Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile
Abstract
:1. Introduction
2. Results
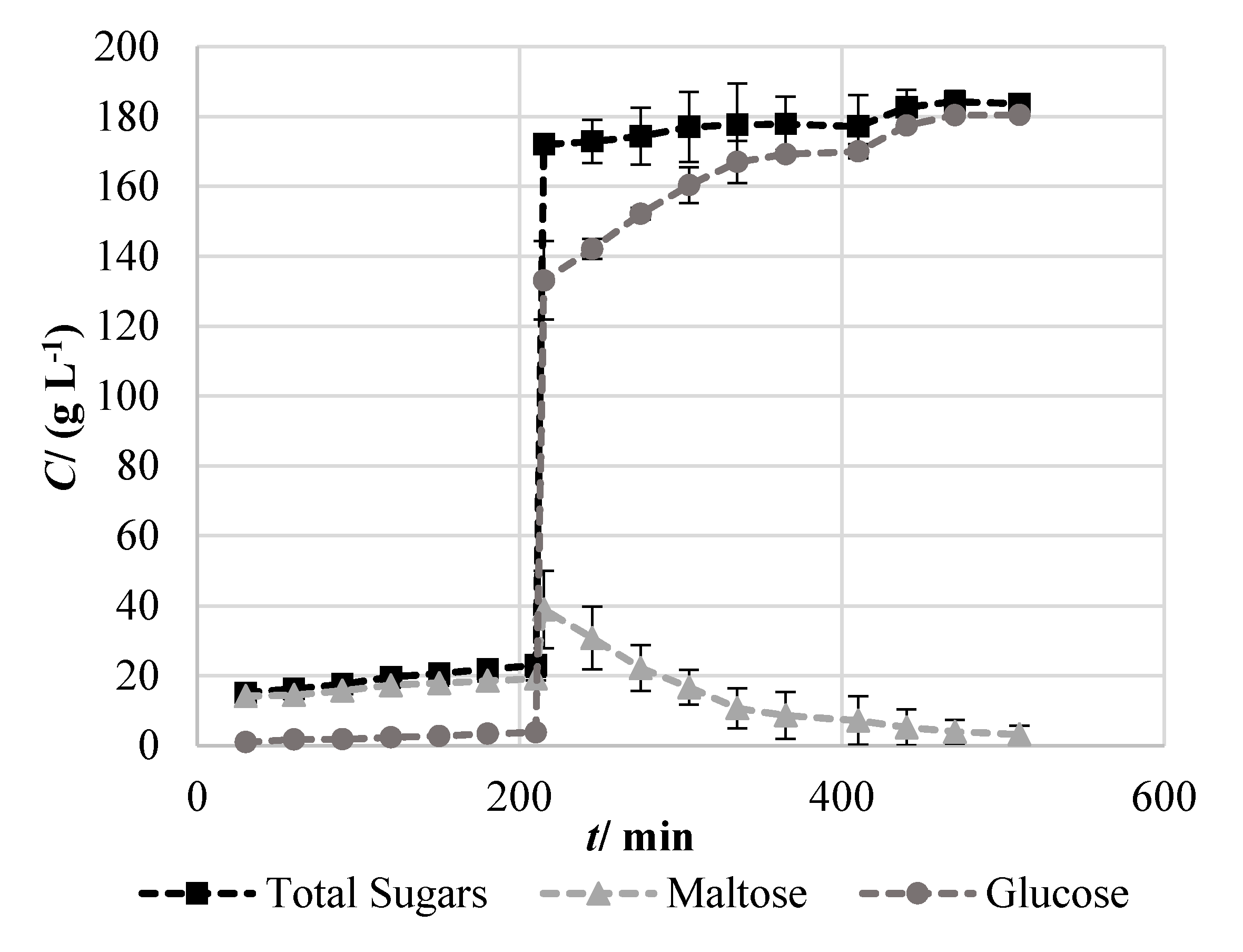
2.1. Production of a Cassava-Based Fermentable Broth
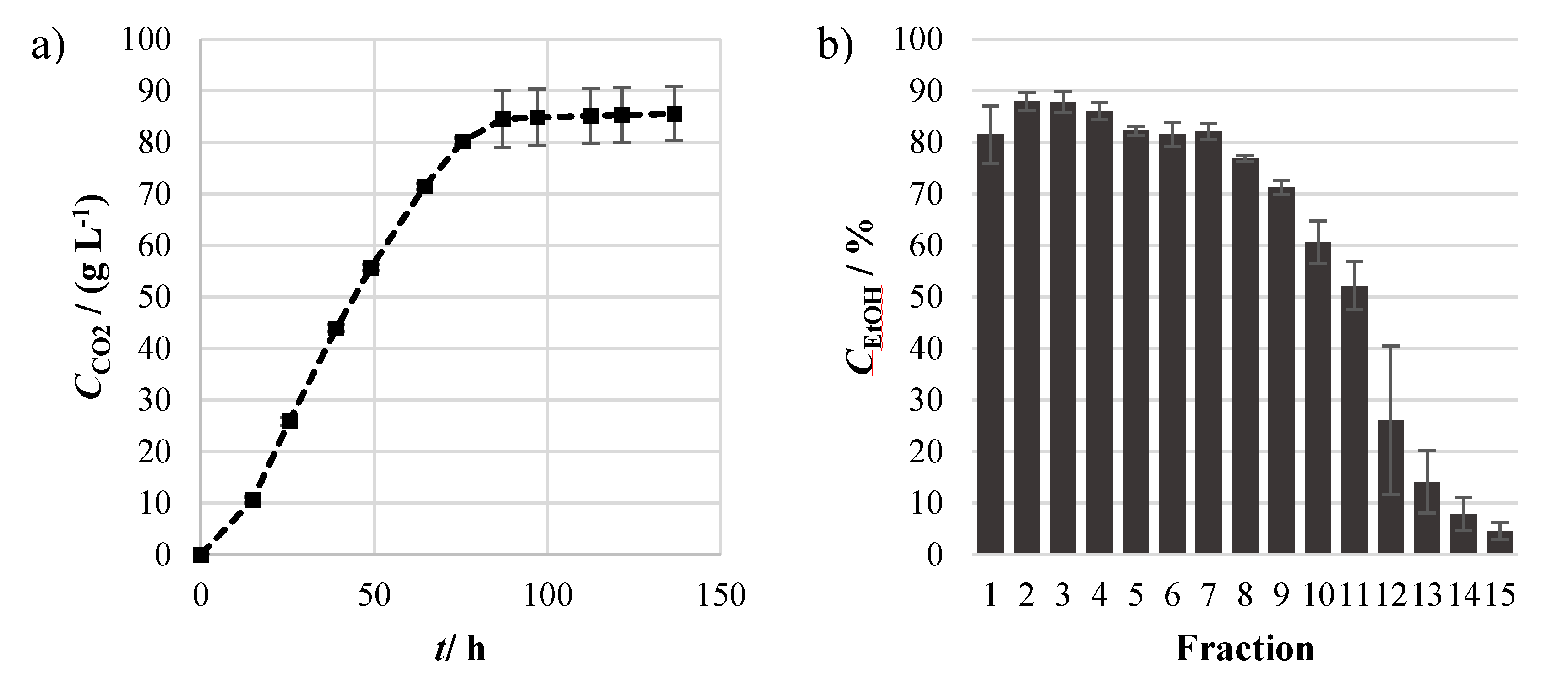
2.2. Fermentation and Distillation for Production of a Cassava Spirit
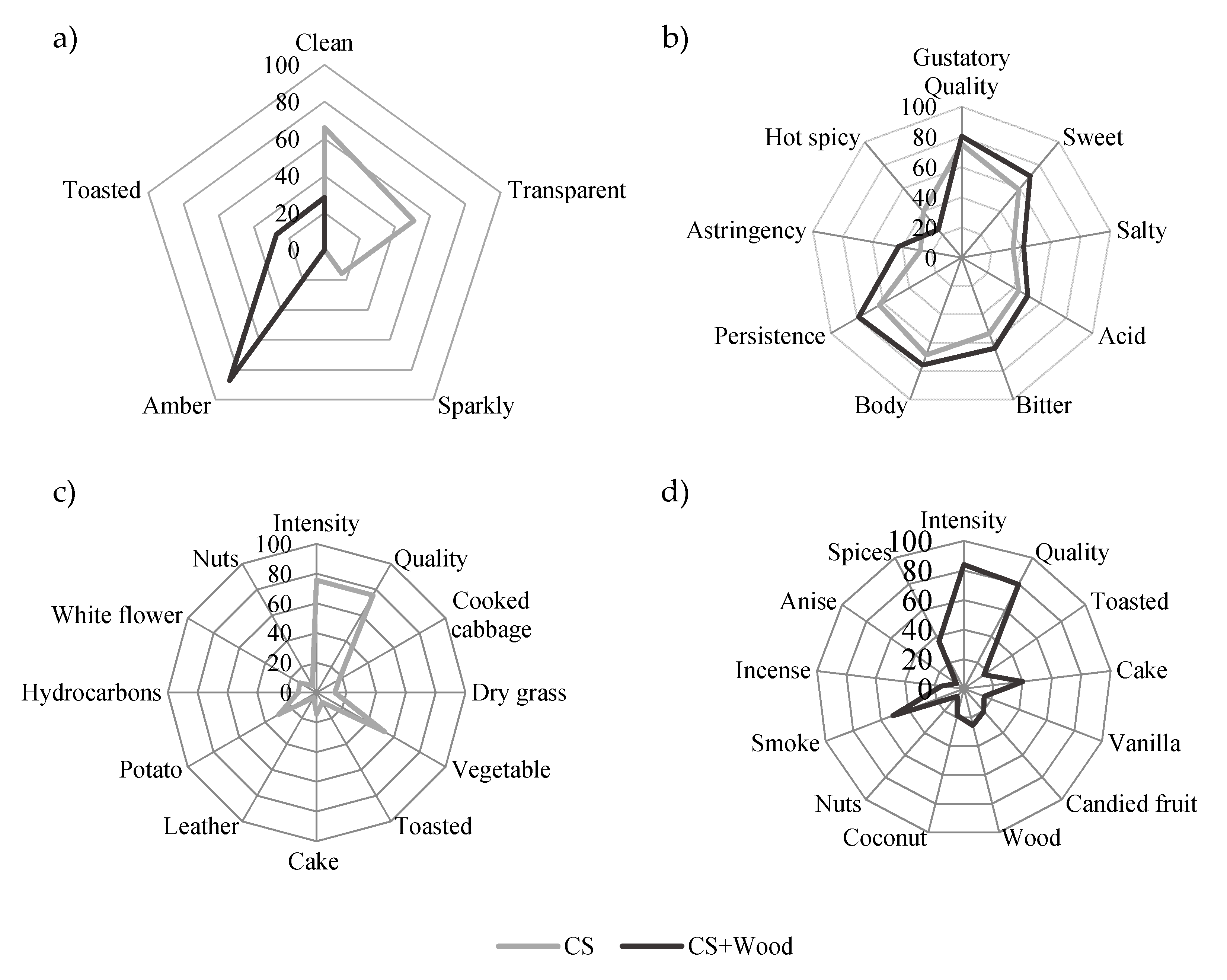
2.3. Characterization of Cassava Spirits
3. Materials and Methods
3.1. Chemicals, Strains, and Raw Materials
3.2. Preparation of a Fermentable Cassava Broth
3.3. Alcoholic Fermentation
3.4. Distillation
3.5. Contact with Wood
3.6. Quantification of Fermentable Sugars and Ethanol
3.7. Analysis of Major Volatile Compounds
3.8. Analysis of Minor Volatile Compounds
3.9. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Liu, J.; Zhang, P.; He, S. Root and Tuber Crops. Encycl. Agric. Food Syst. 2014, 5, 46–61. [Google Scholar]
- Parmar, A.; Sturm, B.; Hensel, O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- Uchechukwu-Agua, A.D.; Caleb, O.J.; Opara, U.L. Postharvest Handling and Storage of Fresh Cassava Root and Products: a Review. Food Bioprocess Technol. 2015, 8, 729–748. [Google Scholar] [CrossRef]
- Jakrawatana, N.; Pingmuangleka, P.; Gheewala, S.H. Material flow management and cleaner production of cassava processing for future food, feed and fuel in Thailand. J. Clean. Prod. 2016, 134, 633–641. [Google Scholar] [CrossRef]
- Alene, A.D.; Abdoulaye, T.; Rusike, J.; Labarta, R.; Creamer, B.; del Río, M.; Ceballos, H.; Becerra, L.A. Identifying crop research priorities based on potential economic and poverty reduction impacts: The case of cassava in Africa, Asia, and Latin America. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Enete, A.; Igbokwe, E. Cassava Market Participation Decisions of Producing Households in Africa. Tropicultura 2009, 27, 129–136. [Google Scholar]
- Colehour, A.M.; Meadow, J.F.; Liebert, M.A.; Cepon-Robins, T.J.; Gildner, T.E.; Urlacher, S.S.; Bohannan, B.J.M.; Snodgrass, J.J.; Sugiyama, L.S. Local domestication of lactic acid bacteria via cassava beer fermentation. PeerJ 2014, 2014, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Das, A.J.; Seth, D.; Miyaji, T.; Deka, S.C. Fermentation optimization for a probiotic local northeastern Indian rice beer and application to local cassava and plantain beer production. J. Inst. Brew. 2015, 121, 273–282. [Google Scholar] [CrossRef]
- Ray, R.C.; Sivakumar, P.S. Traditional and novel fermented foods and beverages from tropical root and tuber crops: review. Int. J. Food Sci. Technol. 2009, 44, 1073–1087. [Google Scholar] [CrossRef]
- Kubo, R.; Funakawa, S.; Araki, S.; Kitabatake, N. Production of indigenous alcoholic beverages in a rural village of Cameroon. J. Inst. Brew. 2014, 120, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Brito, V.H. dos S.; Cereda, M.P. Fermented Foods and Beverages from Cassava (Manihot esculenta Crantz) in South America: Abstract; CRC Press: Boca Raton, FL, USA, 2017; pp. 202–223. [Google Scholar]
- Lyumugabe, F.; Songa, E.B. Traditional Fermented Alcoholic Beverages of Rwanda (Ikigage, Urwagwa, and Kanyanga): Production and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166857. [Google Scholar]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, F.D.; Gasparotto, J.M.; Klauck, E.; Werle, L.B.; Jahn, S.L.; Hoffmann, R.; Mazutti, M.A. Conversion of cassava starch to ethanol and a byproduct under different hydrolysis conditions. Starch/Staerke 2015, 67, 620–628. [Google Scholar] [CrossRef]
- Ruiz, M.I.; Sanchez, C.I.; Torrresa, R.G.; Molina, D.R. Enzymatic hydrolysis of cassava starch for production of bioethanol with a colombian wild yeast strain. J. Braz. Chem. Soc. 2011, 22, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Coelho, E.; Teixeira, J.A.; Domingues, L.; Tavares, T.; Oliveira, J.M. Factors affecting extraction of adsorbed wine volatile compounds and wood extractives from used oak wood. Food Chem. 2019, 295, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoph, N.; Bauer-Christoph, C. Flavour of spirit drinks: Raw materials, fermentation, distillation, and ageing. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–239. ISBN 9783540493389. [Google Scholar]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, E.S.P.; Cardoso, D.R.; Franco, D.W. Quantitative Ester Analysis in Cachaça and Distilled Spirits by Gas Chromatography−Mass Spectrometry (GC−MS). J. Agric. Food Chem. 2008, 56, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Domingues, L.; Teixeira, J.A.; Oliveira, J.M.; Tavares, T. Understanding wine sorption by oak wood: Modeling of wine uptake and characterization of volatile compounds retention. Food Res. Int. 2019, 116, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Cadahía, E.; Fernández de Simón, B.; Jalocha, J. Volatile compounds in Spanish, French, and American oak woods after natural seasoning and toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Silva, R.; Aguiar, T.Q.; Coelho, E.; Jiménez, A.; Revuelta, J.L.; Domingues, L. Metabolic engineering of Ashbya gossypii for deciphering the de novo biosynthesis of γ-lactones. Microb. Cell Fact. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Lyu, J.; Nam, P.W.; Lee, S.J.; Lee, K.G. Volatile compounds isolated from rice beers brewed with three medicinal plants. J. Inst. Brew. 2013, 119, 271–279. [Google Scholar] [CrossRef]
- Marconi, O.; Sileoni, V.; Ceccaroni, D.; Perretti, G. The Use of Rice in Brewing. Adv. Int. Rice Res. 2017. [Google Scholar]
- Poisson, L.; Schieberle, P. Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Santos, R.; Ricardo-Da-Silva, J.M.; Anjos, O.; Mira, H.; Belchior, A.P.; Canas, S. Kinetics of odorant compounds in wine brandies aged in different systems. Food Chem. 2016, 211, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cai, J.; Duan, C.Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Kelbert, M.; Romaní, A.; Coelho, E.; Pereira, F.B.; Teixeira, J.A.; Domingues, L. Simultaneous Saccharification and Fermentation of Hydrothermal Pretreated Lignocellulosic Biomass: Evaluation of Process Performance Under Multiple Stress Conditions. Bioenergy Res. 2016, 9, 750–762. [Google Scholar] [CrossRef] [Green Version]
- International Organisation of Vine and Wine. Determination of sugars in spirit drinks of viti-vinicultural origin (OIV-MA-BS-11). In Compendium of International Methods of Analysis of Spirituous Beverages of Vitivinicultural Origin; International Organisation of Vine and Wine: Paris, France, 2019. [Google Scholar]
- International Organisation of Vine and Wine. Determination of the principal volatile substances of spirit drinks of viti-vinicultural origin (OIV-MA-BS-14). In Compendium of International Methods of Analysis of Spirituous Beverages of Vitivinicultural Origin; International Organisation of Vine and Wine: Paris, France, 2019. [Google Scholar]
- Coelho, E.; Lemos, M.; Genisheva, Z.; Domingues, L.; Vilanova, M.; Oliveira, J.M. Validation of a LLME/GC-MS methodology for quantification of volatile compounds in fermented beverages. Molecules 2020, 25, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization. Sensory Analysis-General Guidance for the Design of Test Rooms (ISO 8589:2007); ISO, International Organization for Standardization: Paris, France, 2007. [Google Scholar]
- Lawless, H.T. Heymann Sensory Evaluation of Food Principles and Practices Second Edition; Springer: New York, NY, USA, 1998. [Google Scholar]
- International Organization for Standardization. Sensory Analysis-Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional approach (ISO 11035:1994); ISO, International Organization for Standardization: Paris, France, 1994. [Google Scholar]
- Dravnieks, A.; Bock, F.C.; Powers, J.J.; Tibbetts, M.; Ford, M. Comparison of odors directly and through profiling. Chem. Senses 1978, 3, 191–225. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Cassava Spirit | Cassava Spirit + Oak Chips | PT | Descriptor | |
|---|---|---|---|---|
| C (mg L−1) | C (mg L−1) | C (mg L−1) | ||
| acetaldehyde | 39.2 ± 25.6 | 44.0 ± 33.1 | 10 | Fresh, green |
| ethyl acetate | 101.0 ± 55.2 | 124.6 ± 73.3 | 7.5 | Solvent, fruity |
| methanol | 23.7 ± 1.7 | 24.6 ± 0.5 | ||
| 1-propanol | 22.1 ± 4.7 | 24.3 ± 5.3 | 830 | |
| 2-methyl-1-propanol | 88.1 ± 5.0 | 101.6 ± 14.1 | 40;75 | Malty |
| 2-methyl-1-butanol | 77.0 ± 4.3 | 85.9 ± 7.9 | 7;30 | Malty, solvent |
| 3-methyl-1-butanol | 478.4 ± 15.9 | 522.6 ± 37.6 | 7;30 | Malty |
| 2-phenylethanol | 24.5 ± 3.1 | 27.0 ± 3.2 | 7.5;10 | Flowery |
| Compound | LRI | Cassava Spirit | Cassava Spirit+ Oak Chips | II | PT | Descriptor |
|---|---|---|---|---|---|---|
| C (µg L−1) | C (µg L−1) | (m/z) | ||||
| Esters | ||||||
| isoamyl acetate | 1119 | 1605.5 ± 30.6 | 1451.1 ± 229.3 | 43 + 55 + 70 | 30 | Banana |
| ethyl hexanoate | 1229 | 863.1 ± 26.9 | 848.3 ± 40.4 | 43 + 88 + 145 | 5 | Fruity, Green Apple |
| ethyl octanoate | 1429 | 350.9 ± 9.1 | 398.8 ± 15.7 | 55 + 88 + 127 | 2; 26 | Apple, fruity, sweet |
| diethyl succinate | 1668 | - | 33.3 ± 8.5 | 101 + 129 | 100,000 | |
| 2-phenylethyl acetate | 1801 | 335.4 ± 9.8 | 249.3 ± 14.3 | 43 + 104 | 250 | Flowery, sweet |
| ethyl hexadecanoate | 2249 | 488.8 ± 35.6 | 80.6 ± 13.4 | 55 + 101 + 157 + 241 + 284 | ||
| Alcohols | ||||||
| 1-hexanol | 1344 | 172.1 ± 1.1 | 179.1 ± 2.1 | 56 + 69 | 500; 2500 | |
| Furan compounds | ||||||
| furfural | 1471 | 413.0 ± 53.0 | 1381.4 ± 37.3 | 39 + 95 | 8000 | Smoky, almond |
| 5-methylfurfural | 1564 | - | 207.8 ± 14.1 | 53 + 109 | ||
| Acids | ||||||
| hexanoic acid | 1851 | 215.4 ± 25.9 | 406.8 ± 36.9 | 60 + 99 | 3000;8000 | Fatty acids, vegetable oil |
| octanoic acid | 2065 | 351.8 ± 28.7 | 438.2 ± 27.8 | 60 + 101 | 8800; 10,000 | Fatty acids, vegetable oil |
| Phenolic compounds | ||||||
| guaiacol | 1848 | - | 79.5 ± 22.4 | 81 + 109 + 124 | 5 | Smoky, almond |
| eugenol | 2150 | - | 40.3 ± 4.6 | 77 + 103 + 164 | 7 | Spicy, clove |
| 2,6-dimethoxyphenol | 2249 | - | 293.9 ± 16.3 | 65 + 93 + 154 | ||
| Lactones | ||||||
| cis-oak lactone | 1870 | - | 944.4 ± 3.7 | 41 + 71 + 99 | 20 | Oak, wood |
| trans-oak lactone | 1938 | - | 378.2 ± 16.2 | 41 + 69 + 99 | 20 | |
| γ-nonalactone | 2009 | 1038.0 ± 24.1 | 1193.2 ± 221.2 | 85 | ||
| γ -decalactone | 2122 | 40.6 ± 0.3 | 63.1 ± 4.4 | 85 | 5; 10 | Fruity, peach |
| Aldehydes | ||||||
| 5-acetoxymethyl-2-furaldehyde | 2183 | - | 130.6 ± 16.3 | 43 + 79 + 126 | ||
| vanillin | 2543 | - | 566.3 ± 10.3 | 151 | 100 | Vanilla, spicy |
| syringaldehyde | 2929 | - | 4419.4 ± 60.0 | 182 | ||
| sinapaldehyde | 3510 | - | 10009.3 ± 1072.4 | 45 + 165 + 208 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, E.; Ballesteros, L.F.; Domingues, L.; Vilanova, M.; Teixeira, J.A. Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile. Molecules 2020, 25, 3228. https://doi.org/10.3390/molecules25143228
Coelho E, Ballesteros LF, Domingues L, Vilanova M, Teixeira JA. Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile. Molecules. 2020; 25(14):3228. https://doi.org/10.3390/molecules25143228
Chicago/Turabian StyleCoelho, Eduardo, Lina F. Ballesteros, Lucília Domingues, Mar Vilanova, and José A. Teixeira. 2020. "Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile" Molecules 25, no. 14: 3228. https://doi.org/10.3390/molecules25143228
APA StyleCoelho, E., Ballesteros, L. F., Domingues, L., Vilanova, M., & Teixeira, J. A. (2020). Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile. Molecules, 25(14), 3228. https://doi.org/10.3390/molecules25143228







