Inorganic Arsenic Exposure Decreases Muscle Mass and Enhances Denervation-Induced Muscle Atrophy in Mice
Abstract
:1. Introduction
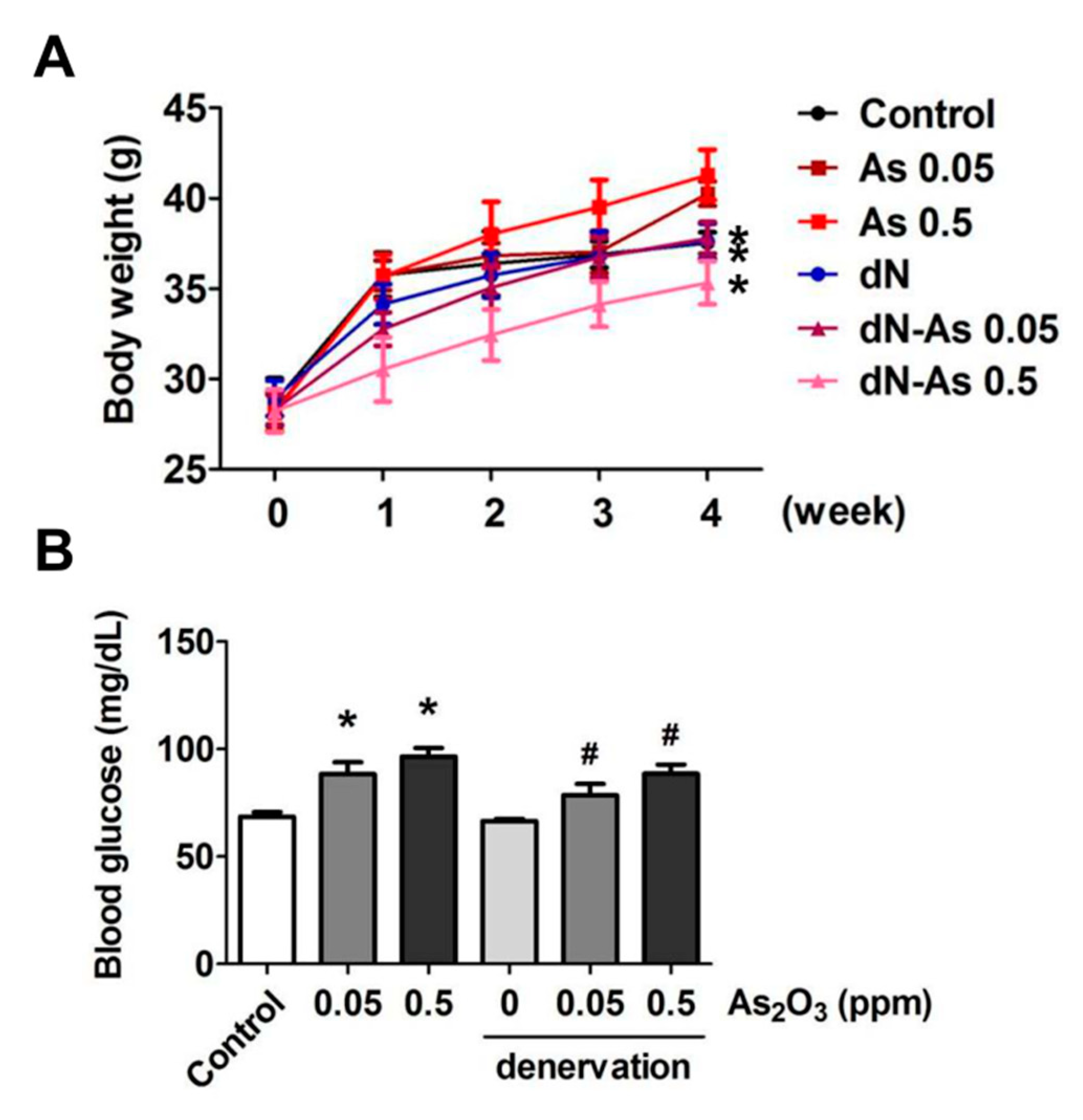
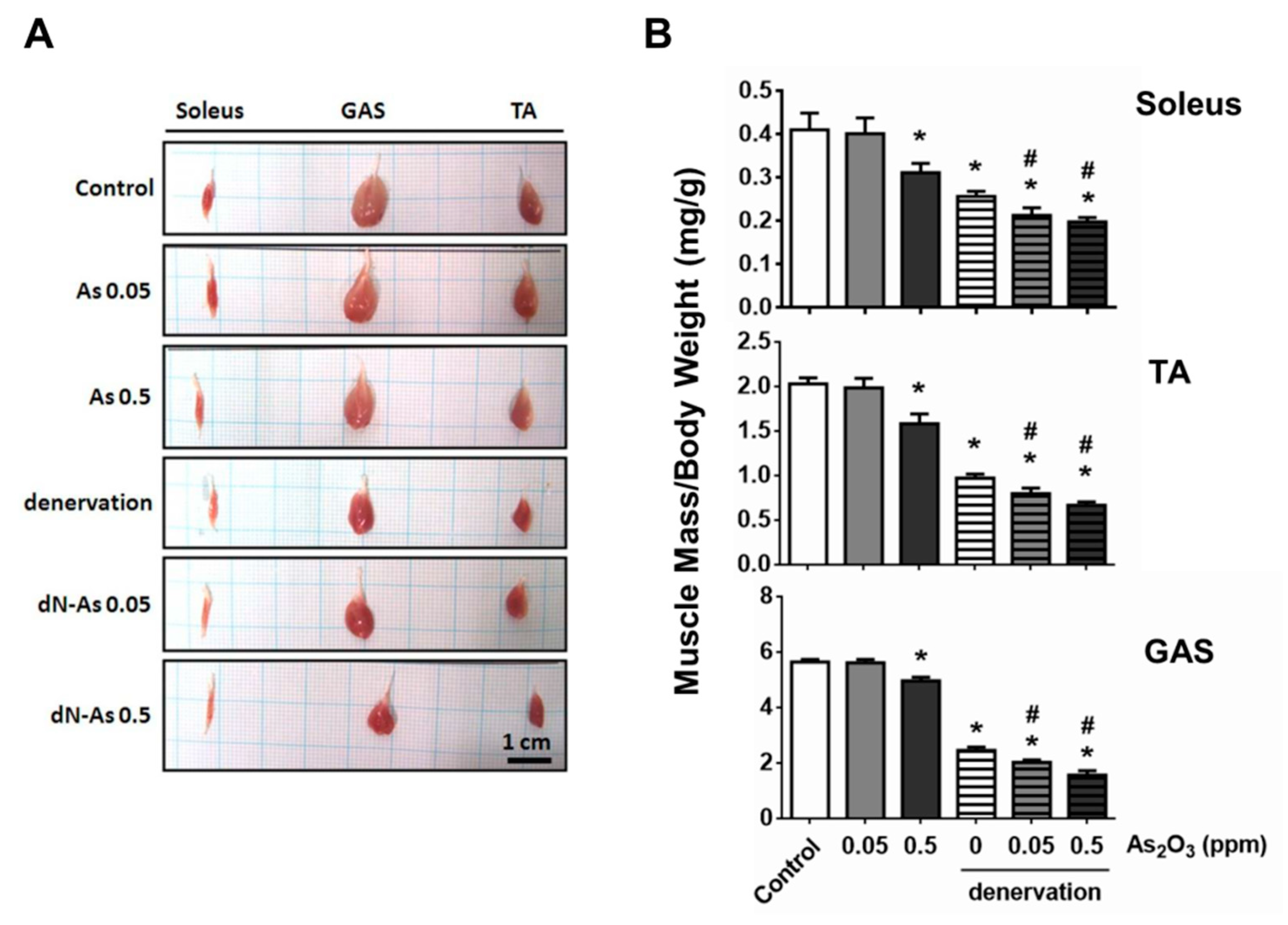
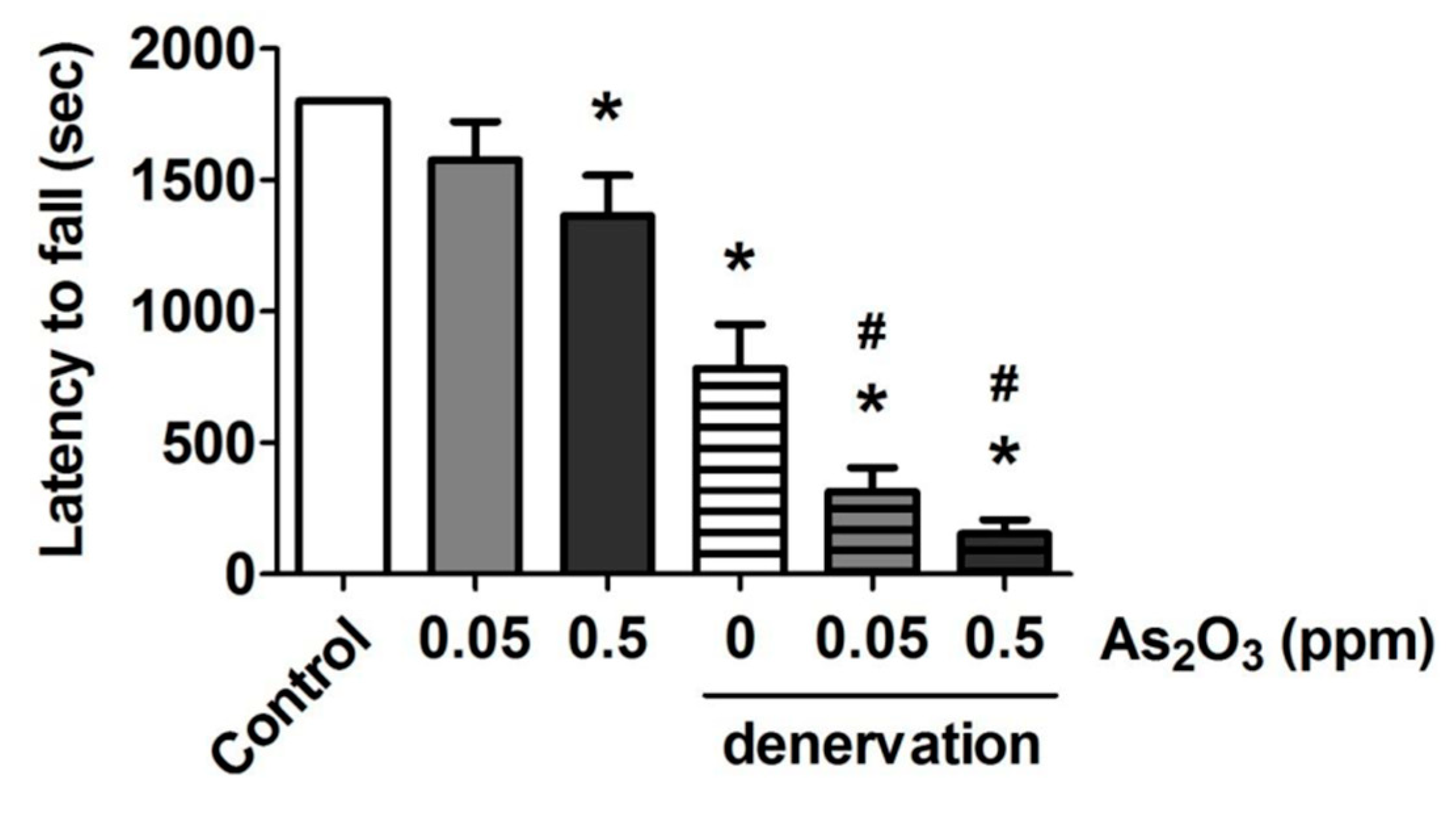
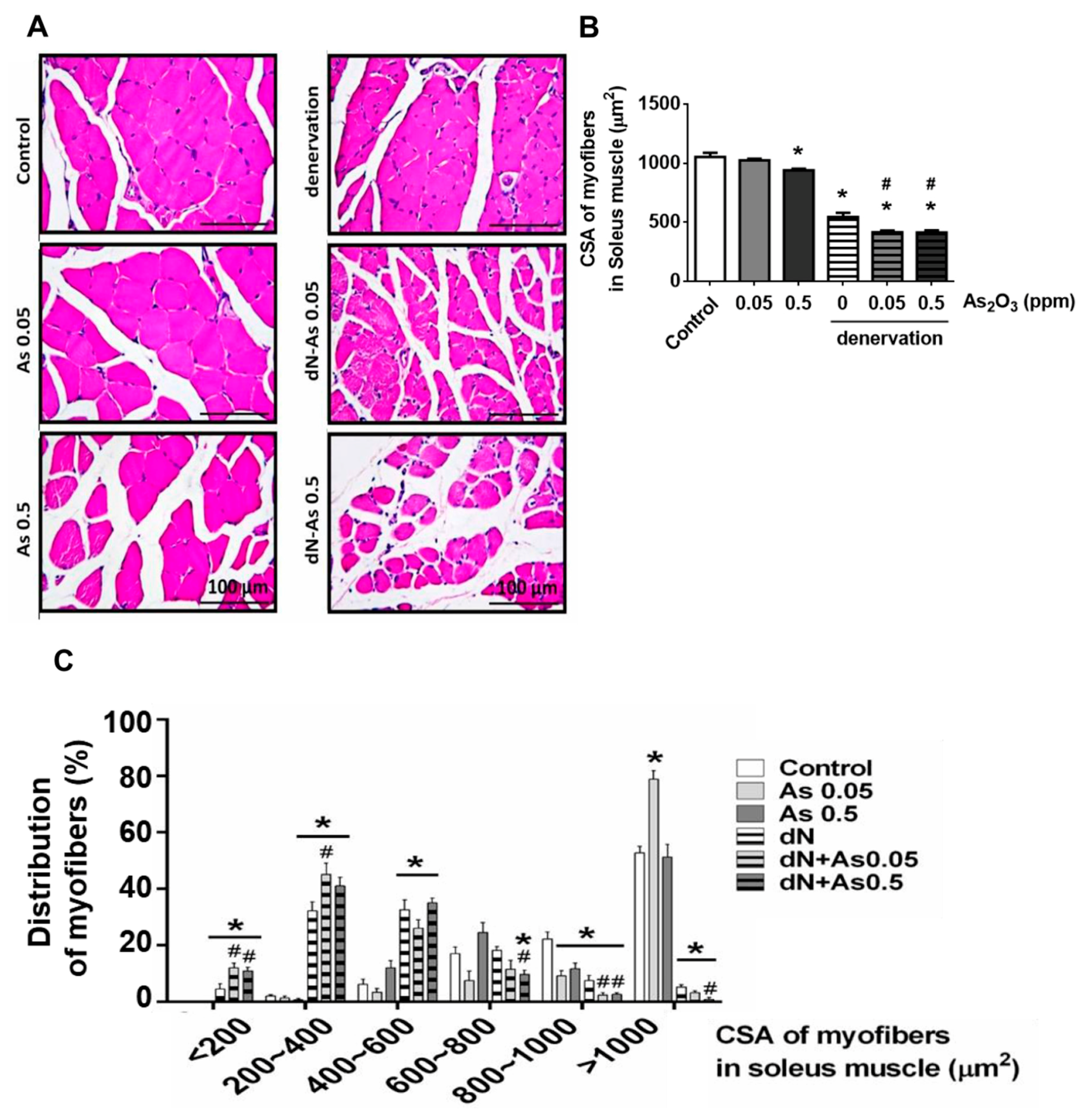
2. Results and Discussion
3. Materials and Methods
3.1. Animal Experiment
3.2. Muscle Denervation Model
3.3. Detection of Muscle Fatigue Task
3.4. Histological and Immunohistochemical Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Arsenic. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 6 June 2020).
- Chakraborti, D.; Mukherjee, S.C.; Pati, S.; Sengupta, M.K.; Rahman, M.M.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Chakraborti, A.K.; Basu, G.K. Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: A future danger? Environ. Health Perspect. 2003, 111, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Alimoghaddam, K. A review of arsenic trioxide and acute promyelocytic leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 44–54. [Google Scholar]
- Huang, S.Y.; Chang, C.S.; Tang, J.L.; Tien, H.F.; Kuo, T.L.; Huang, S.F.; Yao, Y.T.; Chou, W.C.; Chung, C.Y.; Wang, C.H.; et al. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br. J. Haematol. 1998, 103, 1092–1095. [Google Scholar] [CrossRef]
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharmacol. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef]
- Palus, S.; von Haehling, S.; Springer, J. Muscle wasting: an overview of recent developments in basic research. J. Cachexia Sarcopenia Muscle 2014, 5, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.P.; Tsai, K.S.; Chen, Y.W.; Huang, C.F.; Yang, R.S.; Liu, S.H. Arsenic inhibits myogenic differentiation and muscle regeneration. Environ. Health Perspect. 2010, 118, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chung, M.N.; Lan, K.C.; Yang, R.S.; Liu, S.H. Exposure of low-concentration arsenic induces myotube atrophy by inhibiting an Akt signaling pathway. Toxicol. In Vitro. 2020, 65, 104829. [Google Scholar] [CrossRef]
- Chen, S.L.; Dzeng, S.R.; Yang, M.H.; Chiu, K.H.; Shieh, G.M.; Wai, C.M. Arsenic species in groundwaters of the blackfoot disease area. Taiwan Environ. Sci. Technol. 1994, 28, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Arsenic Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; Biological Report 85 (1.12); U.S. Fish and Wildlife Service: Laurel, MD, USA, 1988; pp. 4–6.
- O’Day, P.A. Chemistry and mineralogy of arsenic. Elements (Que) 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Jiménez-Capdeville, M.E.; Giordano, M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003, 145, 1–18. [Google Scholar] [CrossRef]
- Prakash, C.; Soni, M.; Kumar, V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016, 36, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H. Arsenic neurotoxicity in humans. Int. J. Mol. Sci. 2019, 20, 3418. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias, R.; Simmers, J.L.; Mi, R.; Park, J.S.; Ying, S.; Hoke, A.; Lee, S.J.; Cohn, R.D. Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis. Model Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, K.A.; Marshall, J.A.; Hokanson, J.E.; Meliker, J.R.; Zerbe, G.O.; Byers, T.E. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ. Res. 2013, 123, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yang, C.Y.; Chan, D.C.; Wang, C.C.; Huang, K.H.; Wu, C.C.; Tsai, K.S.; Yang, R.S.; Liu, S.H. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female Mice. Environ. Health Perspect. 2015, 123, 1138–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, C.B.; Storgaard, H.; Madsbad, S.; Richter, E.A.; Vaag, A.A. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J. Clin. Endocrinol. Metab. 2007, 92, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Umegaki, H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J. Diabetes Investig. 2015, 6, 623–624. [Google Scholar] [CrossRef]
- Jang, H.C. Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab. J. 2016, 40, 182–189. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladstone, J.N.; Bishop, J.Y.; Lo, I.K.; Flatow, E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am. J. Sports Med. 2007, 35, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Dilisio, M.F.; Agrawal, D.K. Immunobiological factors aggravating the fatty infiltration on tendons and muscles in rotator cuff lesions. Mol. Cell Biochem. 2016, 417, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Venkatanarasimha, N.; Walsh, M.A.; Hughes, P.M. MRI appearance of muscle denervation. Skeletal Radiol. 2008, 37, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Sishi, B.J.; Engelbrecht, A.M. Tumor necrosis factor alpha (TNF-α) inactivates the PI3-kinase/PKB pathway and induces atrophy and apoptosis in L6 myotubes. Cytokine 2011, 54, 173–184. [Google Scholar] [CrossRef]
- Rocchi, C.; Greco, V.; Urbani, A.; Di Giorgio, A.; Priori, M.; Massa, R.; Bernardi, G.; Marfia, G.A. Subclinical autonomic dysfunction in spinobulbar muscular atrophy (Kennedy disease). Muscle Nerve 2011, 44, 737–740. [Google Scholar] [CrossRef]
- Parodi, S.; Pennuto, M. Neurotoxic effects of androgens in spinal and bulbar muscular atrophy. Front. Neuroendocrinol. 2011, 32, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Storbeck, M.; Horsberg Eriksen, B.; Unger, A.; Hölker, I.; Aukrust, I.; Martínez-Carrera, L.A.; Linke, W.A.; Ferbert, A.; Heller, R.; Vorgerd, M.; et al. Phenotypic extremes of BICD2-opathies: from lethal, congenital muscular atrophy with arthrogryposis to asymptomatic with subclinical features. Eur. J. Hum. Genet. 2017, 25, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Fischmann, A.; Hafner, P.; Fasler, S.; Gloor, M.; Bieri, O.; Studler, U.; Fischer, D. Quantitative MRI can detect subclinical disease progression in muscular dystrophy. J. Neurol. 2012, 259, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Yang, R.S.; Sheu, M.L.; Chan, D.C.; Yang, T.H.; Tsai, K.S.; Chiang, C.K.; Liu, S.H. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 2016, 238, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jacques, N.; Parker, L.; Brown, P.; Dummer, T.J. Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environ. Health 2014, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- UNEP (United Nations Environment Programme). Water Global Environment Outlook-5 (GEO5); United Nations Environment Programme: Nairobi, Kenya, 2012; Volume 4, pp. 97–132. [Google Scholar]
- Mukherjee, S.C.; Rahman, M.M.; Chowdhury, U.K.; Sengupta, M.K.; Lodh, D.; Chanda, C.R.; Saha, K.C.; Chakraborti, D. Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 165–183. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-M.; Chung, M.-N.; Chiu, C.-Y.; Liu, S.-H.; Lan, K.-C. Inorganic Arsenic Exposure Decreases Muscle Mass and Enhances Denervation-Induced Muscle Atrophy in Mice. Molecules 2020, 25, 3057. https://doi.org/10.3390/molecules25133057
Chen C-M, Chung M-N, Chiu C-Y, Liu S-H, Lan K-C. Inorganic Arsenic Exposure Decreases Muscle Mass and Enhances Denervation-Induced Muscle Atrophy in Mice. Molecules. 2020; 25(13):3057. https://doi.org/10.3390/molecules25133057
Chicago/Turabian StyleChen, Chang-Mu, Min-Ni Chung, Chen-Yuan Chiu, Shing-Hwa Liu, and Kuo-Cheng Lan. 2020. "Inorganic Arsenic Exposure Decreases Muscle Mass and Enhances Denervation-Induced Muscle Atrophy in Mice" Molecules 25, no. 13: 3057. https://doi.org/10.3390/molecules25133057





