Safety and Efficacy of New Oximes to Reverse Low Dose Diethyl-Paraoxon-Induced Ventilatory Effects in Rats
Abstract
:1. Introduction
2. Results
2.1. Study 1: Safety of New Oximes on Ventilation Parameters at Rest in Awaken and Unrestrained Rats
2.2. Study 2: Efficiency of Novel Oximes on Ventilation Parameters at Rest in Awaken and Unrestrained Diethyl-Paraoxon-Poisoned Rats
3. Discussion
- Owing to the rapid onset and long-lasting effect of KO-27, the results suggest that one single dose of KO-27 may relevantly replace the continuous or repeated injections of PRX in a context where healthcare resources are limited. However, this proposal is hampered by the lack of data supporting the safety of KO-27 in human.
- Figure 3—Panel B suggests that a combination of PRX and BI-6 may reverse DEPO-induced increase of expiratory time during the study period of 210 min. Indeed, PRX led to a rapid reversal of expiratory time increase consequently to PRX injection without any noticeable effect of BI-6. Thereafter, PRX lasted at the time the antidotal effect of BI-6 occurred at a time when PRX antidotal effect disappeared with long-lasting antidotal of BI-6 and later increase until the end of the experiment. As expected, all altered respiratory parameters improved in the same manner. These data suggest that a combination of PRX and BI-6 should also be considered in humans. However, this proposal is hampered by the absence of knowledge regarding to the pharmacokinetic drug–drug interaction in addition to the elimination of PRX and BI-6 administered at the same time. Also the lack of data supporting the safety of BI-6 in humans requires to be addressed.
4. Material and Methods
4.1. Animals and Housing Conditions
4.2. Chemicals and Drugs
4.3. Safety Precaution
4.4. Clinical Examination
4.5. Whole Body Plethysmography
4.6. Telemetry Applied to Plethysmography
4.7. Study Designs
4.7.1. Study 1: Safety of New Oximes on Ventilation Parameters at Rest in Awaken and Unrestrained Rats
4.7.2. Study 2: Efficiency of New Oximes on Ventilation Parameters at Rest in Awaken and Unrestrained Diethyl-Paraoxon-Poisoned Rats
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satoh, T. Global epidemiology of organophosphate and carbamate poisonings. In Toxicology of Organophosphate and Carbamate Compounds, 1st ed.; Gupta, R.C., Ed.; Academic Press: New York, NY, USA, 2006; pp. 89–100. [Google Scholar]
- Kayouka, M.; Houze, P.; Debray, M.; Baud, F.J. Acute renal failure enhances the antidotal activity of pralidoxime towards paraoxon-induced respiratory toxicity. Toxicol. Lett. 2009, 189, 48–56. [Google Scholar] [CrossRef]
- Lerman, Y.; Gutman, H. The use of respiratory stimulants in organophosphates’ intoxication. Med. Hypotheses 1988, 26, 267–269. [Google Scholar] [CrossRef]
- Yamashita, M.; Tanaka, J.; Ando, Y. Human mortality in organophosphate poisonings. Vet. Hum. Toxicol. 1997, 39, 84–85. [Google Scholar] [PubMed]
- Pawar, K.S.; Bhoite, R.R.; Pillay, C.P.; Chavan, S.C.; Malshikare, D.S.; Garad, S.G. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: A randomised controlled trial. Lancet 2006, 368, 2136–2141. [Google Scholar] [CrossRef]
- Bartholomew, P.M.; Gianutsos, G.; Cohen, S.D. Differential cholinesterase inhibition and muscarinic receptor changes in CD-1 mice made tolerant to malathion. Toxicol. Appl. Pharmacol. 1985, 81, 147–155. [Google Scholar] [CrossRef]
- Eddleston, M.; Buckley, N.; Eyer, P.; Dawson, A. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Vale, A.; Lotti, M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 131, 149–168. [Google Scholar]
- King, A.M.; Aaron, C.K. Organophosphate and carbamate poisoning. Emerg. Med. Clin. North Am. 2015, 33, 133–151. [Google Scholar] [CrossRef]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem. Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef]
- Eddleston, M.; Chowdhury, F.R. Pharmacological treatment of organophosphorus insecticide poisoning: The old and the (possible) new. Br. J. Clin. Pharmacol. 2016, 81, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Lorke, D.E.; Petroianu, G.A. The Experimental Oxime KO-27-A Promising Protector from Organophosphate Pesticide Poisoning. A Review Comparing KO-27, KO-48, Pralidoxime, and Obidoxime. Front. Neurosci. 2019, 13, 427. [Google Scholar] [CrossRef]
- Barelli, A.; Soave, P.M.; Del Vicario, M.; Barelli, R. New experimental Oximes in the management of organophosphorus pesticides poisoning. Minerva Anestesiol. 2011, 77, 1197–1203. [Google Scholar]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Malinak, D.; Nepovimova, E.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. SAR Study to Find Optimal Cholinesterase Reactivator Against Organophosphorous Nerve Agents and Pesticides. Arch Toxicol. 2016, 90, 2831–2859. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.A.; Eddleston, M.; Szinicz, L. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst. Rev. 2005, 1. [Google Scholar] [CrossRef] [Green Version]
- Buckley, N.A.; Eddleston, M.; Li, Y.; Bevan, M.; Robertson, J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst. Rev. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.F.; Houzé, P.; Monier, C.; Risede, P.; Sarhan, H.; Borron, S.W.; Megarbane, B.; Garnier, R.; Baud, F.J. Toxic doses of paraoxon alter the respiratory pattern without causing respiratory failure in rats. Toxicology 2007, 232, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Houzé, P.; Pronzola, L.; Kayouka, M.; Villa, A.; Debray, M.; Baud, F.J. Ventilatory effects of low dose paraoxon result from central muscarinic effects. Toxicol. Appl. Pharmacol. 2008, 233, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kayouka, M.; Houzé, P.; Risède, P.; Debray, M.; Baud, F.J. Acute Renal Failure Alters the Kinetics of Pralidoxime in Rats. Toxicol. Lett. 2009, 184, 61–66. [Google Scholar] [CrossRef]
- Kayouka, M.; Houzé, P.; Baud, F.J.; Cisternino, S.; Debray, M.; Risède, P.; Schinkel, A.H.; Warnet, J.M. Does modulation of organic cation transporters improve pralidoxime activity in an animal model of organophosphate poisoning? Crit. Care Med. 2011, 9, 803–811. [Google Scholar] [CrossRef]
- Duarte, T.; Martin, C.; Baud, F.J.; Laprévote, O.; Houzé, P. Follow up studies on the respiratory pattern and total cholinesterase activities in dichlorvos-poisoned rats. Toxicol. Lett. 2012, 213, 142–150. [Google Scholar] [CrossRef]
- Houzé, P.; Borron, S.W.; Eric Kercji, E.; Baud, F.J. Dose-effect study of the reversal by antimuscarinic agents of methomyl-induced respiratory toxicity. Asian J. Pharmacol. Toxicol. 2017, 5, 1–14. [Google Scholar]
- Houzé, P.; Berthin, T.; Raphalen, J.H.; Hutin, A.; Baud, J.F. High Dose of Pralidoxime Reverses Paraoxon-Induced Respiratory Toxicity in Mice. Turk. J. Anaesthesiol. Reanim. 2018, 46, 131–138. [Google Scholar] [CrossRef]
- Houzé, P.; Hutin, A.; Lejay, M.; Baud, F.J. Comparison of the Respiratory Toxicity and Total Cholinesterase Activities in Dimethyl Versus Diethyl Paraoxon-Poisoned Rats. Toxics 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassa, J.; Kuca, K.; Karasova, J.; Musilek, K. The development of new oximes and the evaluation of their reactivating, therapeutic and neuroprotective efficacy against tabun. Mini Rev. Med. Chem. 2008, 8, 1134–1143. [Google Scholar] [CrossRef]
- Kurt, T.L. Epidemiological association in US veterans between Gulf War illness and exposures to anticholinesterases. Toxicol. Lett. 1998, 102–103, 523–526. [Google Scholar] [CrossRef]
- Hulse, E.J.; Haslam, J.D.; Emmett, S.R.; Woolley, T. Organophosphorus nerve agent poisoning: Managing the poisoned patient. Br. J. Anaesth. 2019, 123, 457–463. [Google Scholar] [CrossRef]
- Namba, T.; Nolte, C.T.; Jackrel, J.; Grob, D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am. J. Med. 1971, 50, 475–492. [Google Scholar] [CrossRef]
- Kumar, H. High dose atropine in organophosphorus poisoning. J. Assoc. Physicians India 1992, 40, 281–285. [Google Scholar]
- Houzé, P.; Mager, D.E.; Risède, P.; Baud, F.J. Pharmacokinetics and toxicodynamics of pralidoxime effects on paraoxon-induced respiratory toxicity. Toxicol. Sci. 2010, 116, 660–672. [Google Scholar] [CrossRef]
- Bohnert, S.; van den Berg, R.M.; Mikler, J.; Klaassen, S.D.; Joosen, M.J. Pharmacokinetics of Three Oximes in a Guinea Pig Model and Efficacy of Combined Oxime Therapy. Toxicol. Lett. 2020, 324, 86–94. [Google Scholar] [CrossRef]
- Thiermann, H.; Eyer, F.; Felgenhauer, N.; Pfab, R.; Zilker, T.; Eyer, P. Pharmacokinetics of obidoxime in patients poisoned with organophosphorus compounds. Toxicol. Lett. 2010, 197, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zunec, S.; Kopjar, N.; Zeljezić, D.; Kuca, K.; Musilek, K.; Lucić Vrdoljak, A. In vivo evaluation of cholinesterase activity, oxidative stress markers, cyto- and genotoxicity of K048 oxime–A promising antidote against organophosphate poisoning. Basic Clin. Pharmacol. Toxicol. 2014, 114, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroianu, G.A.; Nurulain, S.M.; Shafiullah, M.; Hasan, M.Y.; Kuča, K.; Lorke, D.E. Usefulness of administration of non-organophosphate cholinesterase inhibitors before acute exposure to organophosphates: Assessment using paraoxon. J. Appl. Toxicol. 2013, 33, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuča, K.; Petroianu, G.A. Combined Pre- and Post-treatment of Paraoxon Exposure. Molecules 2020, 25, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, F.J.; Houzé, P.; Villa, A.; Borron, S.W.; Carli, P. Toxicodynetics: A new discipline in clinical toxicology. Ann. Pharm. Fr. 2016, 74, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Nepovimova, E.; Malinak, D.; Kucera, T.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. Progress in Acetylcholinesterase Reactivators and in the Treatment of Organophosphorus Intoxication: A Patent Review (2006–2016). Expert Opin. Ther. Pat. 2017, 27, 971–985. [Google Scholar] [CrossRef]
- De Candole, C.A.; Douglas, W.W.; Evans, C.L.; Holmes, R.; Spencer, K.E.; Torrance, R.W.; Wilson, K.M. The failure of respiration in death by anticholinesterase poisoning. Br. J. Pharmacol. Chemother. 1953, 8, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, D., Jr.; Tenney, S.M. Control of breathing in experimental anemia. Respir. Physiol. 1970, 10, 384–395. [Google Scholar] [CrossRef]
- Tallarida, R.; Murray, R.B. Area under a curve: Simpson’s Rule. In Manual of Pharmacologic Calculations with Computer Programs; Springer: New York, NY, USA, 1981; pp. 47–49. [Google Scholar]
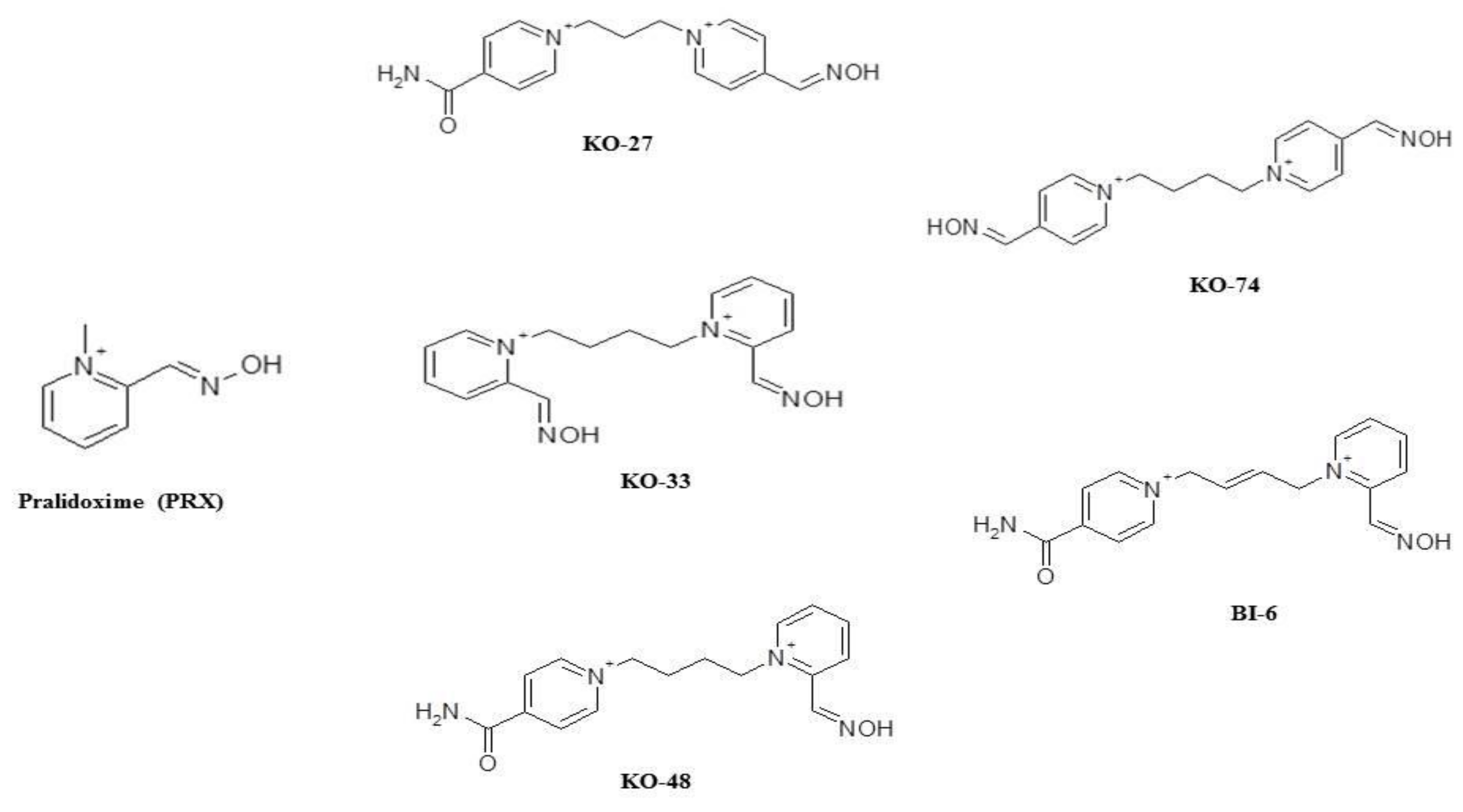
Sample Availability: Samples of the compounds KO-27, KO-33, KO-48, KO-74, and BI-6 are available from the authors. |



| Control Group | Pralidoxime Group | KO-27 Group | KO-33 Group | KO-48 Group | KO-74 Group | BI-6 Group | |
|---|---|---|---|---|---|---|---|
| AUC0-210min (Celsius.min) | 7919 ± 590 | 7855 ± 513 | 7976 ± 542 | 8102 ± 447 | 8023 ± 470 | 7915 ± 503 | 8050 ± 585 |
| Control Group | Pralidoxime Group | KO-27 Group | KO-33 Group | KO-48 Group | KO-74 Group | BI-6 Group | |
|---|---|---|---|---|---|---|---|
| AUC0-210min frequency (cycles) | 17321 ± 4370 | 17374 ± 4638 | 17633 ± 4733 | 17132 ± 4857 | 17268 ± 4629 | 17720 ± 4748 | 17321 ± 4370 |
| AUC0-210min total time (second.min) | 85 ± 23 | 78 ± 21 | 78 ± 21 | 79 ± 21 | 80 ± 21 | 76 ± 21 | 82 ± 22 |
| AUC0-20mn expiratory time (second.min) | 61 ± 17 | 55 ± 15 | 54 ± 15 | 54 ± 15 | 54 ± 15 | 53 ± 14 | 58 ± 16 |
| AUC0-210min inspiratory time (second.min) | 24 ± 6 | 23 ± 6 | 24 ± 7 | 25 ± 7 | 25 ± 7 | 23 ± 6 | 24 ± 6 |
| AUC0-210min tidal volume (µL.min) | 217210 ± 58324 | 221008 ± 59493 | 216971 ± 58241 | 202218 ± 46189 | 217133 ± 58391 | 204421 ± 52174 | 221185 ± 59502 |
| AUC0-210min minute ventilation (µL) | 23489827 | 25086912 ± 6699540 | 25629140 ± 6868978 | 21482414 ± 5751740 | 24726875 ± 6628236 | 22729783 ± 6063776 | 24721913 ± 6620647 |
| Control Group | DEPO Group | DEPO+PRX Group | DEPO+KO-27 Group | DEPO+KO-33 Group | DEPO+KO-48 Group | DEPO+KO-74 Group | DEPO+BI-6 Group | |
|---|---|---|---|---|---|---|---|---|
| AUC0-210min (Celsius.min) | 7919 ± 640 | 7125 ± 513 *** | 7245 ± 398 +++ | 7076 ± 548 +++ | 7035 ± 447 +++ | 7243 ± 860 +++ | 6915 ± 906 +++ | 7250 ± 506 +++ |
| Control Group | DEPO Group | DEPO+PRX Group | DEPO+KO-33 Group | DEPO +KO-48 Group | DEPO+KO-74 Group | |
|---|---|---|---|---|---|---|
| AUC0-210min frequency (cycles) | 22570 ± 6287 | 17315 ± 4280 *** | 17839 ± 4982 +++ | 16091 ± 4778 +++ | 17068 ± 4352 +++ | 17820 ± 4856 +++ |
| AUC0-210min total time (second.min) | 90 ± 34 | 65 ± 32 *** | 80 ± 25 +++ | 63 ± 18 +++ | 68 ± 21 +++ | 66 ± 21 +++ |
| AUC0-20mn expiratory time (second.min) | 68 ± 15 | 47 ± 9 *** | 57 ± 6 +++ | 43 ± 10 +++ | 44 ± 9 +++ | 43 ± 7 +++ |
| AUC0-210min inspiratory time (second.min) | 22 ± 8 | 18 ± 9 | 23 ± 8 | 20 ± 9 | 24 ± 10 | 23 ± 9 |
| AUC0-210min tidal volume (µL.min) | 214231 ± 49325 | 20942 ± 48923 | 222008 ± 56723 | 204376 ± 41897 | 213422 ± 49391 | 23475 ± 51656 |
| AUC0-210min minute ventilation (µL) | 23489827 | 25567785 ± 683214 | 25086912 ± 6699540 | 24765409 ± 5751740 | 24346784 ± 6628236 | 23678954 ± 6063776 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayouka, M.; Houzé, P.; Lejay, M.; Baud, F.J.; Kuca, K. Safety and Efficacy of New Oximes to Reverse Low Dose Diethyl-Paraoxon-Induced Ventilatory Effects in Rats. Molecules 2020, 25, 3056. https://doi.org/10.3390/molecules25133056
Kayouka M, Houzé P, Lejay M, Baud FJ, Kuca K. Safety and Efficacy of New Oximes to Reverse Low Dose Diethyl-Paraoxon-Induced Ventilatory Effects in Rats. Molecules. 2020; 25(13):3056. https://doi.org/10.3390/molecules25133056
Chicago/Turabian StyleKayouka, Maya, Pascal Houzé, Marc Lejay, Frédéric J. Baud, and Kamil Kuca. 2020. "Safety and Efficacy of New Oximes to Reverse Low Dose Diethyl-Paraoxon-Induced Ventilatory Effects in Rats" Molecules 25, no. 13: 3056. https://doi.org/10.3390/molecules25133056






