020. "LC/MS Analysis of Saponin Fraction from the Leaves of Elaeagnus rhamnoides (L.) A. Nelson and Its Biological Properties in Different In Vitro Models" Molecules 25, no. 13: 3004. https://doi.org/10.3390/molecules25133004
APA StyleŻuchowski, J., Skalski, B., Juszczak, M., Woźniak, K., Stochmal, A., & Olas, B. (2020). LC/MS Analysis of Saponin Fraction from the Leaves of Elaeagnus rhamnoides (L.) A. Nelson and Its Biological Properties in Different In Vitro Models. Molecules, 25(13), 3004. https://doi.org/10.3390/molecules25133004







