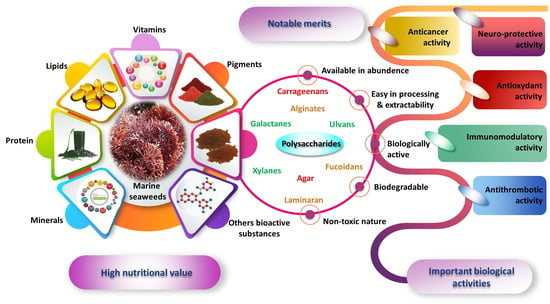
Bioactive Polysaccharides from Seaweeds
Abstract
:1. Introduction
2. Marine-Algal Bioactive Compounds
2.1. Pigments and Phenolic Compounds
2.2. Lipids and Proteins
2.3. Vitamins and Minerals
2.4. Carbohydrates
3. Main Structural Features of Algal Polysaccharides
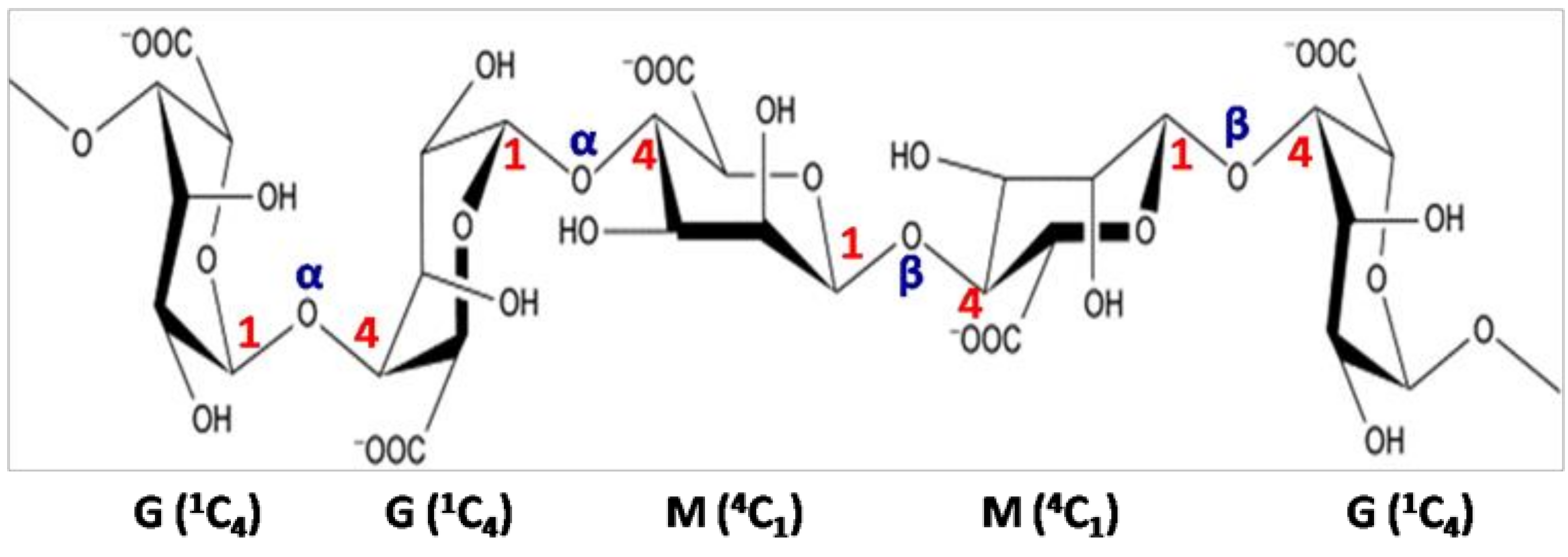
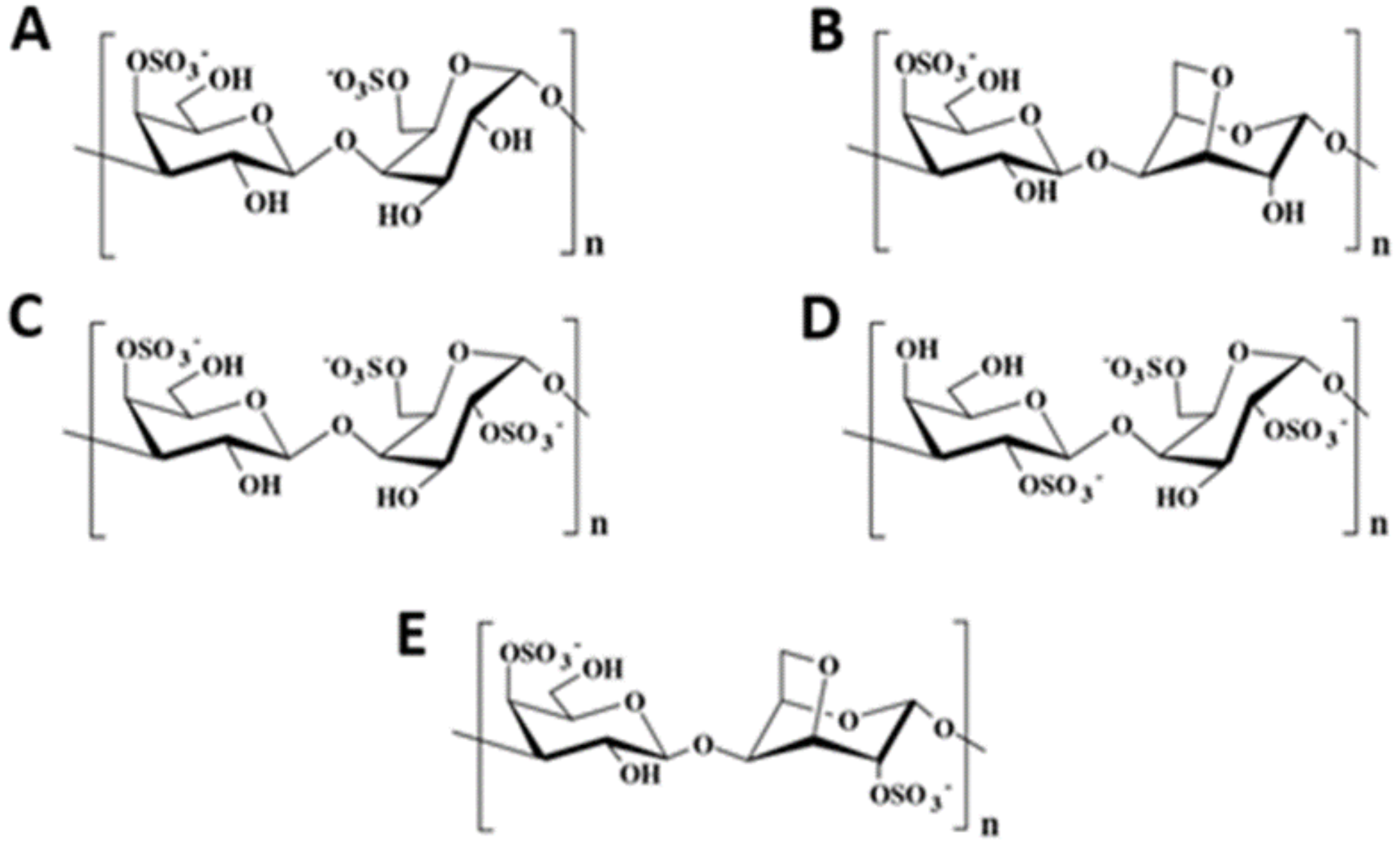
3.1. Brown Seaweed Polysaccharides
3.2. Red Seaweed Polysaccharides
3.3. Green Seaweed Polysaccharides
4. Health Claims of Algal Polysaccharides
4.1. Antioxidant Activity
4.2. Anticoagulant and Antithrombotic Activities
4.3. Anticancer and Antitumor Activities
4.4. Immunomodulatory Property
4.5. Neuroprotective Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Academic Press: London, UK, 2016; pp. 223–247. [Google Scholar]
- The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2016; p. 205.
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability; Academic Press: London, UK, 2015; pp. 7–25. [Google Scholar]
- Zubia, M.; Mattio, L. Macroalgues tropicales: Une ressource durable d’avenir. Tech. De L’ingénieurs 2019, 9040, 9040. [Google Scholar]
- Buschmann, A.H. The need for a balanced ecosystem approach to blue revolution aquaculture. Environment: Sci. Policy Sustain. Dev. 2017, 49, 36–43. [Google Scholar]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aumeerun, S.; Soulange-Govinden, J.; Driver, M.F.; Rao, A.R.; Ravishankar, G.A.; Neetoo, H. Macroalgae and Microalgae. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; p. 207. [Google Scholar]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Butt, M.S.; Amjad, N.; Yasmin, I.; Suleria, H.A.R. Marine-Algal Bioactive Compounds: A Comprehensive Appraisal. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 71–80. [Google Scholar]
- Cui, K.; Tai, W.; Shan, X.; Hao, J.; Li, G.; Yu, G. Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens. Int. J. Biol. Macromol. 2018, 120, 1817–1822. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for extraction of bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Becerra, M.; Boutefnouchet, S.; Córdoba, O.; Vitorino, G.P.; Brehu, L.; Lamour, I.; Laimay, F.; Efstathiou, A.; Smirlis, D.; Michel, S.; et al. Antileishmanial activity of fucosterol recovered from Lessonia vadosa Searles (Lessoniaceae) by SFE, PSE and CPC. Phytochem. Lett. 2015, 11, 418–423. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of Galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Choi, I.W.; Qian, Z.J.; Heo, S.J.; Kang, D.H.; Oh, C.; Jeon, Y.J.; Jang, C.H.; Park, W.S.; Kang, K.H.; et al. Antiinflammatory effect of polyphenol-rich extract from the red alga Callophyllis japonica in lipopolysaccharide-induced RAW 264.7 macrophages. Algae 2014, 29, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jager, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of alpha-glucosidase inhibitors by combined use of high resolution alpha-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Jebali, A.; Karray, F.; Hassairi, I.; Abdelkafi, S.; Ayadi, H.; Sayadi, S.; Dhouib, A. Characterization of Amphora sp.; a newly isolated wild strain potentially usable for biodiesel production. Bioprocess Biosyst. Eng. 2015, 29, 1381–1392. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds-A review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Fitz Gerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Černá, M. Seaweed proteins and amino acids as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 297–312. [Google Scholar]
- Admassu, H.; Zhao, W.; Yang, R.; Gasmalla, M.; Alsir, E. Development of functional foods: Seaweeds (algae) untouched potential and alternative resource - a review. Int. J. Sci. Technol. Res. 2015, 4, 108–115. [Google Scholar]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Hentati, F.; Barkallah, M.; Ben Atitallah, A.; Dammak, M.; Louati, I.; Pierre, G.; Fendri, I.; Attia, H.; Michaud, P.; Abdelkafi, S. Quality characteristics and functional and antioxidant capacities of algae-fortified fish burgers prepared from common barbel (Barbus barbus). Biomed Res. Int. 2019, 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Smith, J.L.; Summers, G.; Wong, R. Nutrient and heavy metal content of edible seaweeds in New Zealand. New Zealand J. Crop Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds 1. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in central west coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed Sargassum naozhouense Tseng et Lu. Mar. Drugs 2013, 11, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parjikolaei, B.R.; Bruhn, A.; Eybye, K.L.; Larsen, M.M.; Rasmussen, M.B.; Christensen, K.V.; Fretté, X.C. Valuable biomolecules from nine north Atlantic red macroalgae: Amino acids, fatty acids, carotenoids, minerals and metals. Nat. Resour. 2016, 7, 157–183. [Google Scholar] [CrossRef] [Green Version]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Fayaz, M.; Namitha, K.K.; Murthy, K.C.; Swamy, M.M.; Sarada, R.; Khanam, S.; Subbaraeo, P.V.; Ravishankar, G.A. Chemical composition, iron bioavailability, and antioxidant activity of Kappaphycus alvarezzi (Doty). J. Agric. Food Chem. 2005, 53, 792–797. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Kokilam, G.; Vasuki, S. Biochemical and phytochemical analysis on Ulva fasciata and Caulerpa taxifolia. Int. J. Pharm. Sci. Res. 2014, 4, 7–11. [Google Scholar]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Ratana-arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Agric. Nat. Resour. 2006, 40 (Suppl.), 75–83. [Google Scholar]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae, Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.J.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef]
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [Green Version]
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical composition and potential use of Fucus vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural features and rheological properties of a sulfated xylogalactan-rich fraction isolated from tunisian red seaweed. Jania adhaerens. Appl. Sci. 2020, 10, 1655. [Google Scholar] [CrossRef] [Green Version]
- Kraan, S. Algal Polysaccharides, Novel applications and outlook. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012; Chapter 22; pp. 489–524. [Google Scholar]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyrin isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Nakazono, S.; Cho, K.; Isaka, S.; Abu, R.; Yokose, T.; Murata, M.; Ueno, M.; Tachibana, K.; Hirasaka, K.; Kim, D.; et al. Anti-obesity effects of enzymatically-digested alginate oligomer in mice model fed a high-fat-diet. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Deng, Z.; Geng, L.; Wang, J.; Zhang, Q. In vitro evaluation of the neuroprotective effect of oligo-porphyran from Porphyra yezoensis in PC12 cells. J. Appl. Phycol. 2019, 31, 2559–2571. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response Surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Ammar, H.H.; Lajili, S.; Said, R.B.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. Daru J. Pharm. Sci. 2015, 23, 1. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhu, Z.Y.; Sun, H.Q.; Chen, L.J. Structural characterization and inhibition on α-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017, 174, 1–12. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C. Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Sellimi, S.; Kadri, N.; Barragan-Montero, V.; Laouer, H.; Hajji, M.; Nasri, M. Fucans from a Tunisian brown seaweed Cystoseira barbata: Structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014, 66, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Susquehanna, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Kornprobst, J.M. Substances naturelles d’origine marine: Chimiodiversité. In Pharmacodiversité, biotechnologies; Tec & Doc: Paris, France, 2006. [Google Scholar]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Hentati., F.; Ursu, A.V.; Pierre, G.; Delattre, C.; Bogdan, T.; Abdelkafi, S.; Gholamereza, D.; Tanase, D.; Michaud, P. Production, extraction and characterization of alginates from seaweeds. In Handbook of Algal Technologies and Phytochemicals; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press (Taylor & Francis group, Royaume-Uni): Boca Raton, FL, USA, 2019; pp. 33–42. [Google Scholar]
- Rinaudo, M. Seaweed polysaccharides. In Comprehensive Glycoscience; Kamerling, J.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Khajouei, R.A.; Keramat, J.; Hamdami, N.; Ursu, A.V.; Delattre, C.; Laroche, C.; Gardarin, C.; Lecerf, D.; Desbrières, J.; Djelveh, G.; et al. Extraction and characterization of an alginate from the Iranian brown seaweed Nizimuddinia zanardini. Int. J. Biol. Macromol. 2018, 118, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Rees, D. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv. Carbohydr. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [PubMed]
- Knutsen, S.; Myslabodski, D.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and its applications. Polysacch. Springer Int. Publ. Cham Switz. 2014, 1–37. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis–A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Craigie, J.S. Cell walls. In Biology of the Red Algae; Cole, K.M., Sheath, R.G., Eds.; Cambridge University Press: London, UK, 1990; pp. 221–251. [Google Scholar]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, H.; Zhao, T.; Deslandes, E.; Ismaeli, N.M.; Molloy, F.; Critchley, A.T. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr. Res. 2005, 340, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Miladi, R.; Manghisi, A.; Minicante, S.A.; Genovese, G.; Abdelkafi, S.; Morabito, M. A DNA barcoding survey of Ulva (Chlorophyta) in Tunisia and Italy reveals the presence of the overlooked alien U. ohnoi. Cryptogam. Algol. 2018, 39, 85–107. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Briand, X.; Cluzet, S.; Dumas, B.; Esquerre-Tugaye, M.T.; Salamagne, S. Use of Ulvans as Activators of Plant Defence and Resistance Reactions against Biotic and Abiotic Stresses. US Patent 0232494 A1, 13 October 2005. [Google Scholar]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313318. [Google Scholar] [CrossRef]
- Ngo, D.H.; Wijesekara, I.; Vo, T.S.; Van Ta, Q.; Kim, S.K. Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Res. Int. 2011, 44, 523–529. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitz Gerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Athukorala, Y.; Jeon, Y.J.; Senevirathne, M.; Cho, K.R.; Kim, S.H. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum. Prev. Nutr. Food Sci. 2007, 12, 65–73. [Google Scholar] [CrossRef]
- Sudharsan, S.; Giji, S.; Seedevi, P.; Vairamani, S.; Shanmugam, A. Isolation, characterization and bioactive potential of sulfated galactans from Spyridia hypnoides (Bory) Papenfuss. Int. J. Biol. Macromol. 2018, 109, 589–597. [Google Scholar] [CrossRef]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Ashayerizadeh, O.; Dastar, B.; Pourashouri, P. Study of antioxidant and antibacterial activities of depolymerized fucoidans extracted from Sargassum Tenerrimum. Int. J. Biol. Macromol. 2020, 151, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, P.; Li, Z.; Zhang, H.; Xu, Z.; Li, P. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 2003, 15, 305310. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Souza, B.W.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef] [Green Version]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; Da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Rivas, G.G.; Gutierrez, C.M.G.; Arteaga, G.A.; Mercado, I.E.S.; Sanchez, N.E.A. Screening for anticoagulant activity in marine algae from the Northwest Mexican pacific coast. J. Appl. Phycol. 2011, 23, 495–503. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Fan, L.; Jiang, L.; Xu, Y.; Zhou, Y.; Shen, Y.; Xie, W.; Long, Z.; Zhou, J. Synthesis and anticoagulant activity of sodium alginate sulfates. Carbohydr. Polym. 2011, 83, 11797–11803. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Karmakar, P.; Ghosh, T.; Sinha, S.; Saha, S.; Mandal, P.; Ghosal, P.K.; Ray, B. Polysaccharides from the brown seaweed Padina tetrastromatica: Characterization of a sulfated fucan. Carbohydr. Polym. 2009, 78, 416–421. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 5, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, R.V.; Ermakova, S.P.; Awada, S.M.; Zvyagintseva, T.N.; Kanaan, H.M. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Compd. 2011, 47, 329–334. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.B.; Sankawa, U. An antivirally active sulfated polysaccharide from Sargassum horneri (Turner) C. Agardh. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Ecklonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Yamamoto, I.; Takahashi, M.; Tamura, E.; Maruyama, H.; Mori, H. Antitumor activity of edible marine algae: Effect of crude fucoidan fractions prepared from edible brown seaweed against L-1210 leukemia. Hydrobiology 1984, 116–117, 145–148. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.L.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.A.; Leite, E.L.; Nader, H.B.; Rocha, H.A.O. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luescher-Mattli, M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr. Med. Chem. 2003, 2, 219–225. [Google Scholar] [CrossRef]
- Caceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zuniga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; De Almeida, A.A.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Lins, K.O.; Bezerra, D.P.; Alves, A.P.; Alencar, N.M.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef]
- Takano, R.; Iwane-Sakata, H.; Hayashi, K.; Hara, S.; Hirase, S. Concurrence of agaroid and carrageenan chains in funoran from the red seaweed Gloiopeltis furcata Post. Et Ruprecht (Cryptonemiales, Rhodophyta). Carbohydr. Polym. 1998, 35, 81–87. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef]
- Usui, T.; Asari, K.; Mizuno, T. Isolation of highly purified fucoidan from Eisenia bicyclis and its anticoagulant and antitumor activities. Agric. Biol. Chem. 1980, 44, 2. [Google Scholar] [CrossRef]
- Xing, R.G.; Liu, S.; Yu, H.H.; Guo, Z.Y.; Li, Z.; Li, P.C. Preparation of high-molecular weight and high-sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydr. Polym. 2005, 61, 148–154. [Google Scholar] [CrossRef]
- Yu, P.Z.; Li, N.; Liu, X.G.; Zhou, G.F.; Zhang, Q.B.; Li, P.C. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef]
- Mao, W.; Li, H.; Li, Y.; Zhang, H.; Qi, X.; Sun, H.; Chen, Y.; Guo, S. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int. J. Biol. Macromol. 2009, 44, 70–74. [Google Scholar] [CrossRef]
- Zhang, H.J.; Mao, W.J.; Fang, F.; Li, H.Y.; Sun, H.H.; Chen, Y.; Qi, X.H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Jung, W.K.; Athukorala, Y.; Lee, Y.J.; Cha, S.H.; Lee, C.H.; Vasanthan, T.; Choi, K.S.; Yoo, S.H.; Kim, S.K.; Jean, Y.J. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. J. Appl. Phycol. 2007, 19, 425–430. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Nishino, T.; Nagumo, T. Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydr. Res. 1992, 229, 355–362. [Google Scholar] [CrossRef]
- Qui, X.; Amarasekara, A.; Doctor, V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr. Polym. 2006, 63, 224–228. [Google Scholar]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Uehara, T.; Harada, N.; Sekiguchi, M.; Hiraoka, A. Heparinoid-active sulphated polysaccharides from Monostroma nitidum and their distribution in the chlorophyta. Phytochemistry 1991, 30, 3611–3614. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.; Zhang, X.; Qi, X.; Chen, Y.; Chen, Y.; Xu, J.; Zhao, C.; Hou, Y.; Yang, Y.; et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr. Polym. 2011, 85, 394–400. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsuura, Y.; Bacic, A.; Liao, M.L.; Hori, K.; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int. J. Biol. Macromol. 2001, 28, 395–399. [Google Scholar] [CrossRef]
- Pereira, M.G.; Benevides, N.M.; Melo, M.R.; Valente, A.P.; Melo, F.R.; Mourão, P.A. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005, 340, 2015–2023. [Google Scholar] [CrossRef]
- Sudharsan, S.; Subhapradha, N.; Seedevi, P.; Shanmugam, V.; Madeswaran, P.; Shanmugam, A.; Srinivasan, A. Antioxidant and anticoagulant activity of sulfated polysaccharide from Gracilaria debilis (Forsskal). Int. J. Biol. Macromol. 2015, 81, 1031–1038. [Google Scholar] [CrossRef]
- Fonseca, R.J.; Oliveira, S.N.M.; Melo, F.R.; Pereira, M.G.; Benevides, N.M.; Mourão, P.A. Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb. Haemost. 2008, 99, 539545. [Google Scholar] [CrossRef]
- Shanmugam, M.; Mody, K.H. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and in vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef]
- Kwon, M.J.; Nam, T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Ji, C.F.; Ji, Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014, 7, 1728–1732. [Google Scholar] [CrossRef]
- Ji, C.F.; Ji, Y.B.; Meng, D.Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Yan, M.D.; Yao, C.J.; Chow, J.M.; Chang, C.L.; Hwang, P.A.; Chuang, S.E.; Whang-Peng, J.; Lai, G.M. Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.E.; Choi, E.S.; Shin, J.; Lee, S.O.; Park, K.S.; Cho, N.P.; Cho, S.D. Fucoidan induces caspase-dependent apoptosis in MC3 human mucoepidermoid carcinoma cells. Exp. Ther. Med. 2014, 7, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H. Missing self-recognition and self-tolerance of natural killer (NK) cells. In Seminar in Immunology; Academic Press: London, UK, 2006; Volume 18, pp. 145–150. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchib, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. Fucoidan and its health benefits. In Bioactive Seaweeds Food Applications; Academic Press: London, UK, 2018; pp. 223–238. [Google Scholar]
- Okai, Y.; Ishizaka, S.; Higashi-Okai, K. Detection of immunomodulating activities in an extract of Japanese edible seaweed, Laminaria japonica (Makonbu). J. Sci. Food Agric. 1996, 72, 455–460. [Google Scholar] [CrossRef]
- Choi, E.M.; Kim, A.J.; Kim, Y.O.; Hwang, J.K. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process Biochem. 2017, 58, 289–297. [Google Scholar] [CrossRef]
- Yermak, I.M.; Barabanova, A.O.; Aminin, D.L.; Davydova, V.N.; Sokolova, E.V.; Solov’eva, T.F.; Kim, Y.H.; Shin, K.S. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydr. Polym. 2012, 87, 713–720. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1,6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef]
- Yin, G.; Li, W.; Lin, Q.; Lin, X.; Lin, J.; Zhu, Q.; Jiang, H.; Huang, Z. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish Shellfish Immunol. 2014, 41, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Surayot, U.; You, S. Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiao, G.; Yang, Y.; Li, M.; Li, Q.; Wang, X.; Cai, C.; Li, J.; Hao, J.; Yu, G. Structure and immunomodulatory activity of a sulfated agarose with pyruvate and xylose substitutes from Polysiphonia senticulosa Harvey. Carbohydr. Polym. 2017, 176, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Shah, J.; Mishra, V.; Tambuwala, M.; Kesharwani, P. Nanoneuromedicine for management of neurodegenerative disorder. J. Drug Deliv. Sci. Technol. 2019, 49, 477–490. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Zhang, Y.; Liu, X.; Wang, C.; Teng, L.; Wang, D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int. J. Biol. Macromol. 2019, 121, 29–37. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; & Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Shimohama, S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004, 64, 99–106. [Google Scholar]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders—memantine, a new hope. Pharmacol. Res. 2005, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.; Marco-Contelles, J. A step further towards multitarget drugs for alzheimer and neuronal vascular diseases: Targeting the cholinergic system, amyloid-β aggregation and Ca2+ dyshomeostasis. Curr. Med. Chem. 2011, 18, 552–576. [Google Scholar] [CrossRef] [PubMed]
- Han, R.W.; Chang, M.; Peng, Y.L.; Wang, P.; Hu, S.Q.; Choi, C.L.; Han, Y.F. Reversal of scopolamine-induced spatial and recognition memory deficits in mice by novel multifunctional dimers bis-cognitins. Brain Res. 2012, 1470, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]













| Macroalgae | Country | Proteins (%) | Lipids (%) | Carbohydrates (%) | Fibers (%) | References |
|---|---|---|---|---|---|---|
| Brown Seaweeds (Pheophyceae) | ||||||
| Colpomenia sinuosa | Iran | 9.20 | 1.50 | 32.10 | - | Rohani-Ghadikolaei et al. [33] |
| Cystoseira compressa | Tunisia | 9.98 | 2.80 | 39.11 | 57.33 | Hentati et al. [34] |
| Durvillaea antarctica | Chile | 10.40 | 0.80 | 70.90 | 71.40 | Ortiz et al. [35] |
| Ecklonia radiata | New Zealand | 9.60 | 1.80 | 66.90 | - | Smith et al. [36] |
| Fucus spiralis | Portugal | 9.71 | 5.23 | 17.59 | - | Paiva et al. [37] |
| Hormosira banksii | New Zealand | 6.07 | 2.63 | 62.90 | - | Smith et al. [36] |
| Padina pavonica | Iran | 11.83 | 1.79 | - | 11.00 | Tabarsa et al. [38] |
| Saccorhiza polyschides | Portugal | 14.44 | 1.10 | 45.60 | - | Rodrigues et al. [39] |
| Sargassum naozhouense | China | 11.20 | 1.06 | 47.43 | 4.83 | Peng et al. [40] |
| Red Seaweeds (Rhodophyceae) | ||||||
| Ahnfeltia plicata | Denmark | 31.10 | 1.10 | 59.10 | - | Parjikolaei et al. [41] |
| Dumontia contorta | United Kingdom | 31.70 | 0.12 | - | 34.30 | Marsham et al. [42] |
| Gracilaria cervicornis | Brazil | 19.70 | 0.43 | 63.10 | 5.65 | Marinho-Soriano et al. [43] |
| Jania adhaerens | Tunisia | 9.81 | 2.76 | 34.54 | 51.68 | Hentati et al. [34] |
| Kappaphycus alvarezii | India | 16.24 | 0.74 | 27.40 | 29.40 | Fayaz et al. [44] |
| Osmundea pinnatifida | Portugal | 20.79 | 7.53 | 17.61 | - | Paiva et al. [37] |
| Porphyra columbina | Argentina | 24.61 | 0.25 | - | 48.02 | Cian et al. [45] |
| Green Seaweeds (Chlorophyceae) | ||||||
| Caulerpa lentillifera | Borneo | 10.41 | 1.11 | 38.66 | 32.99 | Matanjun et al. [46] |
| Caulerpa taxifolia | India | 12.44 | 0.32 | 23.86 | - | Kokilam and Vasuki. [47] |
| Ulva lactuca | Tunisia | 8.46 | 7.87 | - | 54.90 | Yaich et al. [48] |
| Ulva reticulata | Thailand | 21.06 | 0.75 | 55.77 | 4.84 | Ratana-Arporn and Chirapart [49] |
| Ulva rigida | Spain | 17.80 | 0.90 | 42.60 | 11.90 | Taboada et al. [50] |
| Type of PS | Source | Main Monosaccharide | Main Backbone | Biological Properties | References |
|---|---|---|---|---|---|
| Brown macroalgae | |||||
| S-fucan | Padina tetrastromatica | Fucp, Galp, Xylp, GlcpAc | (1,2)- and (1,3)-α-l-Fucp | Nd | Karmakar et al. [117] |
| S-galactofucans | Spatoglossum schröederi | Galp, Fucp, Xylp | (1,4)- and (1,3)-α-l-Fucp | Anti-thrombotic | Costa et al. [109] |
| S-galactofucans | Adenocystis utricularis | Galp, Fucp, Rhap, uronic acids | (1,3)-α-l-Fucp | Antiviral | Ponce et al. [69] |
| S-fucans | Ascophyllum nodosum | Fucp, Xylp, Galp, GlcpAc, Glcp | (1,3)- and (1,4)-α-l-Fucp | Immunomodulatory, anti-inflammatory, anticoagulant, anti-thrombotic | Cumashi et al. [118] |
| S-fucans | Fucus spp. | Fucp, Xylp, Galp, GlcpAc | (1,3)- and (1,4)-α-l-Fucp | Immunostimulant, antiviral, antitumor, antiproliferative, antiadhesive | Costa et al. [109] |
| S-galactofucans | Sargassum sp. | Galp, Fucp, Rhap, GlcpAc | (1,6)-β-d-Galp and (1,2)-β-d-Manp | Antitumor | Sokolova et al. [119] Ale et al. [120] |
| S-fucoidan | Sargassum horneri | Fucp | (1,3)-α-l-Fucp, (1,3)- and (1,4)-α-l-Fucp | Antitumor, antiviral | Ale et al. [120] Hoshino et al. [121] |
| S-fucans | Ecklonia cava Ecklonia kurome | Fucp, Rhap, Galp, GlcpAc | (1,3)- or (1,6)-, and (1,4)-α-l-Fucp | Anti-proliferative, antitumor, anticoagulant, antioxidant, antithrombotic, anti-inflammatory | Ermakova et al. [122] Yamamoto et al. [123] |
| S-galactofucan | Laminaria japonica | Galp, Fucp | (1,3)- and (1,4)-α-l-Fucp | Anti-lipidaemic, antiviral, antitumor, immunomodulator, antioxidant neuroprotective | Fedorov et al. [124] Cumashi et al. [118] Wang et al. [125] |
| Red macroalgae | |||||
| S-λ-carrageenan | Chondrus crispus | Galp, AnGalp | (1,3)-α-d-Galp, and (1,4)-β-3,6-AnGalp or (1,4)-β-d-Galp | Antiviral, anticoagulant, antithrombotic | Albuquerque et al. [126] Luescher-Mattli [127] |
| S-κ-carrageenan | E. spinosa | Galp, AnGalp | (1,3)-α-d-Galp, and (1,4)-β-3,6-AnGalp or (1,4)-β-d-Galp | Anticoagulant, anti-thrombotic | Prajapati et al. [86] Campo et al. [87] |
| S-carrageenans | Stenogramme interrupta | Galp, AnGalp | (1,3)-α-d-Galp, and (1,4)-β-3,6-AnGalp or (1,4)-β-d-Galp | Antiviral | Prajapati et al. [86] Caceres et al. [128] |
| Carrageenan | Hypnea musciformis | Galp, AnGalp | (1,3)-α-d-Galp, and (1,4)-β-3,6-AnGalp or (1,4)-β-d-Galp | Anticancer | Souza et al. [129] |
| LMW-carrageenans | Champia feldmannii | Galp, AnGalp | (1,3)-α-d-Galp, and (1,4)-β-3,6-AnGalp or (1,4)-β-d-Galp | Antitumor | Lins et al. [130] Prajapati et al. [86] Campo et al. [87] |
| Green macroalgae | |||||
| S-arabinogalactans | Codium spp. | Galp, Araf | (1,3)-β-d-Gal | Anticoagulant, antithrombotic, antiviral | Takano et al. [131] Lee et al. [132] |
| S-ulvans | Ulva pertusa | Rhap, Xylp, GlcpAc, IdoAc | [→4)-β-d-GlcpAc-(1,4)-α-l-Rhap3S-(1→], and [→4)-α-l-IdoAc-(1,4)-α-l-Rhap3S-(1→] | Antioxidant, anti-proliferative, hypocholesterolaemic | Usui et al. [133] Xing et al. [134] Yu et al. [135] |
| S-PS | Ulva rigida | Rhap, GlcpAc | β-d-GlcpAc-(1,4)-l-Rhap | Immunostimulatory | Lahaye and Robic [94] Leiro et al. [136] |
| S-rhamnans | Monostroma latissimum | Rhap | (1,3)-α-l-Rhap, and (1,3)-α-l-Rhap or (1,2)-α-l-Rhap or (1→2,3)-α-l-Rhap | Antiviral, anticoagulant | Lee et al. [137] Mao et al. [138] Zhang et al. [139] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. https://doi.org/10.3390/molecules25143152
Hentati F, Tounsi L, Djomdi D, Pierre G, Delattre C, Ursu AV, Fendri I, Abdelkafi S, Michaud P. Bioactive Polysaccharides from Seaweeds. Molecules. 2020; 25(14):3152. https://doi.org/10.3390/molecules25143152
Chicago/Turabian StyleHentati, Faiez, Latifa Tounsi, Djomdi Djomdi, Guillaume Pierre, Cédric Delattre, Alina Violeta Ursu, Imen Fendri, Slim Abdelkafi, and Philippe Michaud. 2020. "Bioactive Polysaccharides from Seaweeds" Molecules 25, no. 14: 3152. https://doi.org/10.3390/molecules25143152