Optimization and Characterization of Microwave-Assisted Hydro-Distillation Extraction of Essential Oils from Cinnamomum camphora Leaf and Recovery of Polyphenols from Extract Fluid
Abstract
1. Introduction
2. Results and Discussion
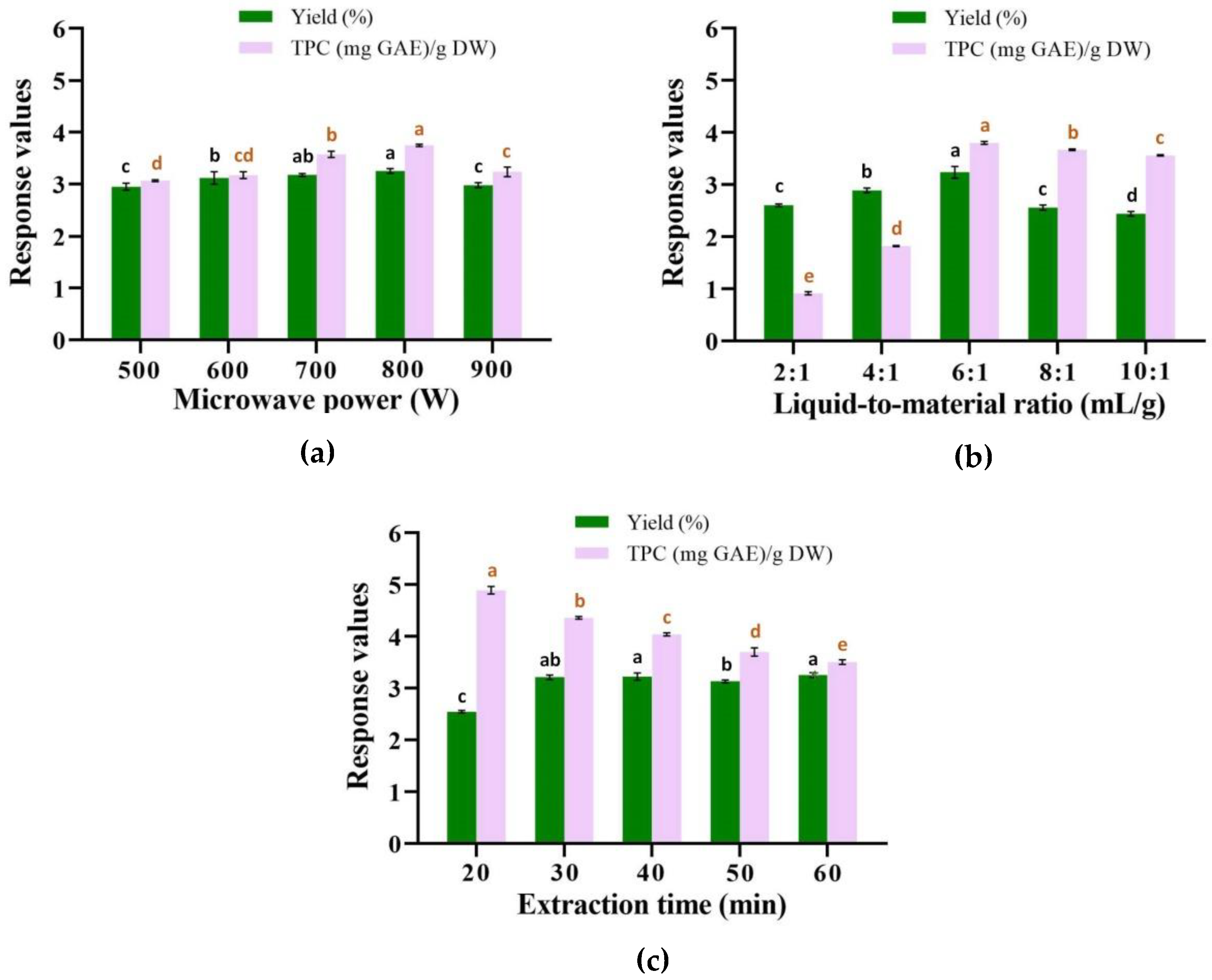
2.1. Analysis of Single-Factor Experiments
2.1.1. Effect of Microwave Power
2.1.2. Effect of Liquid-to-Material Ratio
2.1.3. Effect of Extraction Time
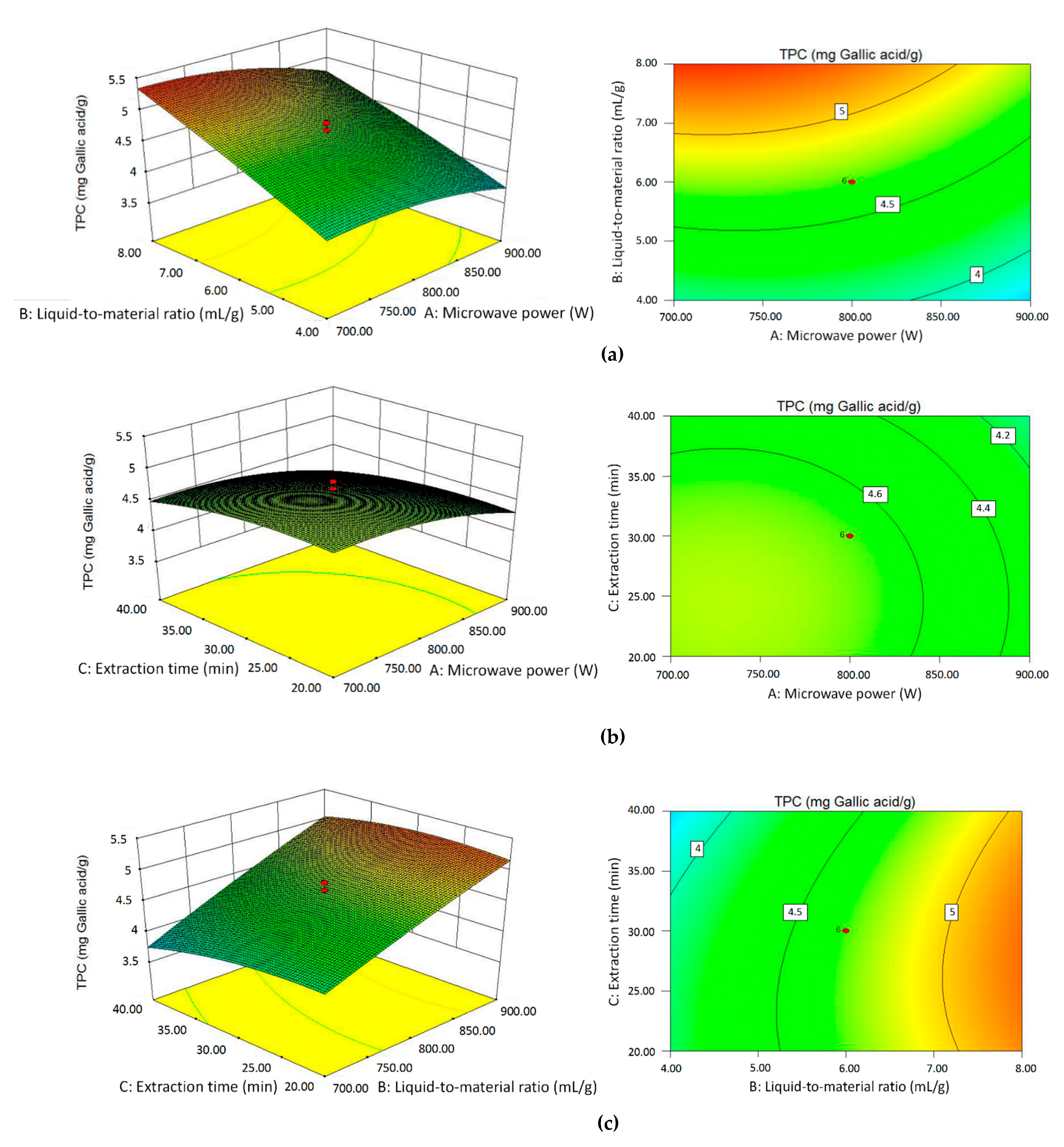
2.2. Analysis of Response Surface Methodology
2.2.1. Results of Central Composite Design (CCD)
2.2.2. Model Fitting and Analysis of Variance (ANOVA)
2.2.3. Effects of Independent Variables on Essential Oil Yield
2.2.4. Effects of Independent Variables on TPC Value
2.2.5. Verification of the Predicted Value
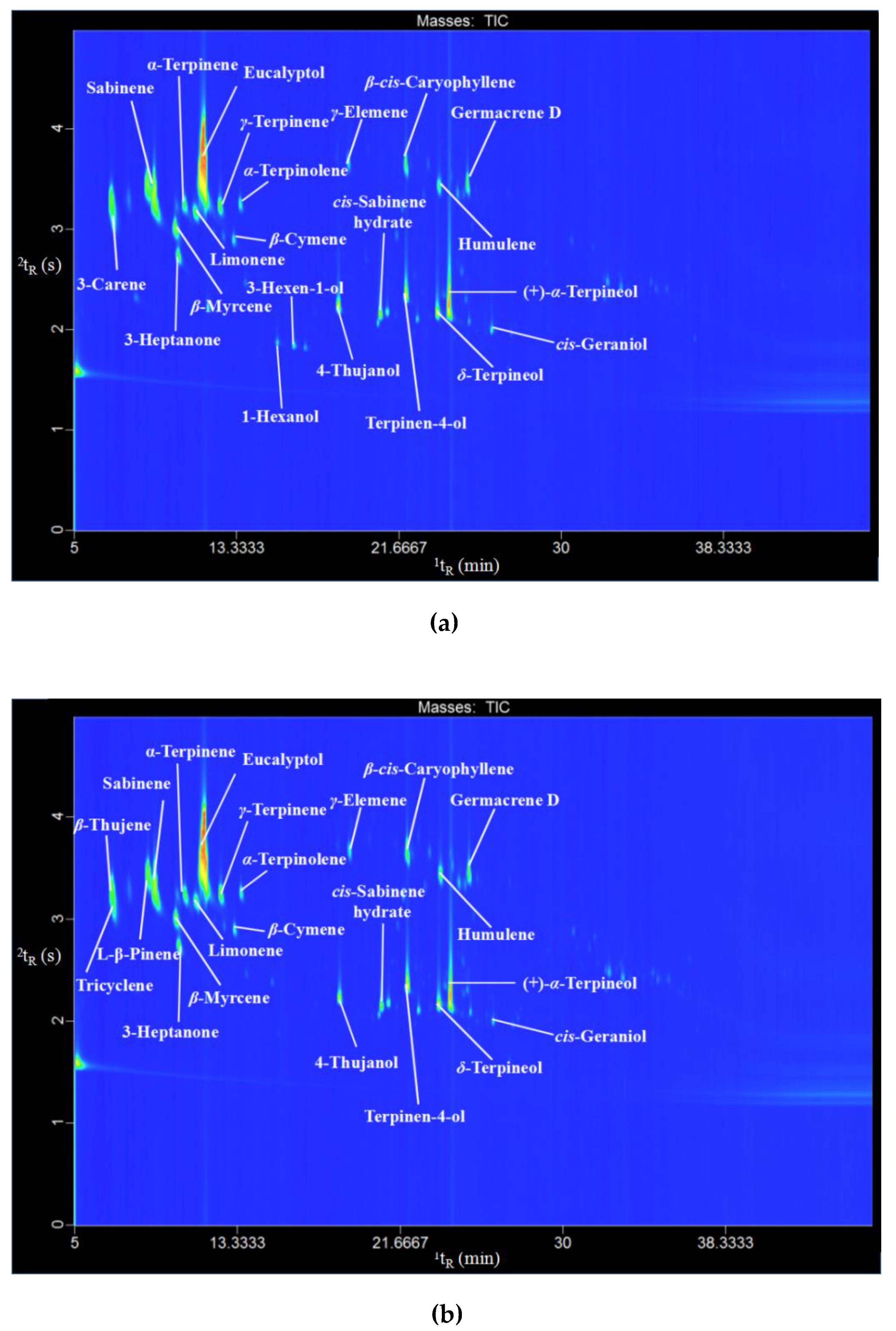
2.3. Chemical Profile of C. camphora Essential Oils
3. Materials and Methods
3.1. Plant and Reagents
3.2. Microwave-Assisted Hydro-Distillation (MAHD)
3.3. Total Phenolic Content Measured by the Folin–Ciocalteu Method
3.4. Single-Factor Experiment Design
3.5. Response Surface Methodology (RSM) Design
3.6. GC×GC-TOFMS Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.X.; Ren, J.L.; Li, Z.H. Antibacterial activity and mechanism of pinoresinol from Cinnamomum camphora leaves against food-related bacteria. Food Control. 2017, 79, 192–199. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, W.; Hu, J.W.; Wu, J.; Li, Z.J.; Gao, Y.; Liu, D.; Liu, Y.; Liu, W.; Liang, M.; et al. Secondary metabolites from the twigs of Cinnamomum camphora. Chem. Nat. Compd. 2019, 55, 345–347. [Google Scholar] [CrossRef]
- Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H.; Shen, Y.F. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour. Technol. 2006, 97, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.; Singh, P.; Shukla, R.; Dubey, N.K. A novel combination of the essential oils of Cinnamomum camphora and Alpinia galanga in checking aflatoxin B-1 production by a toxigenic strain of Aspergillus flavus. World J. Microbiol. Biotechnol. 2008, 24, 693–697. [Google Scholar] [CrossRef]
- Chen, Y.J.; Dai, G.H. Acaricidal activity of compounds from Cinnamomum camphora (L.) Presl against the carmine spider mite, Tetranychus cinnabarinus. Pest Manag. Sci. 2015, 71, 1561–1571. [Google Scholar] [CrossRef]
- Jawaid, T.; Kamal, M.; Singh, R.; Shukla, D.; Devanathadesikan, V.; Sinha, M. Anticonvulsant and neuroprotective effects of methanolic extract of Cinnamomum camphora leaves in rat brain. Orient. Pharm. Exp. Med. 2018, 18, 237–246. [Google Scholar] [CrossRef]
- Kang, N.J.; Han, S.C.; Yoon, S.H.; Sim, J.Y.; Maeng, Y.H.; Kang, H.K.; Yoo, E.S. Cinnamomum camphora leaves alleviate allergic skin inflammatory responses in vitro and in vivo. Toxicol. Res. 2019, 35, 279–285. [Google Scholar] [CrossRef]
- Chen, H.P.; Yang, K.; You, C.X.; Lei, N.; Sun, R.Q.; Geng, Z.F.; Ma, P.; Cai, Q.; Du, S.S.; Deng, Z.W. Chemical constituents and insecticidal activities of the essential oil of Cinnamomum camphora leaves against Lasioderma serricorne. J. Chem. 2014, 2014, 963729. [Google Scholar] [CrossRef]
- Guo, S.S.; Geng, Z.F.; Zhang, W.J.; Liang, J.Y.; Wang, C.F.; Deng, Z.W.; Du, S.S. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Baudoux, D.; Idaomar, M. Antigenotoxic effects of three essential oils in diploid yeast (Saccharomyces cerevisiae) after treatments with UVC radiation, 8-MOP plus UVA and MMS. Mutat. Res. Toxicol. Environ. Mutagen. 2006, 606, 27–38. [Google Scholar] [CrossRef]
- Chen, J.L.; Tang, C.L.; Zhang, R.F.; Ye, S.X.; Zhao, Z.M.; Huang, Y.Q.; Xu, X.J.; Lan, W.J.; Yang, D.P. Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Li, D.X.; Huang, X.Q.; Yang, H.X.; Qiu, Z.W.; Zou, L.T.; Liang, Q.; Shi, Y.; Wu, Y.X.; Wu, S.H.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.G.; Lin, Y.H.; Chai, X.H.; Duan, X.J.; Zhao, X.X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crop. Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Liu, C.H.; Mishra, A.K.; He, B.; Tan, R.X. Composition and antifungal activity of essential oils from Artemisia princeps and Cinnamomum camphora. Int. Pest. Control. 2001, 43, 72–74. [Google Scholar]
- Jiang, H.; Wang, J.; Song, L.; Cao, X.S.; Yao, X.; Tang, F.; Yue, Y.D. GCxGC-TOFMS Analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules 2016, 21, 423. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramirez-Corona, N.; Palou, E.; Lopez-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Mollaei, S.; Sedighi, F.; Habibi, B.; Hazrati, S.; Asgharian, P. Extraction of essential oils of Ferulago angulata with microwave-assisted hydrodistillation. Ind. Crop. Prod. 2019, 137, 43–51. [Google Scholar] [CrossRef]
- Chen, F.L.; Du, X.Q.; Zu, Y.G.; Yang, L.; Wang, F. Microwave-assisted method for distillation and dual extraction in obtaining essential oil, proanthocyanidins and polysaccharides by one-pot process from Cinnamomi cortex. Sep. Purif. Technol. 2016, 164, 1–11. [Google Scholar] [CrossRef]
- Bustamante, J.; van Stempvoort, S.; Garcia-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, Z.T.; Wang, Y.; Li, R. Composition comparison of essential oils extracted by hydro distillation and microwave-assisted hydrodistillation from Amomum kravanh and Amomum compactum. J. Essent. Oil Bear. Plants 2011, 14, 354–359. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Rezaei, K. Comparison of microwave-assisted hydro distillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.T.; Feng, X.; Li, R.; Wang, Y. Composition comparison of essential oils extracted by classical hydro distillation and microwave-assisted hydrodistillation from Pimenta dioica. J. Essent. Oil Bear. Plants 2013, 16, 45–50. [Google Scholar] [CrossRef]
- Brokl, M.; Fauconnier, M.L.; Benini, C.; Lognay, G.; du Jardin, P.; Focant, J.F. Improvement of Ylang-Ylang essential oil characterization by GC×GC-TOFMS. Molecules 2013, 18, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.L.; Polidoro, A.d.S.; Schneider, J.K.; da Cunha, M.E.; Saucier, C.; Jacques, R.A.; Cardoso, C.A.L.; Mota, J.S.; Caramao, E.B. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC/TOFMS) for the analysis of volatile compounds in Piper regnellii (Miq.) C. DC. essential oils. Microchem. J. 2015, 118, 242–251. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, R.Z.; Qu, R.F.; Li, Z.Y. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the analysis of volatile components in Neroli essential oil. Mendeleev. Commun. 2012, 22, 45–46. [Google Scholar] [CrossRef]
- Zhu, S.K.; Lu, X.; Xing, J.; Zhang, S.W.; Kong, H.W.; Xu, G.W.; Wu, C.Y. Comparison of comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry and gas chromatography-mass spectrometry for the analysis of tobacco essential oils. Anal. Chim. Acta 2005, 545, 224–231. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, R.; Mei, Y.; Liu, R.; Yu, W. Characterization of crude and ethanol-stabilized bio-oils before and after accelerated aging treatment by comprehensive two-dimensional gas-chromatography with time-of-flight mass spectrometry. J. Energy Inst. 2017, 90, 646–659. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H.B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: Optimization and identification of phenolic compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef]
- Le, X.D.; Nguyen, M.C.; Vu, D.H.; Pham, M.Q.; Pham, Q.L.; Nguyen, Q.T.; Nguyen, T.A.; Pham, V.T.; Bach, L.G.; Nguyen, T.V.; et al. Optimization of microwave-assisted extraction of total phenolic and total flavonoid contents from fruits of Docynia indica (Wall.) Decne. using response surface methodology. Processes 2019, 7, 485. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.H.H.; Nguyen, D.C.; Nguyen, T.Q.; Tan, H.; Nhan, L.T.H.; Nguyen, D.H.; Tran, L.D.; Do, S.T.; Nguyen, T.D. Optimization of microwave-assisted extraction of essential oil from vietnamese basil (Ocimum basilicum L.) using response surface methodology. Processes 2018, 6, 206. [Google Scholar] [CrossRef]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, D.P.; Li, S.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 2015, 20, 20448–20459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Fu, C.L. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005, 92, 701–706. [Google Scholar]
- Sombatpraiwan, S.; Junyusen, T.; Treeamnak, T.; Junyusen, P. Optimization of microwave-assisted alkali pretreatment of cassava rhizome for enhanced enzymatic hydrolysis glucose yield. Food Energy Secur. 2019, 8, e00174. [Google Scholar] [CrossRef]
- Yu, H.; Ren, X.; Liu, Y.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Extraction of Cinnamomum camphora chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: Optimization of extraction, and analysis of chemical constituents. Ind. Crop. Prod. 2019, 141, 111794. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, J.; Wang, P.; Li, Q.; Yu, S.; Zhang, Y.; Wang, Y. Chemical composition and larvicidal activities of essential oil of Cinnamomum camphora (L.) leaf against Anopheles stephensi. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190211. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1777–1784. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Run | X1 | X2 | X3 | Yield (%, w/w) | TPC (mg·GAE/g·DW) |
|---|---|---|---|---|---|
| Microwave Power (W) | Liquid-to-Material Ratio (mL/g) | Extraction Time (min) | |||
| 1 | 700 (−1) | 8:1 (1) | 20 (−1) | 1.94 ± 0.05 | 5.44 ± 0.03 |
| 2 | 631.82 (−1.68) | 6:1 (0) | 30 (0) | 3.17 ± 0.12 | 4.51 ± 0.12 |
| 3 | 800 (0) | 9.36:1 (1.68) | 30 (0) | 2.39 ± 0.08 | 5.44 ± 0.12 |
| 4 | 800 (0) | 6:1 (0) | 46.82 (1.68) | 3.30 ± 0.24 | 4.32 ± 0.05 |
| 5 | 968.18 (1.68) | 6:1 (0) | 30 (0) | 2.98 ± 0.12 | 3.98 ± 0.29 |
| 6 | 800 (0) | 6:1 (0) | 30 (0) | 3.21 ± 0.21 | 4.81 ± 0.03 |
| 7 | 900 (1) | 4:1 (−1) | 40 (1) | 2.62 ± 0.09 | 3.29 ± 0.31 |
| 8 | 900 (1) | 8:1 (1) | 20 (−1) | 2.76 ± 0.18 | 4.81 ± 0.02 |
| 9 | 800 (0) | 6:1 (0) | 30 (0) | 3.44 ± 0.14 | 4.53 ± 0.04 |
| 10 | 900 (1) | 4:1 (−1) | 20 (−1) | 2.22 ± 0.05 | 3.82 ± 0.17 |
| 11 | 800 (0) | 2.64:1 (−1.68) | 30 (0) | 2.63 ± 0.37 | 3.71 ± 0.13 |
| 12 | 900 (1) | 8:1 (1) | 40 (1) | 3.35 ± 0.12 | 4.53 ± 0.05 |
| 13 | 700 (−1) | 8:1 (1) | 40 (1) | 2.94 ± 0.05 | 5.18 ± 0.13 |
| 14 | 800 (0) | 6:1 (0) | 30 (0) | 3.45 ± 0.09 | 4.60 ± 0.12 |
| 15 | 800 (0) | 6:1 (0) | 30 (0) | 3.40 ± 0.18 | 4.64 ± 0.02 |
| 16 | 800 (0) | 6:1 (0) | 30 (0) | 3.20 ± 0.07 | 4.81 ± 0.06 |
| 17 | 700 (−1) | 4:1 (−1) | 20 (−1) | 2.37 ± 0.10 | 4.25 ± 0.10 |
| 18 | 700 (−1) | 4:1 (−1) | 40 (1) | 3.14 ± 016 | 3.67 ± 0.02 |
| 19 | 800 (0) | 6:1 (0) | 13.18 (−1.68) | 2.37 ± 0.09 | 4.39 ± 0.14 |
| 20 | 800 (0) | 6:1 (0) | 30 (0) | 3.14 ± 0.03 | 4.69 ± 0.04 |
| No. | Name | CAS | 1 tR (min) | 2 tR (s) | Fresh Leaf | Fallen Leaf |
|---|---|---|---|---|---|---|
| Peak Area (%) | Peak Area (%) | |||||
| 1 | 2,4-Dimethyl-pentane | 108-08-7 | 5.42 | 1.58 | 0.02–0.1 | 0.02–0.1 |
| 2 | 2,2,4-Trimethyl-oxetane | 23120-44-7 | 5.42 | 2.18 | 0.02–0.1 | 0.02–0.1 |
| 3 | Isopropyl peroxide | 16642-57-2 | 5.58 | 1.58 | 0.02–0.1 | - |
| 4 | 2-Azido-2,3,3-trimethylbutane | 51677-41-9 | 5.67 | 1.54 | 0.1–1.0 | 0.1–1.0 |
| 5 | γ-Acetylpropyl acetate | 5185-97-7 | 5.75 | 1.58 | 0.02–0.1 | 0.1–1.0 |
| 6 | 2-Ethylfuran | 3208-16-0 | 5.75 | 2.02 | 0.02–0.1 | <0.02 |
| 7 | 3-Acetyl-1-chloro-1,1,2,2-hexanetetracarbonitrile | - | 6.17 | 2.04 | - | 0.02–0.1 |
| 8 | (−)-α-Pinene | 7785-26-4 | 6.83 | 3.26 | 0.1–1.0 | <0.02 |
| 9 | β-Thujene | 28634-89-1 | 6.92 | 3.18 | - | >1.0 |
| 10 | Tricyclene | 508-32-7 | 6.92 | 3.3 | - | >1.0 |
| 11 | 3-Carene | 13466-78-9 | 6.92 | 3.3 | >1.0 | - |
| 12 | β-cis-Ocimene | 3338-55-4 | 7 | 3.22 | 0.1–1.0 | - |
| 13 | Camphene | 79-92-5 | 7.83 | 3.28 | 0.1–1.0 | 0.1–1.0 |
| 14 | Hexanal | 66-25-1 | 8.17 | 2.32 | 0.1–1.0 | 0.02–0.1 |
| 15 | 3,5,6-Trichloro-4-isopropylsulfanyl-pyridine-2-carbonitrile | 216242-35-2 | 8.25 | 1.52 | 0.02–0.1 | - |
| 16 | l-β-Pinene | 18172-67-3 | 8.67 | 3.46 | - | >1.0 |
| 17 | β-Pinene | 127-91-3 | 8.83 | 3.48 | 0.02–0.1 | 0.02–0.1 |
| 18 | Sabinene | 3387-41-5 | 9.08 | 3.46 | >1.0 | >1.0 |
| 19 | β-Phellandrene | 555-10-2 | 9.17 | 3.28 | 0.02–0.1 | - |
| 20 | o-Methoxy-α-phenethylamine | - | 9.25 | 3.2 | 0.1–1.0 | - |
| 21 | 4,5,6,7-Tetrahydro-5-benzyl-pyrrolo [3,2-c]pyridine | 272442-27-0 | 9.33 | 3.14 | 0.1–1.0 | - |
| 22 | o-Xylene | 95-47-6 | 9.58 | 2.54 | 0.02–0.1 | 0.02–0.1 |
| 23 | β-Myrcene | 123-35-3 | 10.17 | 3.02 | >1.0 | >1.0 |
| 24 | α-Phellandrene | 99-83-2 | 10.25 | 3.24 | 0.02–0.1 | 0.1–1.0 |
| 25 | 3-Heptanone | 13019-20-0 | 10.33 | 2.74 | >1.0 | >1.0 |
| 26 | 2,2-Dimethylpentanoic acid ethenyl ester | 44970-05-0 | 10.5 | 3.2 | 0.02–0.1 | 0.02–0.1 |
| 27 | α-Terpinene | 99-86-5 | 10.67 | 3.26 | >1.0 | 3.22 |
| 28 | Methyl caproate | 106-70-7 | 10.83 | 2.58 | 0.02–0.1 | <0.02 |
| 29 | 3,7-Bis(ethylidene)-bicyclo [3.3.0]octane | - | 10.92 | 2.76 | 0.02–0.1 | - |
| 30 | Dehydro-1,8-cineole | 92760-25-3 | 10.92 | 3.14 | 0.1–1.0 | 0.1–1.0 |
| 31 | Limonene | 138-86-3 | 11.25 | 3.18 | >1.0 | >1.0 |
| 32 | 5,6-Dimethyl-1,3-cyclohexadiene | 5715-27-5 | 11.42 | 3.38 | 0.02–0.1 | - |
| 33 | 2,4-Dimethoxyaniline | 2735-04-8 | 11.42 | 3.5 | - | 0.02–0.1 |
| 34 | Boldenone sulfate | 87331-43-9 | 11.67 | 4.12 | <0.02 | 0.02–0.1 |
| 35 | 2-Hexenal | 505-57-7 | 11.83 | 2.22 | 0.1–1.0 | 0.1–1.0 |
| 36 | Eucalyptol | 470-82-6 | 11.83 | 3.28 | >1.0 | 0.1–1.0 |
| 37 | α-Pinene | 80-56-8 | 12.17 | 3 | - | 0.02–0.1 |
| 38 | γ-Terpinene | 99-85-4 | 12.5 | 3.26 | >1.0 | >1.0 |
| 39 | β-Ocimene | 13877-91-3 | 12.67 | 2.92 | 0.1–1.0 | 0.1–1.0 |
| 40 | β-Cymene | 535-77-3 | 13.17 | 2.9 | 0.1–1.0 | >1.0 |
| 41 | Terpinolene | 586-62-9 | 13.5 | 3.26 | >1.0 | >1.0 |
| 42 | Methyl 2-hexenoate | 2396-77-2 | 13.75 | 2.48 | 0.1–1.0 | 0.02–0.1 |
| 43 | cis-3-Hexenyl-1-acetate | 3681-71-8 | 14.5 | 2.48 | 0.02–0.1 | - |
| 44 | 6-Methyl-5-hepten-2-one | 110-93-0 | 15.08 | 2.38 | - | 0.1–1.0 |
| 45 | 1-Hexanol | 111-27-3 | 15.42 | 1.88 | 0.1–1.0 | 0.02–0.1 |
| 46 | 3-Hexen-1-ol | 544-12-7 | 16.25 | 1.84 | 0.1–1.0 | <0.02 |
| 47 | trans-2-Pentenol | 1576-96-1 | 16.83 | 1.82 | 0.1–1.0 | <0.02 |
| 48 | 2,5-Dimethyl-3-methylene-hepta-1,5-diene | 74663-83-5 | 18.42 | 3.74 | 0.02–0.1 | 0.02–0.1 |
| 49 | 4-Thujanol | 546-79-2 | 18.5 | 2.24 | >1.0 | 0.1–1.0 |
| 50 | γ-Elemene | 29873-99-2 | 19.08 | 3.66 | 0.02–0.1 | 0.1–1.0 |
| 51 | (−)-Camphor | 464-48-2 | 20 | 2.7 | 0.02–0.1 | - |
| 52 | (−)-β-Bourbonene | 5208-59-3 | 20.08 | 3.78 | 0.02–0.1 | 0.02–0.1 |
| 53 | Benzaldehyde | 100-52-7 | 20.25 | 2.04 | - | 0.02–0.1 |
| 54 | Linalool | 78-70-6 | 20.58 | 2.06 | 0.1–1.0 | 0.1–1.0 |
| 55 | cis-Sabinene hydrate | 17699-16-0 | 20.67 | 2.16 | >1.0 | >1.0 |
| 56 | trans-2-Menthenol | 29803-81-4 | 21.08 | 2.18 | 0.1–1.0 | 0.1–1.0 |
| 57 | Pinocarvone | 30460-92-5 | 21.25 | 2.6 | <0.02 | 0.02–0.1 |
| 58 | Fenchol | 1632-73-1 | 21.5 | 2.14 | - | 0.02–0.1 |
| 59 | trans-Bornyl acetate | 5655-61-8 | 21.5 | 2.96 | 0.1–1.0 | 0.02–0.1 |
| 60 | β-Elemene | 515-13-9 | 21.83 | 3.24 | 0.1–1.0 | 0.1–1.0 |
| 61 | β-cis-Caryophyllene | 118-65-0 | 21.92 | 3.64 | 0.02–0.1 | 0.1–1.0 |
| 62 | Terpinen-4-ol | 562-74-3 | 22 | 2.4 | >1.0 | 0.1–1.0 |
| 63 | Caryophyllene | 87-44-5 | 22.08 | 3.6 | 0.1–1.0 | >1.0 |
| 64 | Patchoulane | 25491-20-7 | 22.25 | 3.62 | <0.02 | 0.02–0.1 |
| 65 | Carvomenthenal | 29548-14-9 | 22.42 | 2.54 | 0.02–0.1 | 0.02–0.1 |
| 66 | cis-Menth-2-en-1-ol | 29803-82-5 | 22.58 | 2.12 | 0.1–1.0 | 0.1–1.0 |
| 67 | α-Gurjenene | 489-40-7 | 22.58 | 3.82 | <0.02 | 0.02–0.1 |
| 68 | Aromandendrene | 489-39-4 | 23.17 | 3.66 | 0.02–0.1 | 0.1–1.0 |
| 69 | δ-Terpineol | 7299-42-5 | 23.58 | 2.18 | >1.0 | >1.0 |
| 70 | 1,11-Dodecadiyne | 20521-44-2 | 23.67 | 3.44 | - | 1.37 |
| 71 | Humulene | 6753-98-6 | 23.75 | 3.42 | >1.0 | 0.1–1.0 |
| 72 | Z,Z,Z-1,5,9,9-tetramethyl-1,4,7-Cycloundecatriene | - | 23.75 | 3.44 | - | 0.1–1.0 |
| 73 | 2,5-Dihydrotoluene | 4313-57-9 | 23.92 | 2.38 | - | 0.02–0.1 |
| 74 | Neral | 106-26-3 | 24 | 2.34 | 0.1–1.0 | 0.02–0.1 |
| 75 | α-Terpineol | 98-55-5 | 24.17 | 2.24 | 0.02–0.1 | >1.0 |
| 76 | (+)-α-Terpineol | 7785-53-7 | 24.25 | 2.34 | >1.0 | >1.0 |
| 77 | Alloaromadendrene | 25246-27-9 | 24.33 | 3.52 | 0.02–0.1 | 0.1–1.0 |
| 78 | 1-(3-Methylenecyclopentyl)-ethanone | 54829-98-0 | 24.42 | 2.1 | - | 0.02–0.1 |
| 79 | Dodecanal | 112-54-9 | 24.67 | 2.66 | 0.02–0.1 | 0.02–0.1 |
| 80 | Germacrene D | 23986-74-5 | 24.67 | 3.36 | 0.1–1.0 | 0.1–1.0 |
| 81 | α-Phellandren-8-ol | 1686-20-0 | 24.83 | 2.08 | 0.02–0.1 | 0.02–0.1 |
| 82 | 2-Hydroxycineol | 18679-48-6 | 24.92 | 2.26 | - | 0.1–1.0 |
| 83 | (−)-Lavandulyl acetate | 20777-39-3 | 24.92 | 2.58 | 0.1–1.0 | 0.02–0.1 |
| 84 | 4-Methyl-2-pentene | 4461-48-7 | 25.08 | 2.32 | 0.1–1.0 | 0.1–1.0 |
| 85 | Bicyclogermacrene | 24703-35-3 | 25.17 | 3.46 | 0.1–1.0 | 0.02–0.1 |
| 86 | Germacrene B | 15423-57-1 | 25.17 | 3.48 | - | 0.1–1.0 |
| 87 | trans-Pipertiol | 16721-39-4 | 25.25 | 2.1 | 0.1–1.0 | 0.1–1.0 |
| 88 | α-Farnesene | 502-61-4 | 25.42 | 3.04 | 0.02–0.1 | 0.02–0.1 |
| 89 | (+)-δ-Cadinene | 483-76-1 | 25.58 | 3.4 | <0.02 | 0.02–0.1 |
| 90 | Citronellol | 106-22-9 | 25.67 | 2 | <0.02 | 0.02–0.1 |
| 91 | cis-Geraniol | 106-25-2 | 26.42 | 2 | 0.1–1.0 | 0.1–1.0 |
| 92 | Pentanoic acid | 109-52-4 | 27.25 | 1.62 | 0.02–0.1 | 0.02 - 0.1 |
| 93 | 4-(2-Aetylamino-1-(acetyloxy)-ethyl)phenyl acetate | 55044-38-7 | 27.42 | 1.96 | 0.1–1.0 | 0.1–1.0 |
| 94 | Prenyl bromide | 870-63-3 | 27.42 | 1.98 | <0.02 | 0.02–0.1 |
| 95 | Tetraethylene glycol | 112-60-7 | 30.33 | 2.08 | 0.1–1.0 | 0.1–1.0 |
| 96 | Caryophyllene oxide | 1139-30-6 | 30.5 | 2.88 | 0.02–0.1 | 0.02–0.1 |
| 97 | 2-Methoxy-ethanol | 109-86-4 | 30.58 | 2.06 | 0.02–0.1 | 0.02–0.1 |
| 98 | Propylene glycol | 57-55-6 | 31.08 | 2 | 0.02–0.1 | 0.1–1.0 |
| 99 | Nerolidol | 142-50-7 | 31.42 | 2.3 | <0.02 | 0.02–0.1 |
| 100 | Humulene epoxide II | 19888-34-7 | 31.58 | 2.82 | <0.02 | 0.02–0.1 |
| 101 | Elemol | 639-99-6 | 32.17 | 2.34 | 0.02–0.1 | 0.02–0.1 |
| 102 | trans-Z-α-Bisabolene epoxide | - | 32.17 | 2.5 | - | 0.02–0.1 |
| 103 | Guaiol | 489-86-1 | 32.33 | 2.48 | 0.1–1.0 | 0.1–1.0 |
| 104 | Rosifoliol | 63891-61-2 | 33 | 2.52 | 0.02–0.1 | 0.02–0.1 |
| 105 | Spathulenol | 6750-60-3 | 33.08 | 2.42 | - | 0.1 - 1.0 |
| 106 | Triethylene glycol | 112-27-6 | 33.17 | 1.86 | 0.1–1.0 | 0.02–0.1 |
| 107 | Ethyl nitrosourethane | 614-95-9 | 33.58 | 1.24 | <0.02 | 0.02–0.1 |
| 108 | 1,2-Ethanediol | 107-21-1 | 34.08 | 1.4 | 0.1–1.0 | 0.1–1.0 |
| 109 | α-Dihydroionone | 31499-72-6 | 33.42 | 2.42 | 0.02–0.1 | 0.1–1.0 |
| 110 | Diethyl-mercury | 627-44-1 | 34.5 | 1.24 | - | 0.02–0.1 |
| 111 | Bulnesol | 22451-73-6 | 34.58 | 2.48 | 0.02–0.1 | <0.02 |
| 112 | Icosa-9,11-diyne | 28393-07-9 | 34.83 | 2.4 | 0.02–0.1 | 0.1–1.0 |
| 113 | Paraldehyde | 123-63-7 | 35.42 | 2.38 | - | 0.02–0.1 |
| 114 | Limonene dioxide | 96-08-2 | 35.42 | 2.4 | 0.02–0.1 | - |
| 115 | 16-Allopregnene-3β,9α-diol-20-one 3-O-acetate | 106068-45-5 | 35.42 | 2.42 | - | 0.1–1.0 |
| 116 | 2-Hydrazinoethanol | 109-84-2 | 36.25 | 1.72 | - | 0.1–1.0 |
| 117 | Isoeugenol | 97-54-1 | 36.83 | 1.92 | 0.02–0.1 | <0.02 |
| 118 | 1-Bromo-2-propanol | 19686-73-8 | 36.92 | 1.32 | - | 0.02–0.1 |
| 119 | (1-Hydroxyethylidene)malonic acid diethyl ester | 31575-84-5 | 37.25 | 1.26 | >1.0 | 0.1–1.0 |
| 120 | Diethylene glycol | 111-46-6 | 37.25 | 3.36 | 0.1–1.0 | 0.1–1.0 |
| 121 | Eicosane | 112-95-8 | 38.08 | 3.78 | 0.1–1.0 | - |
| 122 | Isopropyl alcohol | 67-63-0 | 38.33 | 1.64 | - | 0.02–0.1 |
| 123 | Pentaethylene glycol | 4792-15-8 | 38.42 | 3.02 | - | 0.02–0.1 |
| 124 | R-(−)-1,2-propanediol | 4254-14-2 | 38.83 | 1.3 | - | 0.02–0.1 |
| 125 | Norpseudoephedrine | 36393-56-3 | 38.92 | 3.76 | 0.1–1.0 | 0.1–1.0 |
| 126 | Metacetaldehyde | 108-62-3 | 39 | 4.76 | 0.1–1.0 | 0.1–1.0 |
| 127 | Ethyl2-(methoxyimino)acetoacetate | 60846-14-2 | 39.08 | 1.6 | - | 0.1–1.0 |
| 128 | Ethanolamine | 141-43-5 | 39.42 | 1.32 | >1.0 | 0.02–0.1 |
| 129 | Erythro-3-bromo-2-pentanol | 159475-12-4 | 39.5 | 1.42 | 0.1–1.0 | 0.02–0.1 |
| 130 | Octaethylene glycol | 5117-19-1 | 40.42 | 4.08 | 0.1–1.0 | 0.1–1.0 |
| 131 | Dimethyl ethylboronate | 7318-82-3 | 40.75 | 1.8 | - | 0.02–0.1 |
| 132 | Cysteinylglycine | 19246-18-5 | 41 | 1.74 | 0.02–0.1 | - |
| 133 | Isophytol | 505-32-8 | 41.33 | 2.46 | 0.02–0.1 | 0.02–0.1 |
| 134 | 2,5,8,11-Tetraoxadodecane | 112-49-2 | 41.42 | 1.74 | <0.02 | 0.02–0.1 |
| 135 | 2-ethoxy-propane | 625-54-7 | 41.42 | 3.84 | 0.064 | 0.02–0.1 |
| 136 | α-Methoxyacetaldehyde | 10312-83-1 | 42.42 | 1.42 | - | 0.1–1.0 |
| 137 | Diethylmethylsilane | 760-32-7 | 42.5 | 2.4 | 0.1–1.0 | 0.02–0.1 |
| 138 | 1-Iodotetradecane | 19218-94-1 | 43.08 | 4.62 | 0.02–0.1 | - |
| 139 | 1-Propoxy-2-propanol | 1569-01-3 | 43.17 | 1.78 | 0.02–0.1 | - |
| 140 | β-Nitroethanol | 625-48-9 | 43.42 | 1.56 | <0.02 | 0.1–1.0 |
| 141 | Trimethylsilane | 993-07-7 | 43.42 | 1.74 | 0.1–1.0 | 0.02–0.1 |
| 142 | Carbitol | 111-90-0 | 43.5 | 1.78 | 0.1–1.0 | 0.1–1.0 |
| 143 | 2-Methylamino-1-ethanol | 109-83-1 | 43.92 | 3.74 | - | 0.02–0.1 |
| 144 | 3,6,9,12-Tetraoxatetradecan-1-ol | 5650-20-4 | 43.92 | 3.8 | 0.1–1.0 | 0.1–1.0 |
| 145 | Methoxytriglycol | 112-35-6 | 44 | 2.28 | 0.02–0.1 | - |
| 146 | Cysteine-alanine | - | 44.08 | 1.74 | 0.02–0.1 | 0.02–0.1 |
| 147 | Ethyltriglycol | 112-50-5 | 44.17 | 3.78 | 0.02–0.1 | <0.02 |
| 148 | Dimethylethylhexadecylamm-onium bromide | 124-03-8 | 44.33 | 1.26 | <0.02 | 0.02–0.1 |
| 149 | Formaldehyde | 50-00-0 | 44.42 | 1.2 | >1.0 | >1.0 |
| 150 | (3-Methyl-oxiran-2-yl)-methanol | - | 44.42 | 1.3 | >1.0 | >1.0 |
| 151 | Ethyldimethyl-silane | 758-21-4 | 44.5 | 1.74 | 0.02–0.1 | 0.02–0.1 |
| 152 | (2-Ethoxy-1-methoxyethoxy)-ethene | 54063-18-2 | 44.58 | 2.28 | - | 0.02–0.1 |
| 153 | (2-Pyrrol2-[2-(2-hydroxy-3-ethoxy)-ethoxy]-4-nitro-phenoxymorpho-ethoxy)-ethanol | - | 44.75 | 2.54 | - | 0.1–1.0 |
| 154 | 3,4-Dimethyl-2-hexanone | 19550-10-8 | 44.92 | 1.8 | - | 0.02–0.1 |
| 155 | Pyruvaldehyde | 78-98-8 | 45 | 1.76 | 0.1–1.0 | 0.1–1.0 |
| 156 | 2-(1-Ethoxyethoxy)-2-(2-oxiranyl)ethanol | 109613-58-3 | 45.17 | 1.78 | 0.02–0.1 | 0.02–0.1 |
| 157 | 2-[2-(Ethenyloxy)ethoxy]-ethanol | 929-37-3 | 45.42 | 1.86 | >1.0 | 0.1–1.0 |
| 158 | Isopropyldimethylsilane | 18209-61-5 | 45.58 | 1.74 | 0.02–0.1 | 0.1–1.0 |
| 159 | 15-Crown-5 | 33100-27-5 | 45.75 | 3.86 | - | 0.1–1.0 |
| Total | >89.68 | >87.88 |
| Factors | Levels | Other Conditions |
|---|---|---|
| microwave power (W) | 500, 600, 700, 800, 900 | 6:1 mL/g, 60 min |
| liquid-to-material ratio (mL/g) | 2:1, 4:1, 6:1, 8:1, 10:1 | optimized microwave power, 60 min |
| extraction time (min) | 20, 30, 40, 50, 60 | optimized microwave power and liquid-to-material ratio |
| Factors | Codes | Levels | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| microwave power (W) | X1 | 631.82 | 700 | 800 | 900 | 968.18 |
| liquid-to-material ratio (mL/g) | X2 | 1:2.64 | 4:1 | 6:1 | 8:1 | 9.36:1 |
| extraction time (min) | X3 | 13.18 | 20 | 30 | 40 | 46.82 |
| First Column | Second Column | |
|---|---|---|
| stationary phase | DB-WAX | DB-17 |
| dimension | 30 m × 0.25 mm | 1.7 m × 0.10 mm |
| film thickness | 0.25 μm | 0.10 μm |
| polarity | strong | medium |
| corporation | J&W Scientific, Folsom, CA | J&W Scientific, Folsom, CA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, A.; Gan, R.-Y.; Zhang, J.-R.; Xu, X.-Y.; Luo, M.; Liu, H.-Y.; Li, H.-B. Optimization and Characterization of Microwave-Assisted Hydro-Distillation Extraction of Essential Oils from Cinnamomum camphora Leaf and Recovery of Polyphenols from Extract Fluid. Molecules 2020, 25, 3213. https://doi.org/10.3390/molecules25143213
Shang A, Gan R-Y, Zhang J-R, Xu X-Y, Luo M, Liu H-Y, Li H-B. Optimization and Characterization of Microwave-Assisted Hydro-Distillation Extraction of Essential Oils from Cinnamomum camphora Leaf and Recovery of Polyphenols from Extract Fluid. Molecules. 2020; 25(14):3213. https://doi.org/10.3390/molecules25143213
Chicago/Turabian StyleShang, Ao, Ren-You Gan, Jia-Rong Zhang, Xiao-Yu Xu, Min Luo, Hong-Yan Liu, and Hua-Bin Li. 2020. "Optimization and Characterization of Microwave-Assisted Hydro-Distillation Extraction of Essential Oils from Cinnamomum camphora Leaf and Recovery of Polyphenols from Extract Fluid" Molecules 25, no. 14: 3213. https://doi.org/10.3390/molecules25143213
APA StyleShang, A., Gan, R.-Y., Zhang, J.-R., Xu, X.-Y., Luo, M., Liu, H.-Y., & Li, H.-B. (2020). Optimization and Characterization of Microwave-Assisted Hydro-Distillation Extraction of Essential Oils from Cinnamomum camphora Leaf and Recovery of Polyphenols from Extract Fluid. Molecules, 25(14), 3213. https://doi.org/10.3390/molecules25143213








