Comparison of Antioxidants: The Limited Correlation between Various Assays of Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Lipid Peroxidation
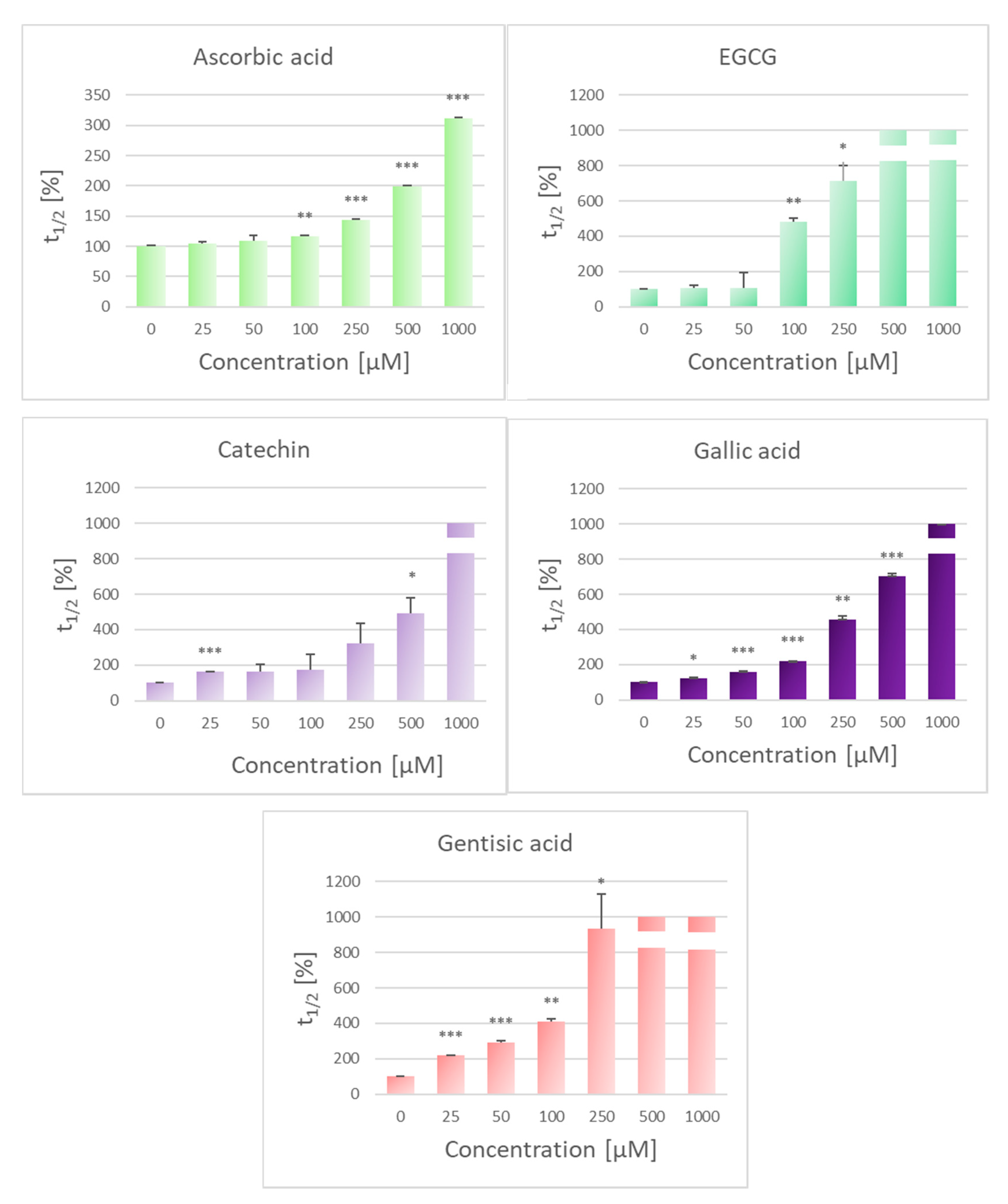
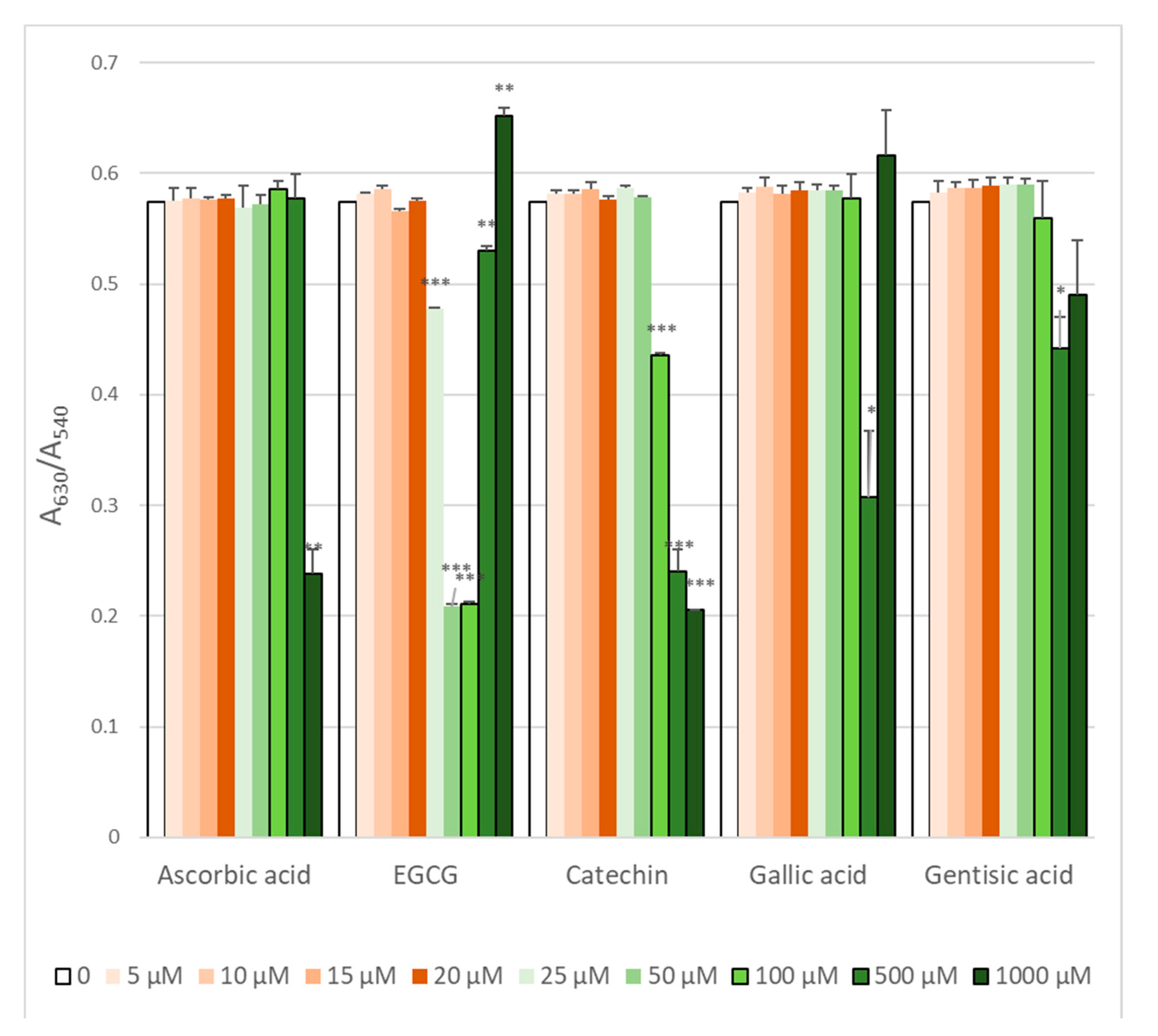
2.2. Protection Against AAPH-Induced Hemolysis
2.3. Attenuation of ROS Level
2.4. Protection against Hemoglobin Oxidation
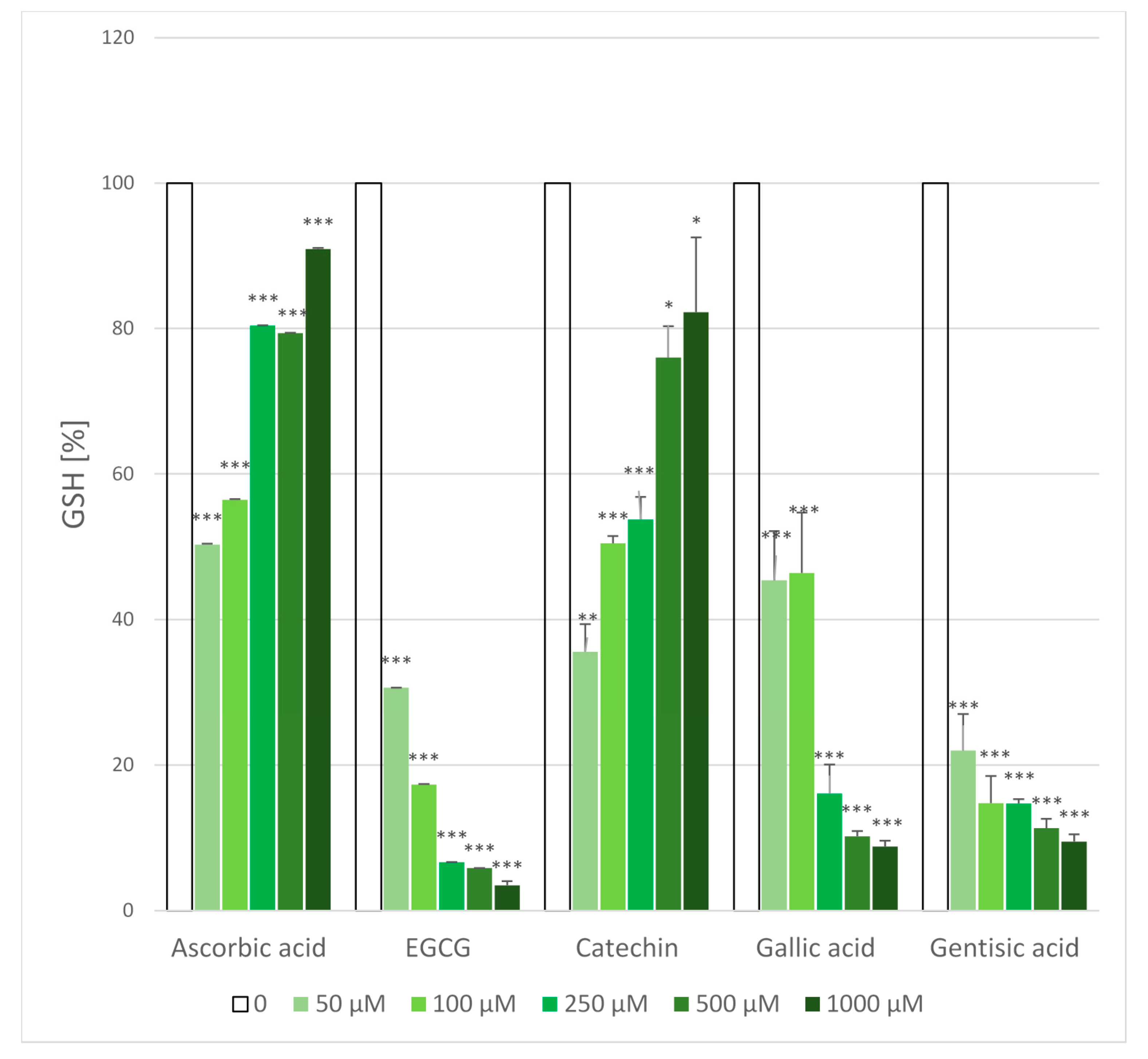
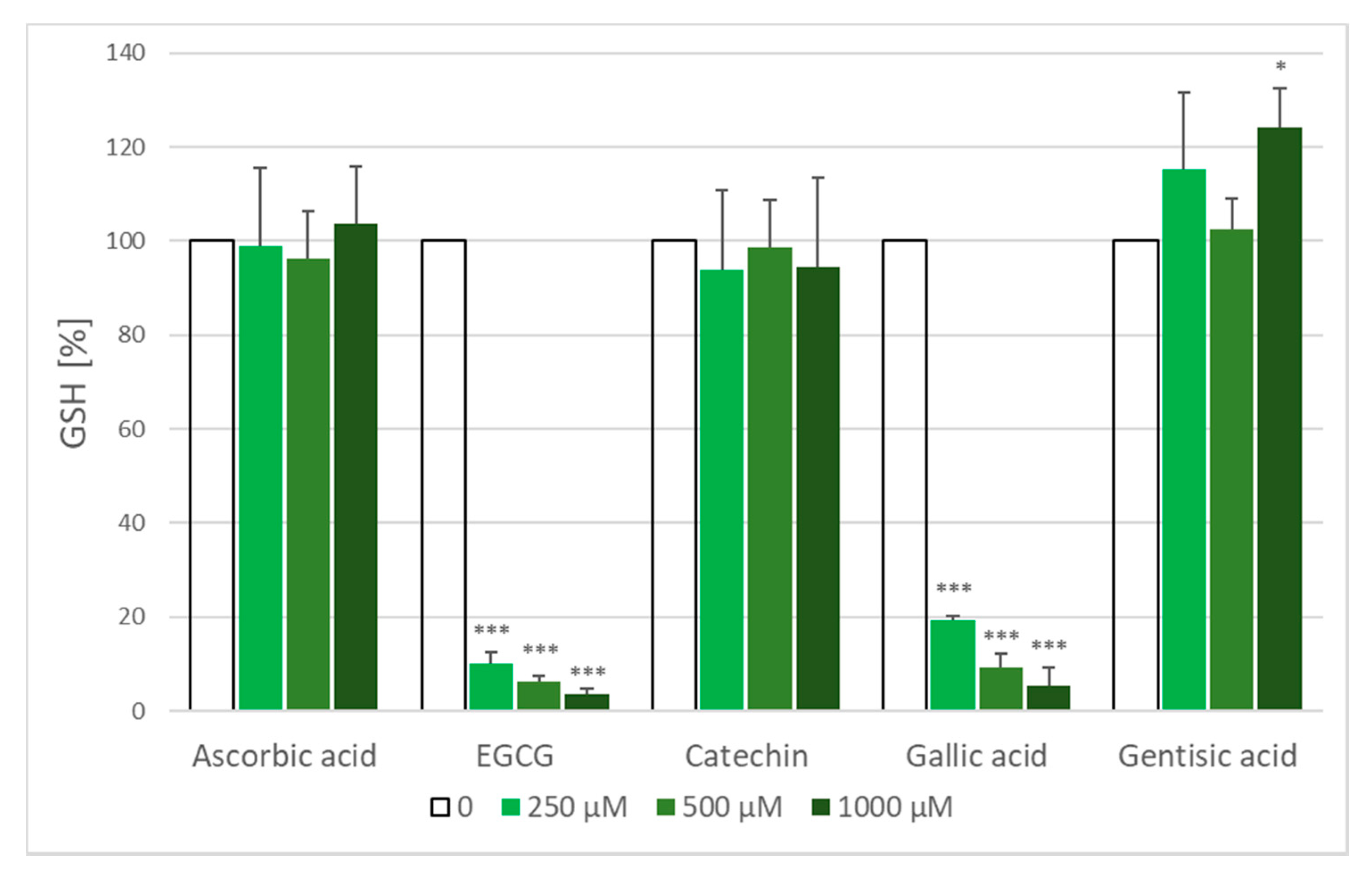
2.5. Glutathione Loss
3. Materials
Chemicals and Equipment
4. Methods
4.1. Experiments in a Cell-Free System
Lipid Peroxidation
4.2. Experiment with Erythrocytes
4.2.1. Ethical Approval
4.2.2. Preparation of Erythrocytes
4.2.3. Preparation of Erythrocyte Ghosts
4.2.4. Estimation of the Protective Effects of Selected Compounds on Erythrocyte Membrane Lipid Peroxidation
4.2.5. The Assay of AAPH-Induced Hemolysis
4.2.6. Determination of Intracellular ROS Generation
4.2.7. Hemoglobin Oxidation
4.2.8. Glutathione Content
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kampa, R.P.; Kicinska, A.; Jarmuszkiewicz, W.; Pasikowska-Piwko, M.; Dolegowska, B.; Debowska, R.; Szewczyk, A.; Bednarczyk, P. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Dermatol. 2019, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT (1). Int. J. Toxicol. 2002, 21 (Suppl. S2), 19–94. [Google Scholar] [PubMed]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef]
- Poulsen, E. Safety evaluation of substances consumed as technical ingredients (food additives). Food Addit. Contam. 1991, 8, 125–133. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Niki, E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990, 186, 100–108. [Google Scholar]
- Zou, C.G.; Agar, N.S.; Jones, G.L. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001, 69, 75–86. [Google Scholar] [CrossRef]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Drummen, G.P.; van Liebergen, L.C.; Op den Kamp, J.A.; Post, J.A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (Micro)Spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Kurtmann, L.; Brüggemann, D.A.; Hoff, S.; Risbo, J.; Skibsted, L.H. Investigation of oxidation in freeze-dried membranes using the fluorescent probe C11-BODIPY(581/591). Cryobiology 2009, 58, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qin, Z.J.; Hu, D.; Munishkina, L.A.; Fink, A.L. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry 2006, 45, 8135–8142. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Sugino, H.; Nitoda, T.; Junoja, L.R. General chemical composition of hen eggs. In Hen Eggs, Their Basic and Applied Science; Yamamoto, T., Juneja, L.R., Hatta, H., Kim, M., Eds.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Ximenes, V.F.; Lopes, M.G.; Petrônio, M.S.; Regasini, L.O.; Silva, D.H.; da Fonseca, L.M. Inhibitory effect of gallic acid and its esters on 2,2′-azobis(2-amidinopropane)hydrochloride (AAPH)-induced hemolysis and depletion of intracellular glutathione in erythrocytes. J. Agric. Food Chem. 2010, 58, 5355–5362. [Google Scholar] [CrossRef]
- Sato, Y.; Kamo, S.; Takahashi, T.; Suzuki, Y. Mechanism of free radical-induced hemolysis of human erythrocytes: Hemolysis by water-soluble radical initiator. Biochemistry 1995, 34, 8940–8949. [Google Scholar] [CrossRef]
- Kalender, Y.; Kaya, S.; Durak, D.; Uzun, F.G.; Demir, F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012, 33, 141–148. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; da Silva Campos, R.A.; Borguini, M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef] [Green Version]
- Milella, L.; Caruso, M.; Galgano, F.; Favati, F.; Padula, M.C.; Martelli, G. Role of the cultivar in choosing Clementine fruits with a high level of health-promoting compounds. J. Agric. Food Chem. 2011, 59, 5293–5298. [Google Scholar] [CrossRef]
- Fujiki, H. Green tea: Health benefits as cancer preventive for humans. Chem. Rec. 2005, 5, 119–132. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Proc. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Reckziegel, P.; Dias, V.T.; Benvegnú, D.M.; Boufleur, N.; Barcelos, R.C.S.; Segat, H.J.; Pase, C.S.; Dos Santos, C.M.M.; Flores, É.M.M.; Bürger, M.E. Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol. Rep. 2016, 3, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Ashidate, K.; Kawamura, M.; Mimura, D.; Tohda, H.; Miyazaki, S.; Teramoto, T.; Yamamoto, Y.; Hirata, Y. Gentisic acid, an aspirin metabolite, inhibits oxidation of low-density lipoprotein and the formation of cholesterol ester hydroperoxides in human plasma. Eur. J. Pharmacol. 2005, 513, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant activity and free radical scavenging reactions of gentisic acid: In-vitro and pulse radiolysis studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef]
- Nakayama, T.; Hashimoto, T.; Kajiya, K.; Kumazawa, S. Affinity of polyphenols for lipid bilayers. Biofactors 2000, 13, 147–151. [Google Scholar] [CrossRef]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramovic, H.; Grobin, B.; Poklar Ulrih, N.; Blaˇz Cigi, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef] [Green Version]
- McCay, P.B. Vitamin E: Interactions with free radicals and ascorbate. Annu. Rev. Nutr. 1985, 5, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Li, K.; Ng, P.C.; Fung, K.P.; Li, C.L.; Wong, R.P.-O.; Chui, K.M.; Gu, G.J.-S.; Yung, E.; Wang, C.C.; et al. Pro-oxidative effects of tea and polyphenols, epigallocatechin-3-gallate and epigallocatechin, on G6PD-deficient erythrocytes in vitro. Int. J. Mol. Med. 2006, 18, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Rodacka, A.; Strumillo, J.; Serafin, E.; Puchala, M. Effect of Resveratrol and Tiron on the Inactivation of Glyceraldehyde-3-phosphate Dehydrogenase Induced by Superoxide Anion Radical. Curr. Med. Chem. 2014, 21, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Strumillo, J.; Nowak, K.E.; Krokosz, A.; Rodacka, A.; Puchala, M.; Bartosz, G. The role of resveratrol and melatonin in the nitric oxide and its oxidation products mediated functional and structural modifications of two glycolytic enzymes: GAPDH and LDH. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 877–885. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y. Protection of wheat bran feruloyl oligosaccharides against free radical-induced oxidative damage in normal human erythrocytes. Food Chem. Toxicol. 2009, 47, 1591–1599. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of caffeic acid in peanut sprouts and evaluation of its in vitro effectiveness against oxidative stress-induced erythrocyte hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef]
- Senft, A.; Dalton, T.; Shertzer, H. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef]
Sample Availability: Samples of the antioxidants are available from the authors on request. |



| Compound | IC50 Egg Yolk [µM] | IC50 Erythrocyte Membranes [µM] |
|---|---|---|
| Synthetic antioxidants | ||
| t-BHQ | 12.04 ± 3.55 | 37.57 ± 1.91 *** |
| Pyrogallol | 60.79 ± 14.17 | 70.80 ± 6.46 |
| Trolox | 62.78 ± 2.90 | 87.46 ± 7.38 ** |
| BHA | 71.42 ± 15.54 | 50.04 ± 5.26 * |
| N-Acetyl-l-cysteine | 231.50 ± 2.21 | 554.99 ± 60.34 *** |
| Phenolic acids | ||
| Gallic acid | 15.33 ± 6.81 | 72.35 ± 4.09 *** |
| Caffeic acid | 15.45 ± 0.33 | 34.92 ± 0.40 *** |
| Propyl gallate | 19.60 ± 3.04 | 65.32 ± 3.27 *** |
| Chlorogenic acid | 22.92 ± 1.91 | 33.33 ± 1.22 *** |
| 2,5-Dihydroxybenzoic acid (Gentisic acid) | 29.38 ± 6.22 | 56.52 ± 0.60 *** |
| Vanillic acid | 34.67 ± 6.34 | 114.72 ± 17.69 *** |
| Ferulic acid | 80.80 ± 2.33 | 74.87 ± 1.12 ** |
| p-Coumaric acid | 89.28 ± 0.12 | 256.39 ± 31.65 *** |
| Sinapic acid | 124.71 ± 4.67 | 40.83 ± 1.6 *** |
| Flavonoids | ||
| (−)-Epicatechin gallate | 13.68 ± 0.51 | 14.46 ± 0.39 |
| (−)-Epicatechin | 16.45 ± 1.22 | 27.06 ± 1.04 *** |
| Quercetin | 17.89 ± 0.83 | 27.67 ± 1.29 *** |
| (−)-Epigallocatechin gallate | 18.16 ± 0.55 | 22.64 ± 0.03 *** |
| (+)-Catechin | 21.26 ± 0.67 | 30.51 ± 1.1 *** |
| Mangiferin | 21.98 ± 6.32 | 35.95 ± 3.59 * |
| (−)-Epigallocatechin | 28.35 ± 0.15 | 61.34 ± 2.48 *** |
| Rutin trihydrate | 34.40 ± 0.63 | 27.94 ± 1.12 *** |
| Daidzein | 67.37 ± 6.36 | 3331.1 ± 429.76 *** |
| Hesperidin | 84.41 ± 7.23 | 242.86 ± 3.72 *** |
| Naringenin | 91.67 ± 5.40 | 441.84 ± 30.72 *** |
| Naringin | 359.11 ± 59.28 | 1782.29 ± 76.34 *** |
| Hesperetin | 426.96 ± 18.13 | 165.58 ± 6.15 *** |
| Other natural antioxidants | ||
| Melatonin | 14.37 ± 4.29 | 1098.96 ± 65.19 *** |
| Resveratrol | 31.38 ± 2.79 | 35.57 ± 3.59 |
| β-Carotene | 66.19 ± 10.40 | 748.75 ± 20.35 *** |
| l-Ascorbic acid | 118.71 ± 5.38 | 138.19 ± 7.24 * |
| l-Cysteine | 241.95 ± 52.86 | 705.92 ± 32.68 *** |
| Glutathione | 341.85 ± 7.09 | 655.90 ± 41.89 *** |
| Compound | IC50 [µM] |
|---|---|
| Ascorbic acid | 209.7 ± 35.2 |
| EGCG | 64.0 ± 18.2 |
| Catechin | 138.5 ± 29.4 |
| Gallic acid | 140.3 ± 20.0 |
| Gentisic acid | 333.3 ± 46.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naparło, K.; Soszyński, M.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of Antioxidants: The Limited Correlation between Various Assays of Antioxidant Activity. Molecules 2020, 25, 3244. https://doi.org/10.3390/molecules25143244
Naparło K, Soszyński M, Bartosz G, Sadowska-Bartosz I. Comparison of Antioxidants: The Limited Correlation between Various Assays of Antioxidant Activity. Molecules. 2020; 25(14):3244. https://doi.org/10.3390/molecules25143244
Chicago/Turabian StyleNaparło, Katarzyna, Mirosław Soszyński, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2020. "Comparison of Antioxidants: The Limited Correlation between Various Assays of Antioxidant Activity" Molecules 25, no. 14: 3244. https://doi.org/10.3390/molecules25143244
APA StyleNaparło, K., Soszyński, M., Bartosz, G., & Sadowska-Bartosz, I. (2020). Comparison of Antioxidants: The Limited Correlation between Various Assays of Antioxidant Activity. Molecules, 25(14), 3244. https://doi.org/10.3390/molecules25143244








