Flavonoids and Mitochondria: Activation of Cytoprotective Pathways?
Abstract
:1. Introduction
2. Flavonoids
3. Mitochondrial Pathways
4. Flavonoids in Mitochondrial Pathways
4.1. Flavonoids as Mitochondrial ROS Scavengers
4.2. Flavonoids Attenuate Mitochondrial ROS Formation
4.3. Antiapoptotic Substances
4.4. Influence on Mitochondrial Biogenesis
4.5. Mitochondrial Autophagy Regulators
4.6. Mitochondrial Fission and Fusion Control
4.7. Mitochondrial Ion Channel Openers
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.; Selker, H.; Thiele, H.; Patel, M.; Udelson, J.; Ohman, E.; Maehara, A.; Eitel, I.; CB, G.; PL, J.; et al. Relationship between infarct size and outcomes following primary PCI: Patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.; Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricker, M.; Tolkovsky, A.; Borutaite, V.; Coleman, M.; Brown, G. Neuronal cell death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Sosa-Ortiz, A.; Acosta-Castillo, I.; Prince, M. Epidemiology of dementias and Alzheimer’s disease. Arch. Med. Res. 2012, 43, 600–608. [Google Scholar] [CrossRef]
- Tysnes, O.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Vasileva, L.; Savova, M.; Amirova, K.; Dinkova-Kostova, A.; Georgiev, M. Obesity and NRF2-mediated cytoprotection: Where is the missing link. Pharm. Res. 2020, 156. [Google Scholar] [CrossRef]
- Lopes, J.E.; Leite, H.; Konstantyner, T. Selenium and selenoproteins: From endothelial cytoprotection to clinical outcomes. Transl. Res. J. Lab. Clin. Med. 2019, 208, 85–104. [Google Scholar] [CrossRef]
- Hakiminia, B.; Goudarzi, A.; Moghaddas, A. Has Vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 2019, 33, e22349. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Jacques, P.; McCullough, M. Improving the estimation of flavonoid intake for study of health outcomes. Nutr. Rev. 2015, 73, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.F.; Spencer, J. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondonno, N.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.; Lewis, J.; Croft, K.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid intake is associated with lower mortality in the danish diet cancer and health cohort. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol Vitr. 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals 2000, 13, 77–83. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules. Free Radic Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kappaB independent of NRF2-mediated mechanism. J. Nutr Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (eNOS) in vitro in human endothelial cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef]
- Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, Y.; Afaq, F.; Johnson, J.J.; Mukhtar, H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis 2009, 30, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Andrade Teles, R.; Diniz, T.; Costa Pinto, T.; De Oliveira Júnior, R.; Gama, E.; Silva, M.; De Lavor, É.; Fernandes, A.; De Oliveira, A.; De Almeida Ribeiro, F.; et al. Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–72. [Google Scholar] [CrossRef]
- McCullough, M.; Peterson, J.; Patel, R.; Jacques, P.; Shah, R.; Dwyer, J. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111. [Google Scholar] [CrossRef] [Green Version]
- Raman, G.; Avendano, E.; Chen, S.; Wang, J.; Matson, J.; Gayer, B.; Novotny, J.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiometabolic health: Systematic review and meta-analysis of randomized trials and prospective cohort studies. Am. J. Clin. Nutr. 2019, 110, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Kimble, R.; Keane, K.; Lodge, J.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3042. [Google Scholar] [CrossRef]
- Heiss, C.; Sansone, R.; Karimi, H.; Krabbe, M.; Schuler, D.; Rodriguez-Mateos, A.; Kraemer, T.; Cortese-Krott, M.; Kuhnle, G.; Spencer, J.; et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age 2015, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.; Geelen, A.; Kromhout, D. Dietary flavonol intake may lower stroke risk in men and women. J. Nutr. 2010, 140, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishtar, E.; Rogers, G.; Blumberg, J.; Au, R.; Jacques, P. Long-term dietary flavonoid intake and risk of alzheimer disease and related dementias in the framingham offspring cohort. Am. J. Clin. Nutr. 2020, nqaa079. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Protective effects of fisetin and other berry flavonoids in Parkinson’s disease. Food Funct. 2017, 8, 3033–3042. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Burton-Freeman, B.; Brzeziński, M.; Park, E.; Sandhu, A.; Xiao, D.; Edirisinghe, I. A Selective Role of Dietary Anthocyanins and Flavan-3-ols in Reducing the Risk of Type 2 Diabetes Mellitus: A review of recent evidence. Nutrients 2019, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Oteiza, P.; Fraga, C.; Mills, D.; Taft, D. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Marais, J.; Deavours, B.; Dixon, R.; Fereira, D. The Stereochemistry of Flavonoids; Grotewold, E., Ed.; Springer Press: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Xiao, L.; Carrillo, J.; Siemann, E.; Ding, J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. Aob Plants 2019, 11, 818–836. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1; US Department of Agriculture: Beltsville, MD, USA, 2014.
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, J.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.; et al. Phenol-explorer 3.0: A major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database J. Biol. Databases Curation 2013, 2013. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.J.; Jackson, T.D.; Stroud, D.A.; Stojanovski, D. Mitochondria-hubs for regulating cellular biochemistry: Emerging concepts and networks. Open Biol. 2019, 9, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Barrett, E.F. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J. Physiol. 2003, 548, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, A.; Szanda, G.; Akhmedov, D.; Mataki, C.; Heizmann, C.W.; Schoonjans, K.; Pozzan, T.; Spat, A.; Wollheim, C.B. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011, 13, 601–611. [Google Scholar] [CrossRef]
- Antony, A.N.; Paillard, M.; Moffat, C.; Juskeviciute, E.; Correnti, J.; Bolon, B.; Rubin, E.; Csordas, G.; Seifert, E.L.; Hoek, J.B.; et al. MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat. Commun. 2016, 7, 10955. [Google Scholar] [CrossRef]
- Cheng, J.; Liao, Y.; Zhou, L.; Peng, S.; Chen, H.; Yuan, Z. Amplified RLR signaling activation through an interferon-stimulated gene-endoplasmic reticulum stress-mitochondrial calcium uniporter protein loop. Sci. Rep. 2016, 6, 20158. [Google Scholar] [CrossRef] [Green Version]
- Collins, Y.; Chouchani, E.T.; James, A.M.; Menger, K.E.; Cocheme, H.M.; Murphy, M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012, 125, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Figueira, T.; Barros, M.; Camargo, A.; Castilho, R.; Ferreira, J.; Kowaltowski, A.; Sluse, F.; Souza-Pinto, N.; Vercesi, A. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2076. [Google Scholar] [CrossRef]
- Brand, M. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, D.F.; Johnson, S.C.; Villarin, J.J.; Chin, M.T.; Nieves-Cintron, M.; Chen, T.; Marcinek, D.J.; Dorn, G.W.; Kang, Y.J.; Prolla, T.A.; et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ. Res. 2011, 108, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Penicaud, L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuan-Pla, A.; Cervera, A.M.; Apostolova, N.; Garcia-Bou, R.; Victor, V.M.; Murphy, M.P.; McCreath, K.J. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005, 579, 2669–2674. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.K.; Cornelius, N.; Gregersen, N. Redox signalling and mitochondrial stress responses; lessons from inborn errors of metabolism. J. Inherit. Metab Dis. 2015, 38, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Peoples, J.; Saraf, A.; Ghazal, N.; Pham, T.; Kwong, J. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Oberst, A.; Bender, C.; Green, D.R. Living with death: The evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 2008, 15, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Del Re, D.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaseva, A.V.; Marchenko, N.D.; Ji, K.; Tsirka, S.E.; Holzmann, S.; Moll, U.M. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012, 149, 1536–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatokun, A.A.; Dawson, V.L.; Dawson, T.M. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharm. 2014, 171, 2000–2016. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef]
- Becker, T.; Wagner, R. Mitochondrial outer membrane channels: Emerging diversity in transport processes. Bioessays News Rev. Mol. Cell. Dev. Biol. 2018, 40, e1800013. [Google Scholar] [CrossRef]
- Palmieri, F.; Pierri, C. Mitochondrial metabolite transport. Essays Biochem. 2010, 47, 37–52. [Google Scholar] [CrossRef]
- Smith, C.; Nehrke, K.; Brookes, P. The slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. Biochem. J. 2017, 474, 2067–2094. [Google Scholar] [CrossRef]
- Szewczyk, A.; Bednarczyk, P.; Jędraszko, J.; Kampa, R.; Koprowski, P.; Krajewska, M.; Kucman, S.; Kulawiak, B.; Laskowski, M.; Rotko, D.; et al. Mitochondrial potassium channels—an overview. Postepy Biochem. 2018, 64, 196–212. [Google Scholar] [CrossRef]
- Gururaja Rao, S.; Ponnalagu, D.; Patel, N.; Singh, H. Three decades of chloride intracellular channel proteins: From organelle to organ physiology. Curr. Protoc. Pharm. 2018, 80. [Google Scholar] [CrossRef]
- Bachmann, M.; Pontarin, G.; Szabo, I. The Contribution of mitochondrial ion channels to cancer development and progression. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2019, 53, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Checchetto, V.; Azzolini, M.; Peruzzo, R.; Capitanio, P.; Leanza, L. Mitochondrial potassium channels in cell death. Biochem. Biophys. Res. Commun. 2018, 500, 51–58. [Google Scholar] [CrossRef]
- Krabbendam, I.; Honrath, B.; Culmsee, C.; Dolga, A. Mitochondrial Ca2+-activated K+ channels and their role in cell life and death pathways. Cell Calcium 2018, 69, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.; Paucek, P.; Yarov-Yarovoy, V.; Murray, H.; Darbenzio, R.; D’Alonzo, A.; Lodge, N.; Smith, M.; Grover, G. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997, 81, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Fryer, R. Sarcolemmal Versus Mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 1999, 84, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskowski, M.; Augustynek, B.; Kulawiak, B.; Koprowski, P.; Bednarczyk, P.; Jarmuszkiewicz, W.; Szewczyk, A. What do we not know about mitochondrial potassium channels? Biochim Biophys Acta 2016, 1857, 1247–1257. [Google Scholar] [CrossRef]
- Murry, C.; Jennings, R.; Reimer, K. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sato, T.; O’Rourke, B.; Marban, E. Mitochondrial ATP-dependent potassium channels: Novel effectors of cardioprotection. Circulation 1998, 97, 2463–2469. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, Q.; Wang, G.; Deng, T.; Chen, R.; Liu, M.; Wang, S. The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxia-ischemia-reperfusion in brain. Neurosci. Lett. 2011, 491, 63–67. [Google Scholar] [CrossRef]
- Grover, G.; Burkett, D.; Parham, C.; Scalese, R.; Sadanaga, K. Protective effect of mitochondrial KATP activation in an isolated gracilis model of ischemia and reperfusion in dogs. J. Cardiovasc. Pharm. 2003, 42, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Kuwata, Y.; Sakamoto, K.; Muraki, K.; Imaizumi, Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca(2+)-activated K(+) channels in cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1635–H1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Liu, Y.; Wang, S.; McDonald, T.; Van Eyk, J.E.; Sidor, A.; O’Rourke, B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science 2002, 298, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Marbán, E. Mitochondria: A new target for K channel openers. Trends Pharm. Sci. 1999, 20, 157–161. [Google Scholar] [CrossRef]
- Borchert, G.; Yang, C.; Kolár, F. Mitochondrial BKCa channels contribute to protection of cardiomyocytes isolated from chronically hypoxic rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H507–H513. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Guo, X.; Jiao, F.; Liu, X.; Liu, Y. Activation of large-conductance Ca(2+)-activated K(+) channels inhibits glutamate-induced oxidative stress through attenuating ER stress and mitochondrial dysfunction. Neurochem. Int. 2015, 90, 28–35. [Google Scholar] [CrossRef]
- Heinen, A.; Aldakkak, M.; Stowe, D.; Rhodes, S.; Riess, M.; Varadarajan, S.; Camara, A. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am. J. Physiol. Heart. Circ. Physiol. 2007, 293, H1400–H1407. [Google Scholar] [CrossRef] [Green Version]
- Facundo, H.; De Paula, J.; Kowaltowski, A. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic. Biol. Med. 2007, 42, 1039–1048. [Google Scholar] [CrossRef]
- Kulawiak, B.; Kudin, A.; Szewczyk, A.; Kunz, W. BK Channel openers inhibit ROS production of isolated rat brain mitochondria. Exp. Neurol. 2008, 212, 543–547. [Google Scholar] [CrossRef]
- Chouchani, E.; Pell, V.; Gaude, E.; Aksentijević, D.; Sundier, S.; Robb, E.; Logan, A.; Nadtochiy, S.; Ord, E.; Smith, A.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Akao, M.; O’Rourke, B.; Marbán, E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: Possible mechanism of cardioprotection. Circ. Res. 2001, 89, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facundo, H.; Fornazari, M.; Kowaltowski, A. Tissue protection mediated by mitochondrial K+ channels. Biochim. Et Biophys. Acta 2006, 1762, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, J.; Pallardo, F.V.; Vina, J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. Iubmb Life 2000, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Takabe, W.; Niki, E.; Uchida, K.; Yamada, S.; Satoh, K.; Noguchi, N. Oxidative stress promotes the development of transformation: Involvement of a potent mutagenic lipid peroxidation product, acrolein. Carcinogenesis 2001, 22, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001, 488, 65–76. [Google Scholar] [CrossRef]
- Gracy, R.W.; Talent, J.M.; Kong, Y.; Conrad, C.C. Reactive oxygen species: The unavoidable environmental insult? Mutat Res. 1999, 428, 17–22. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med. Cell Longev. 2016, 1245049. [Google Scholar] [CrossRef]
- Sichel, G.; Corsaro, C.; Scalia, M.; Di Bilio, A.J.; Bonomo, R.P. In vitro scavenger activity of some flavonoids and melanins against O2−dot. Free Radic Biol Med. 1991, 11, 1–8. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic Biol Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Fraga, C. Plant polyphenols: How to translate their in vitro antioxidant actions to in vivo conditions. Iubmb Life 2007, 59, 308–315. [Google Scholar] [CrossRef]
- Miyagi, Y.; Miwa, K.; Inoue, H. Inhibition of human low-density lipoprotein oxidation by flavonoids in red wine and grape juice. Am. J. Cardiol. 1997, 80, 1627–1631. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastianetto, S.; Quirion, R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging 2002, 23, 891–897. [Google Scholar] [CrossRef]
- Esselun, C.; Bruns, B.; Hagl, S.; Grewal, R.; Eckert, G. Differential effects of silibinin a on mitochondrial function in neuronal PC12 and HepG2 liver cells. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Dudylina, A.; Ivanova, M.; Shumaev, K.; Ruuge, E. Superoxide formation in cardiac mitochondria and effect of phenolic antioxidants. Cell Biochem. Biophys. 2019, 77, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assuncao, M.; Santos-Marques, M.J.; Carvalho, F.; Andrade, J.P. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic. Biol. Med. 2010, 48, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.; Blasina, F.; Antúnez, K.; Jones, D.; Go, Y.; Liang, Y.; Dajas, F. After Cellular internalization, quercetin causes Nrf2 nuclear translocation, increases Glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Schaffer, S.; Asseburg, H.; Kuntz, S.; Muller, W.; Eckert, G. Effects of polyphenols on brain ageing and Alzheimer’s disease: Focus on mitochondria. Mol. Neurobiol. 2012, 46, 161–178. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, H.X.; Jia, A.; Jia, S.S.; Yuan, K. The Protective effect of the total flavonoids of abelmoschus esculentus l. Flowers on transient cerebral ischemia-reperfusion injury is due to activation of the Nrf2-ARE pathway. Oxid Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arulselvan, P.; Fard, M.; Tan, W.; Gothai, S.; Fakurazi, S.; Norhaizan, M.; Kumar, S. Role of antioxidants and natural products in inflammation. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Xie, S.; Saw, W.; Ho, P.; Wang, H.; Lei, Z.; Yi, Z.; Tan, E. The therapeutic implications of tea polyphenols against dopamine (DA) neuron degeneration in Parkinson’s disease (PD). Cells 2019, 8, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- McAnlis, G.; McEneny, J.; Pearce, J.; Young, I. Absorption and antioxidant effects of quercetin from onions, in man. Eur. J. Clin. Nutr. 1999, 53, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zielonka, J.; McAllister, D.; Hardy, M.; Ouari, O.; Joseph, J.; Dwinell, M.; Kalyanaraman, B. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015, 365, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Khodr, H.; Hider, R.C.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem, J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef]
- Hodnick, W.; Bohmont, C.; Capps, C.; Pardini, R. Inhibition of the mitochondrial NADH-oxidase (NADH-coenzyme Q oxido-reductase) enzyme system by flavonoids: A structure-activity study. Biochem. Pharm. 1987, 36, 2873–2874. [Google Scholar] [CrossRef]
- Hodnick, W.; Duval, D.; Pardini, R. Inhibition of mitochondrial respiration and cyanide-stimulated generation of reactive oxygen species by selected flavonoids. Biochem. Pharm. 1994, 47, 573–580. [Google Scholar] [CrossRef]
- Dabaghi-Barbosa, P.; Mariante Rocha, A.; Franco da Cruz Lima, A.; Heleno de Oliveira, B.; Benigna Martinelli de Oliveira, M.; Gunilla Skare Carnieri, E.; Cadena, S.; Eliane Merlin Rocha, M. Hispidulin: Antioxidant properties and effect on mitochondrial energy metabolism. Free Radic. Res. 2005, 39, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Herrerias, T.; de Oliveira, B.; Gomes, M.; de Oliveira, M.; Carnieri, E.; Cadena, S.; Martinez, G.; Rocha, M. Eupafolin: Effect on mitochondrial energetic metabolism. Bioorganic Med. Chem. 2008, 16, 854–861. [Google Scholar] [CrossRef]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Et Biophys. Acta 2011, 1807, 1562–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, D.; Bombicino, S.; Boveris, A.; Valdez, L. (+)-catechin inhibits heart mitochondrial complex i and nitric oxide synthase: Functional consequences on membrane potential and hydrogen peroxide production. Food Funct. 2019, 10, 2528–2537. [Google Scholar] [CrossRef]
- Sharikadze, N.; Jojua, N.; Sepashvili, M.; Zhuravliova, E.; Mikeladze, D. Mitochondrial target of nobiletin’s action. Nat. Prod. Commun. 2016, 11, 1833–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Apple peel polyphenol extract protects against indomethacin-induced damage in Caco-2 cells by preventing mitochondrial complex i inhibition. J. Agric. Food Chem. 2011, 59, 11501–11508. [Google Scholar] [CrossRef]
- Dorta, D.; Pigoso, A.; Mingatto, F.; Rodrigues, T.; Prado, I.; Helena, A.; Uyemura, S.; Santos, A.; Curti, C. The interaction of flavonoids with mitochondria: Effects on energetic processes. Chem. -Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and related chromenone derivatives as monoamine oxidase inhibitors: Targeting neurological and mental disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Garg, V.; Tuli, H.; Yerer, M.; Sak, K.; Sharma, A.; Kumar, M.; Aggarwal, V.; Sandhu, S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in neuroprotection by phytochemicals: Bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, C.; Jiang, W.; Zheng, R.; He, C.; Li, J.; Xing, J. Cardioprotection of tilianin ameliorates myocardial ischemia-reperfusion injury: Role of the apoptotic signaling pathway. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Fang, F.; Li, D.; Pan, H.; Chen, D.; Qi, L.; Zhang, R.; Sun, H. Luteolin inhibits apoptosis and improves cardiomyocyte contractile function through the PI3K/Akt pathway in simulated ischemia/reperfusion. Pharmacology 2011, 88, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tinay, I.; Sener, T.; Cevik, O.; Cadirci, S.; Toklu, H.; Cetinel, S.; Sener, G.; Tarcan, T. Antioxidant agent quercetin prevents impairment of bladder tissue contractility and apoptosis in a rat model of ischemia/reperfusion injury. Low. Urin. Tract Symptoms 2017, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, P.; Yang, A.; Chiang, S.; Tang, C.; Tseng, K.; Huang, C. Baicalein attenuates lung injury induced by myocardial ischemia and reperfusion. Am. J. Chin. Med. 2017, 45, 791–811. [Google Scholar] [CrossRef]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. The antioxidant, anti-inflammatory and anti-apoptotic activities of the Bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2016, 38, 1365–1375. [Google Scholar] [CrossRef]
- Yang, T.; Kong, B.; Gu, J.; Kuang, Y.; Cheng, L.; Yang, W.; Xia, X.; Shu, H. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell. Mol. Neurobiol. 2014, 34, 797–804. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, T.; Su, J.; Zhao, Y.; Chenchen, N.; Wang, N.; Li, X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 2017, 40, 157–162. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, J.; Huang, L.; Luo, N.; Liang, X.; Liang, M.; Xie, W. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clin. Exp. Pharm. Physiol. 2017, 44, 862–871. [Google Scholar] [CrossRef]
- Bournival, J.; Quessy, P.; Martinoli, M. Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zheng, C.; Cai, W.; Cheng, J.; Wang, H.; Li, H.; Sun, Y.; Cui, W.; Wang, Y.; Han, Y.; et al. Multifunction of chrysin in parkinson’s model: Anti-neuronal apoptosis, neuroprotection via activation of MEF2D, and inhibition of monoamine oxidase-B. J. Agric. Food Chem. 2016, 64, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.; Zhu, X.; Wang, Y.; Wu, W.; Zhang, X. Protective effects of hesperidin against amyloid-β (Aβ) induced neurotoxicity through the voltage dependent anion channel 1 (VDAC1)-mediated mitochondrial apoptotic pathway in PC12 cells. Neurochem. Res. 2013, 38, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Pei, H.; Huang, Y.; Li, Y. (−)-Epigallocatechin-3-gallate inhibits arsenic-induced inflammation and apoptosis through suppression of oxidative stress in mice. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Gong, X.; Huang, L.; Wang, Z.; Wan, R.; Zhang, P.; Zhang, Q.; Chen, Z.; Zhang, B. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723–30733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420. [Google Scholar] [CrossRef]
- Zare, M.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019, 232. [Google Scholar] [CrossRef]
- Malik, S.; Bhatia, J.; Suchal, K.; Gamad, N.; Dinda, A.; Gupta, Y.; Arya, D. Nobiletin ameliorates cisplatin-induced acute kidney injury due to its anti-oxidant, anti-inflammatory and anti-apoptotic effects. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2015, 67, 427–433. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, G.; Sun, B.; Wu, Y.; He, L.; Wang, X.; Chen, R.; Cao, L.; Ren, X.; Sun, X. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology 2012, 292, 53–62. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef]
- Simmons, E.; Scholpa, N.; Schnellmann, R. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp. Neurol. 2020, 329, 113309. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Liu, Y.; Liu, X.; Zhu, L.; Cui, Y.; Cui, A.A.Q.; Kong, X.; Liu, Y.; Chen, Q. PGC-1 Beta-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR alpha. Mitochondrion 2010, 10, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Salma, N.; Song, J.; Arany, Z.; Fisher, D. Transcription factor Tfe3 directly regulates Pgc-1alpha in muscle. J. Cell. Physiol. 2015, 230, 2330–2336. [Google Scholar] [CrossRef] [Green Version]
- Rasbach, K.; Schnellmann, R. Isoflavones promote mitochondrial biogenesis. J. Pharm. Exp. 2008, 325, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.; Murphy, E.; Carmichael, M.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.; Williams, A.; Shanely, R.; Jin, F.; McAnulty, S.; Triplett, N.; Austin, M.; Henson, D. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayamajhi, N.; Kim, S.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.; Ryter, S.; Chung, H. Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxidative Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Naka, A.; Sakamoto, Y.; Shibasaki, A.; Toh, M.; Tsukamoto, S.; Kondo, K.; Iida, K. Dietary isoflavone daidzein promotes tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J. Nutr. Biochem. 2015, 26, 1193–1199. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.; Ryu, H.; Choi, B.; Go, Y.; Lee, N.; Lee, D.; Son, H.; Jeon, J.; Kim, S.; et al. Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1α. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y. Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef] [Green Version]
- Kou, G.; Li, Z.; Wu, C.; Liu, Y.; Hu, Y.; Guo, L.; Xu, X.; Zhou, Z. Citrus tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK-PGC1-α pathway in vitro and in vivo: A possible mechanism for its beneficial effect on physical performance. J. Agric. Food Chem. 2018, 66, 11917–11925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, L.; Zhang, W.; Yang, Y.; Zhou, Q.; Du, G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, L.; Wu, Y.; Song, S.; Min, H.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Wei, L.; Sun, X.; Qi, X.; Zhang, Y.; Li, Y.; Xu, Y. Dihydromyricetin ameliorates cardiac ischemia/reperfusion injury through Sirt3 activation. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, H.; Wen, G.; Li, L.; Gao, Y.; Zhuang, Z.; Zhou, M.; Mao, L.; Fan, Y. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1α pathway as a potential mechanism. J. Cell. Mol. Med. 2018, 22, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youle, R.; Narendra, D. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Zhang, S.; Sun, J.; Liu, P.; Xiao, L.; Tang, Y.; Liu, L.; Yao, P. Quercetin attenuates chronic ethanol-induced hepatic mitochondrial damage through enhanced mitophagy. Nutrients 2016, 8, 27. [Google Scholar] [CrossRef]
- Liu, P.; Lin, H.; Xu, Y.; Zhou, F.; Wang, J.; Liu, J.; Zhu, X.; Guo, X.; Tang, Y.; Yao, P. Frataxin-mediated PINK1-parkin-dependent mitophagy in hepatic steatosis: The protective effects of quercetin. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Song, S.; Wang, L.; Liang, Q.; Wang, Y.; Wu, Y.; Gao, Q. Puerarin attenuates palmitate-induced mitochondrial dysfunction, impaired mitophagy and inflammation in L6 myotubes. Life Sci. 2018, 206, 84–92. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Lu, S.; Li, W.; Yang, D.; Su, W.; Wang, X.; Shen, J. Naringin attenuates cerebral ischemia-reperfusion injury through inhibiting peroxynitrite-mediated mitophagy activation. Mol. Neurobiol. 2018, 55, 9029–9042. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, F.; Ryan, M. The constriction and scission machineries involved in mitochondrial fission. J. Cell Sci. 2017, 130, 2953–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernas, L.; Scorrano, L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zou, D.; Yi, L.; Chen, M.; Gao, Y.; Zhou, R.; Zhang, Q.; Zhou, Y.; Zhu, J.; Chen, K.; et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor. Neurol. Neurosci. 2015, 33, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vasc. Pharm. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Chen, C.; Huang, J.; Shen, J.; Bai, Q. Quercetin improves endothelial insulin sensitivity in obese mice by inhibiting drp1 phosphorylation at serine 616 and mitochondrial fragmentation. Acta Biochim. Et Biophys. Sin. 2019, 51, 1250–1257. [Google Scholar] [CrossRef]
- Parrado-Fernández, C.; Sandebring-Matton, A.; Rodriguez-Rodriguez, P.; Aarsland, D.; Cedazo-Mínguez, A. Anthocyanins protect from complex i inhibition and APPswe mutation through modulation of the mitochondrial fission/fusion pathways. Biochim. Et Biophys. Acta 2016, 1862, 2110–2118. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.; Chen, B.; Wang, F.; Yang, Q.; Chen, X. Grape seed proanthocyanidins prevent irradiation-induced differentiation of human lung fibroblasts by ameliorating mitochondrial dysfunction. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Sun, X.; Xu, L.; Sun, R.; Ma, Z.; Deng, X.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Baicalin attenuates in vivo and in vitro hyperglycemia-exacerbated ischemia/reperfusion injury by regulating mitochondrial function in a manner dependent on AMPK. Eur. J. Pharm. 2017, 815, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Luo, H.; Zhou, X.; Cheng, C.; Lin, L.; Liu, B.; Liu, K.; Li, P.; Yang, H. Succinate-induced neuronal mitochondrial fission and hexokinase ii malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin attenuates dexamethasone-induced muscle atrophy by improving mitochondrial function via the PGC-1α pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2018, 49, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Son, E.; Kim, S.; Ryter, S.; Yeo, E.; Kyung, S.; Kim, Y.; Jeong, S.; Lee, C.; Park, J. Quercetogetin protects against cigarette smoke extract-induced apoptosis in epithelial cells by inhibiting mitophagy. Toxicol. Vitr. 2018, 48, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Pan, H.; Qiu, S.; Lu, Y.; Bruce, I.; Luo, J.; Xia, Q. Atractyloside and 5-hydroxydecanoate block the protective effect of puerarin in isolated rat heart. Life Sci. 2006, 79, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, N.; Tissier, R.; Pons, S.; Chenoune, M.; Waintraub, X.; Berdeaux, A.; Ghaleh, B. The ceiling effect of pharmacological postconditioning with the phytoestrogen genistein is reversed by the gsk3beta inhibitor SB 216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione] through mitochondrial ATP-dependent potassium channel opening. J. Pharm. Exp. 2009, 329, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Li, L.; Yin, W.; Shen, L.; You, B.; Gao, H. Protective effect of proanthocyanidins on anoxia-reoxygenation injury of myocardial cells mediated by the PI3K/Akt/GSK-3β pathway and mitochondrial ATP-sensitive potassium channel. Mol. Med. Rep. 2014, 10, 2051–2058. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Ma, H.; Guo, H.; Kong, Q.; Zhang, Y. The cardioprotective effect of naringenin against ischemia-reperfusion injury through activation of ATP-sensitive potassium channel in rat. Can. J. Physiol. Pharm. 2016, 94, 973–978. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Marino, A.; D’Antongiovanni, V.; Ciregia, F.; Giusti, L.; Lucacchini, A.; Chericoni, S.; Breschi, M.; Calderone, V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem. Pharm. 2013, 85, 1634–1643. [Google Scholar] [CrossRef]
- Testai, L.; Da Pozzo, E.; Piano, I.; Pistelli, L.; Gargini, C.; Breschi, M.; Braca, A.; Martini, C.; Martelli, A.; Calderone, V. The citrus flavanone naringenin produces cardioprotective effects in hearts from 1 year old rat, through activation of mitoBK channels. Front. Pharm. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Kampa, R.P.; Kicinska, A.; Jarmuszkiewicz, W.; Pasikowska-Piwko, M.; Dolegowska, B.; Debowska, R.; Szewczyk, A.; Bednarczyk, P. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Derm. 2019, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Kampa, R.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the mitochondrial BKCa channel by the citrus flavonoid naringenin as a potential means of preventing cell damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip Toxicol 2011, 4, 173–183. [Google Scholar] [CrossRef] [PubMed]
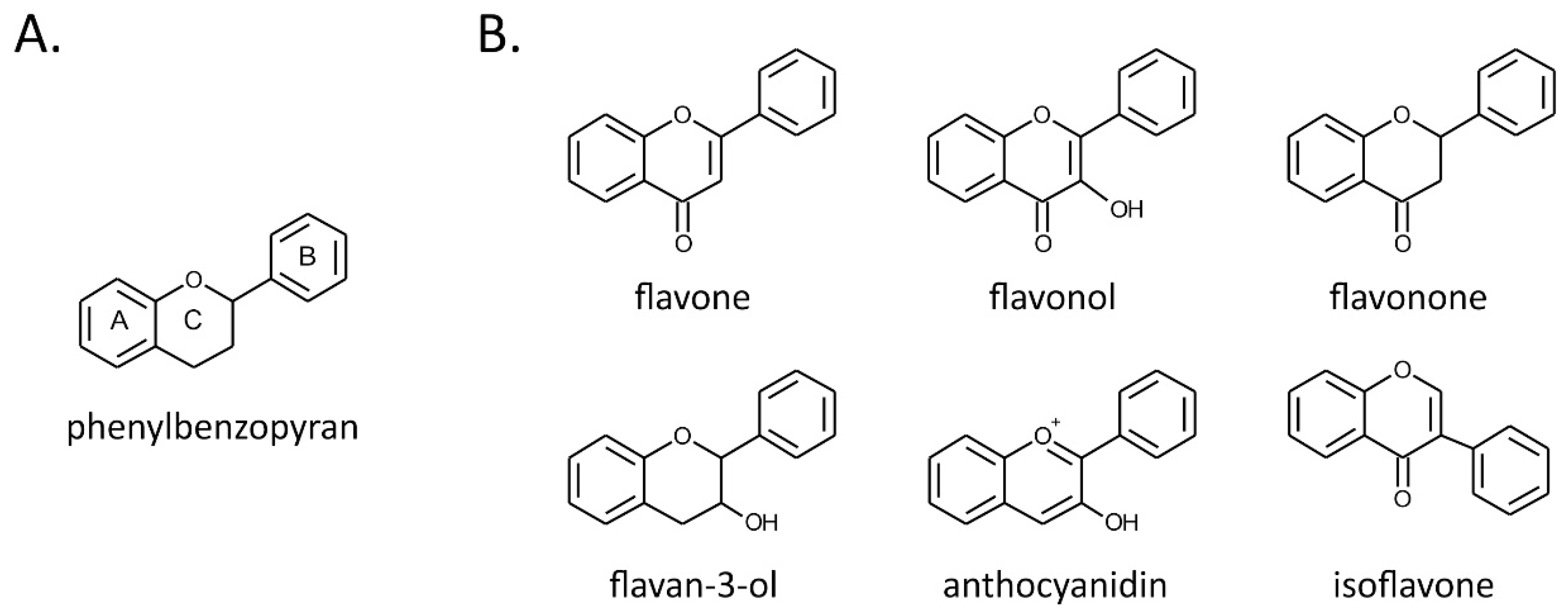
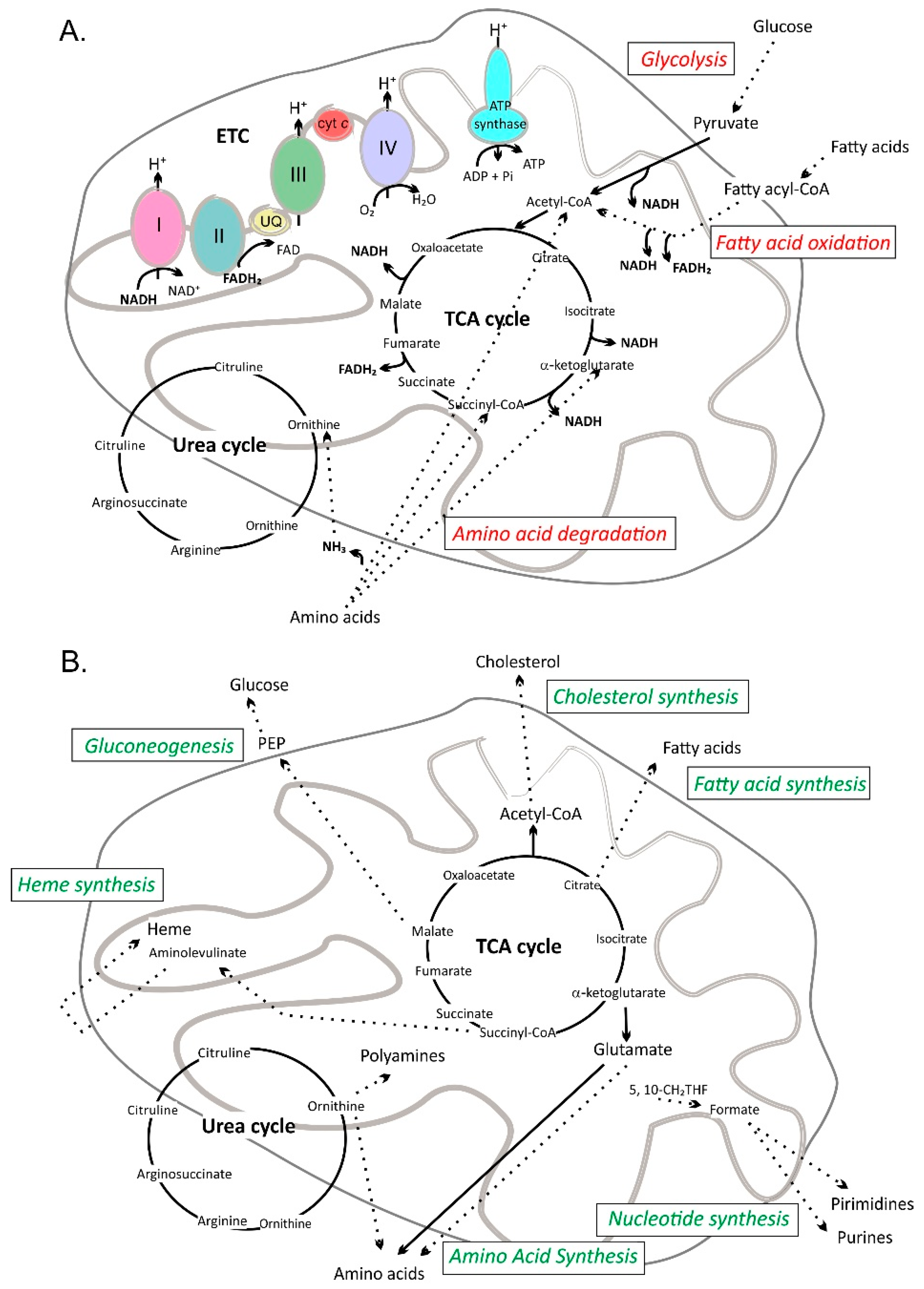
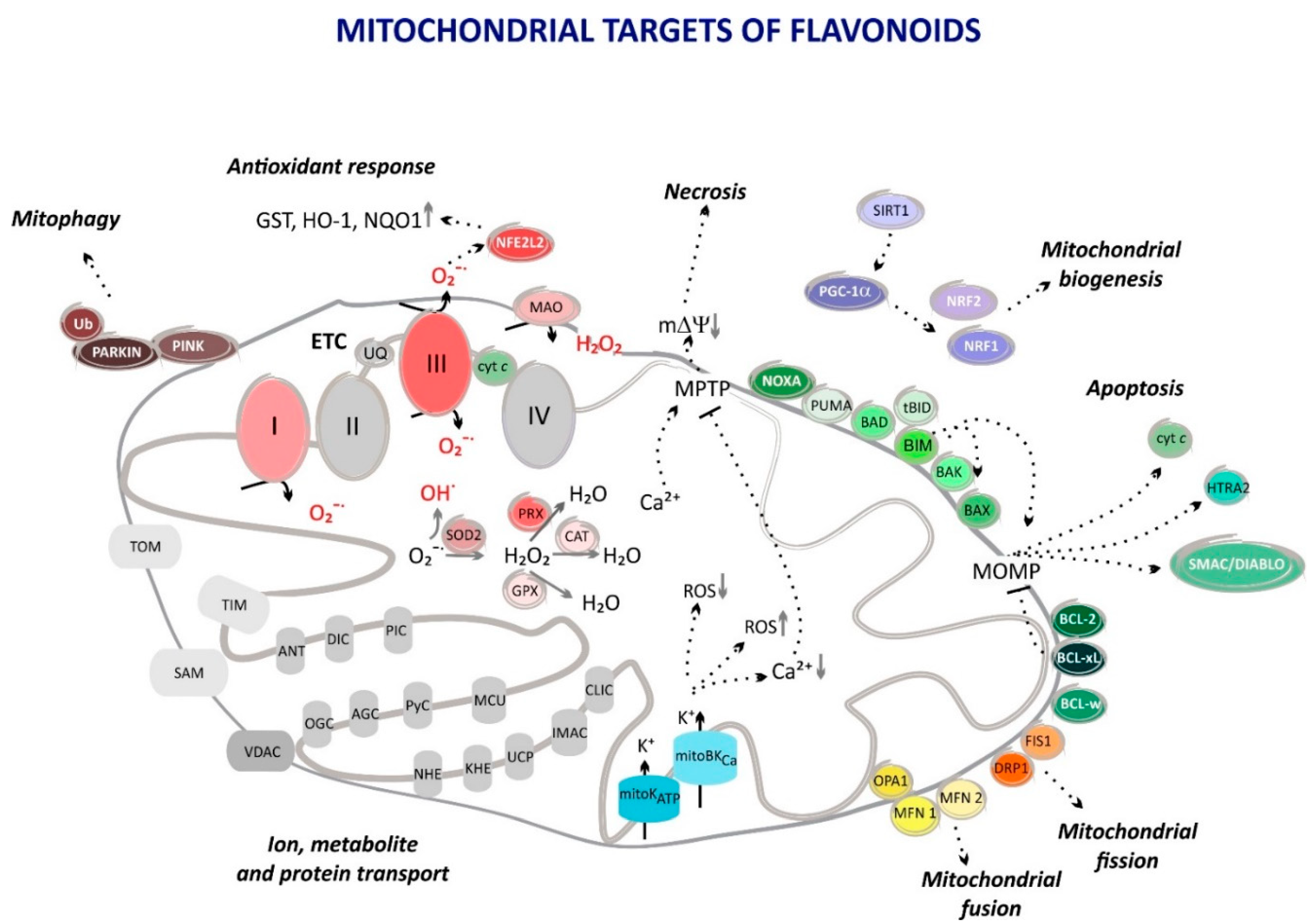
| Flavonoid Class | Selected Compounds | Examples of Dietary Sources |
|---|---|---|
| Flavanones | Hesperetin | Oranges, tangelo, lemons, limes |
| Naringenin | Grapefruit, pomelo, kumquats, oregano | |
| Eriodictyol | Oregano, peppermint, oranges, lemons | |
| Flavones | Apigenin | Parsley, celery, kumquats |
| Baicalein | Welsh onion, Chinese skullcap | |
| Luteolin | Peppers, radicchio, oregano, celery seed | |
| Tangeretin | Tangerines, sweet oranges | |
| Flavonols | Fisetin | Strawberry, apple, persimmon, grape, onion |
| Kaempferol | Capers, saffron, arugula, chard, chives | |
| Myricetin | Cranberries, goji berry | |
| Quercetin | Capers, elderberry, chokeberry | |
| Flavan-3-ols | Catechin | Cocoa, green tea, blueberries, blackberries |
| Epicatechin | Cocoa, green tea, grapes, red wine | |
| Epigallocatechin | Green tea, apples, plums, nuts | |
| Anthocyanidins | Cyanidin | Chokeberries, elderberries, blackberries, red cabbage |
| Delphinidin | Blackcurrants, blueberries, grapes | |
| Pelargonidin | Strawberries, radishes | |
| Isoflavones | Genistein | Soy, red clover, alfalfa |
| Daidzein | Soy, nuts | |
| Glycitein | Soy |
| Flavonoid | Cytotoxicity Model | Cytoprotective Pathway Induced | Reference |
|---|---|---|---|
| Baicalein | Oxidative stress | Iron chelation, ROS scavenging, Inhibition of complex I of ETC | [114] [132,133] |
| I/R | Inhibition of apoptosis (Bcl-2 family proteins) | [148] | |
| Parkinson’s disease model | Mitogenesis | [174] | |
| Catechins | Oxidative stress | ↑ Antioxidant enzyme transcription Inhibition of complex I of ETC | [118,119] [136,137] |
| Arsenic | Inhibition of apoptosis (Bcl-2 family proteins) | [156] | |
| Kaempferol | Oxidative stress | Inhibition of complex I of ETC | [136] |
| Doxorubicin-induced cardiotoxicity | Inhibition of apoptosis (Bcl-2 family proteins) | [161] | |
| Acute rotenone toxicity | Mitophagy | [180] | |
| Ischemic stroke model | Suppression of fission | [194] | |
| Luteolin | I/R | Inhibition of apoptosis (Bcl-2 family proteins) | [146] |
| Oxidative stress | Inhibition of complex I of ETC | [136] | |
| Naringenin | Ischemic stroke | Inhibition of apoptosis (Bcl-2 family proteins) | [152] |
| I/R | Activation of mitochondrial potassium channels | [200,201,202,203] | |
| TNF-α/CHX | Activation of mitochondrial potassium channels | [204] | |
| Quercetin | Oxidative stress | Direct ROS scavenging from complex III of ETC, ↑ Antioxidant enzyme transcription Inhibition of complex I of ETC Inhibition of apoptosis (Bcl-2 family proteins) | [117] [120] [136] [153] |
| I/R injury | Inhibition of apoptosis (Bcl-2 family proteins) | [147,150] | |
| Osteoarthritis | Mitogenesis | [176] | |
| Traumatic brain injury | Mitogenesis | [178] | |
| Chronic ethanol treatment | Mitophagy | [181] | |
| Non-alcoholic fatty liver disease model | Mitophagy | [182] | |
| Acute hypobaric hypoxia | Mitogenesis, inhibition of fission | [188] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. https://doi.org/10.3390/molecules25133060
Kicinska A, Jarmuszkiewicz W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules. 2020; 25(13):3060. https://doi.org/10.3390/molecules25133060
Chicago/Turabian StyleKicinska, Anna, and Wieslawa Jarmuszkiewicz. 2020. "Flavonoids and Mitochondria: Activation of Cytoprotective Pathways?" Molecules 25, no. 13: 3060. https://doi.org/10.3390/molecules25133060
APA StyleKicinska, A., & Jarmuszkiewicz, W. (2020). Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules, 25(13), 3060. https://doi.org/10.3390/molecules25133060






