Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy
Abstract
1. Introduction
2. Synthesis of New Corrole Derivatives
2.1. Classical Synthetic Methodologies
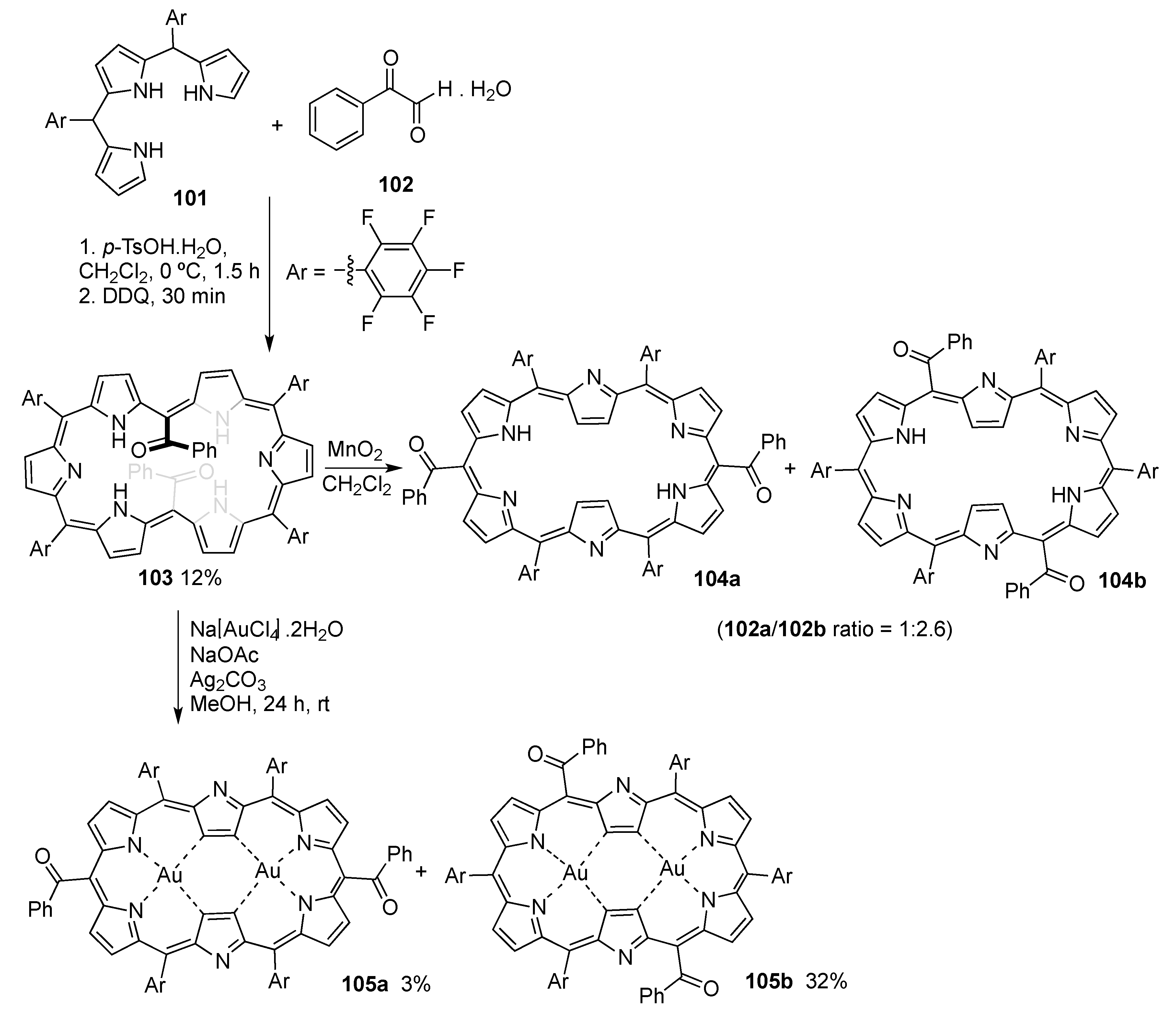
2.2. New Synthetic Methodologies
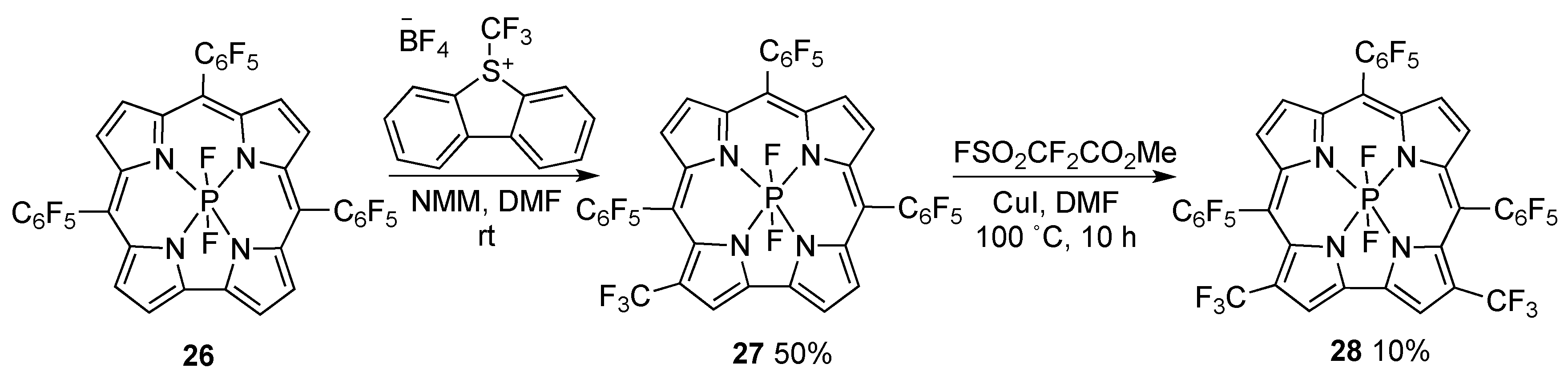
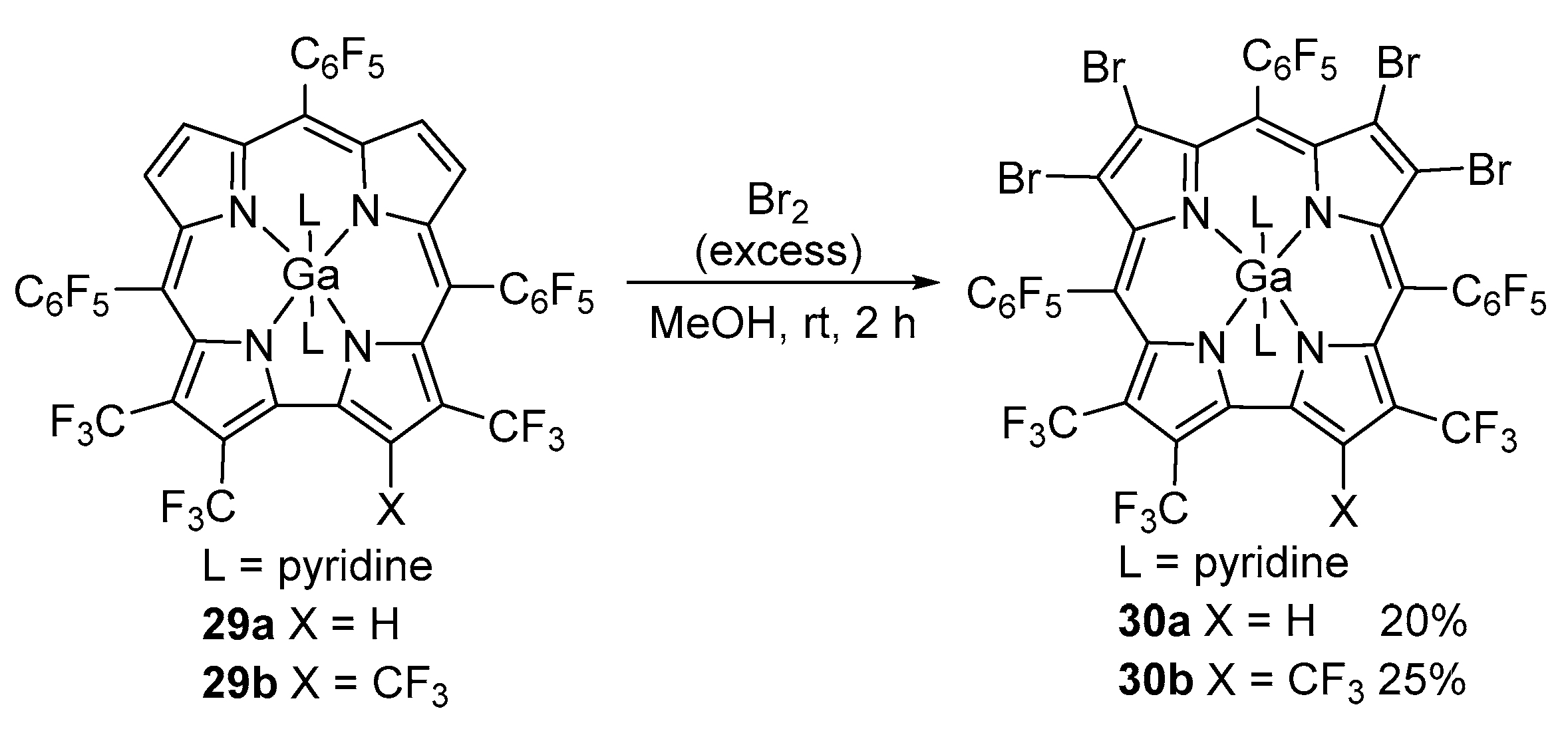
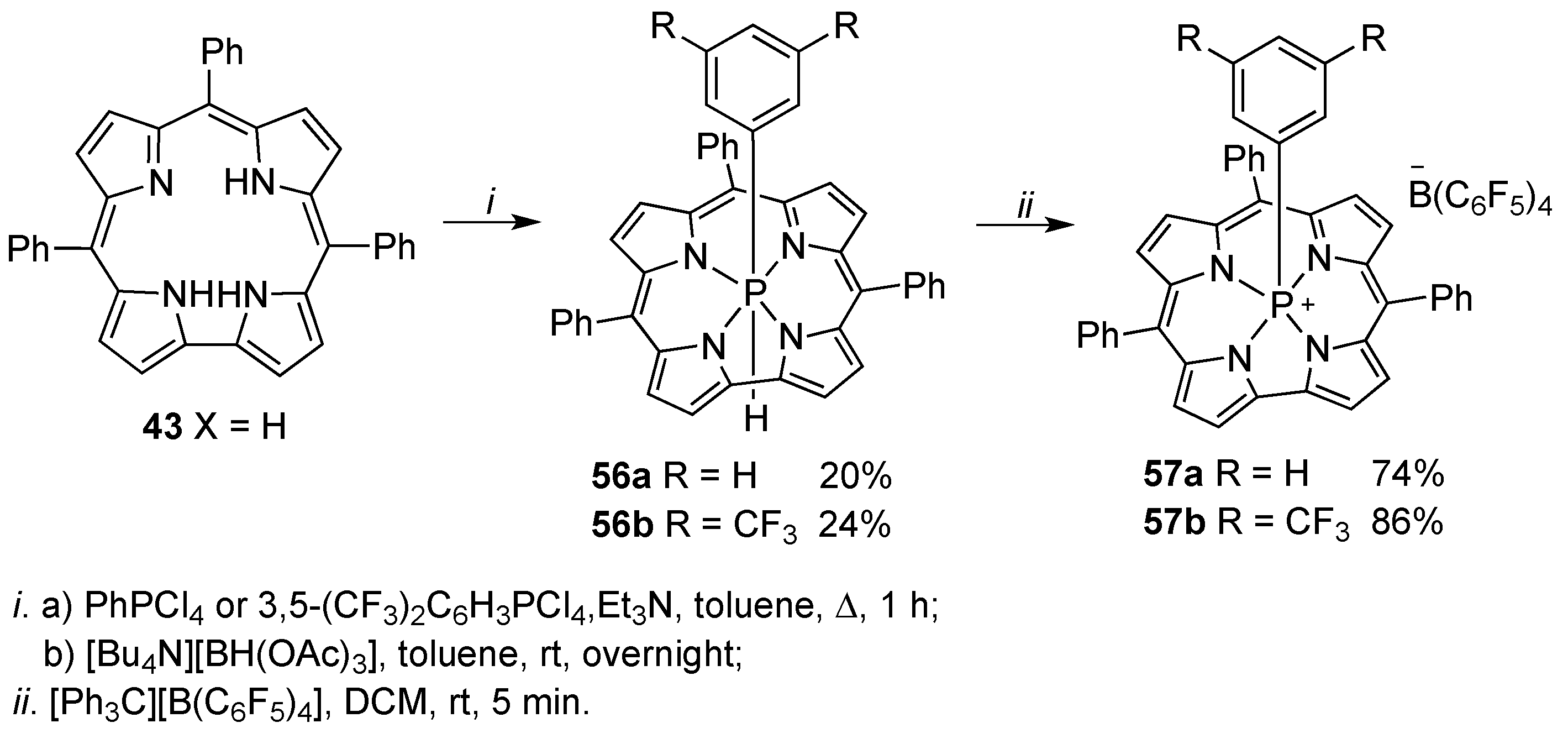
3. Functionalization at the Inner Core and Peripheral Positions of Corroles
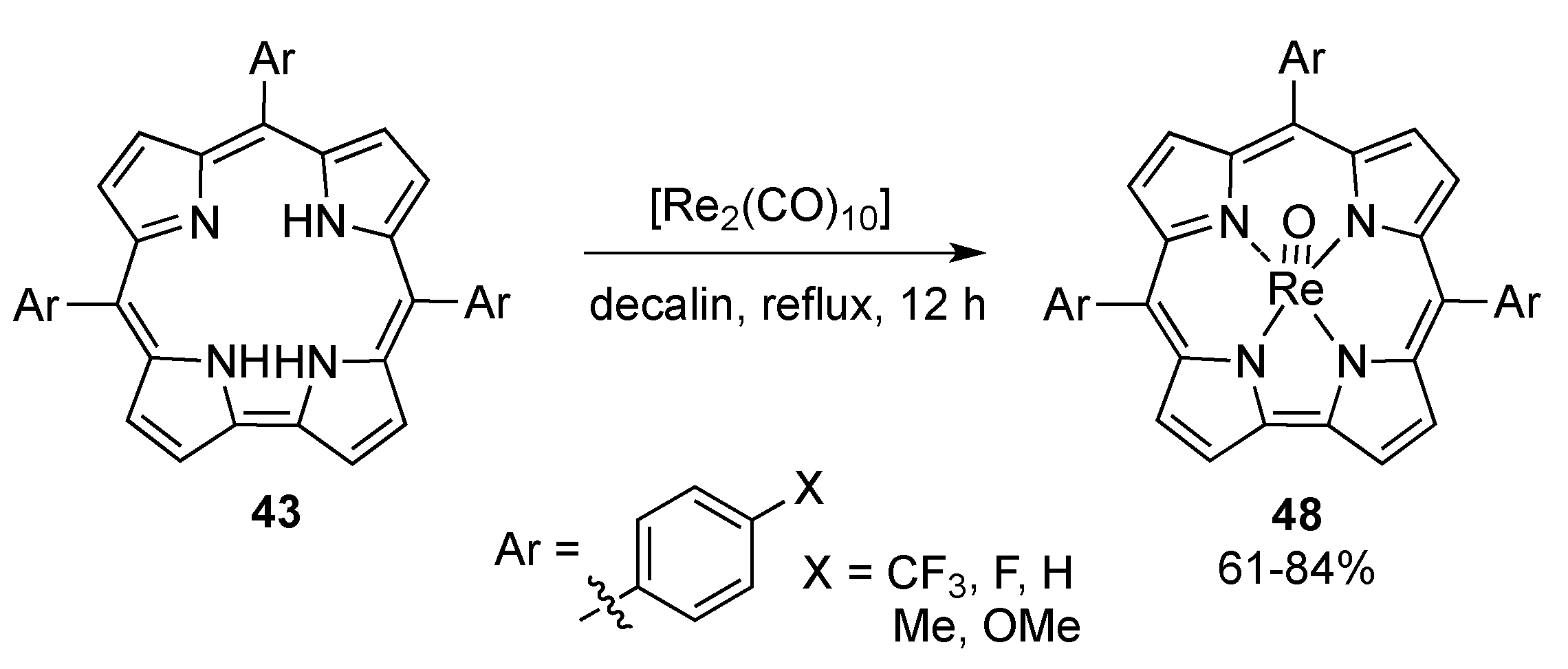
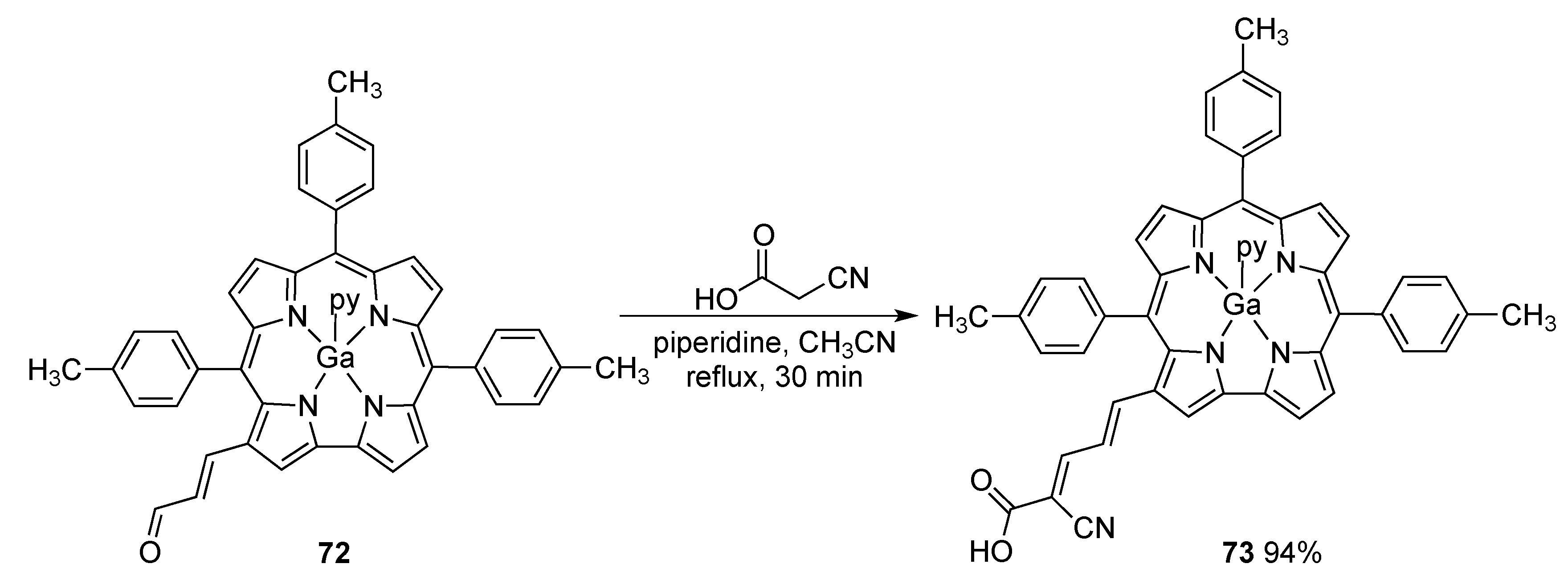
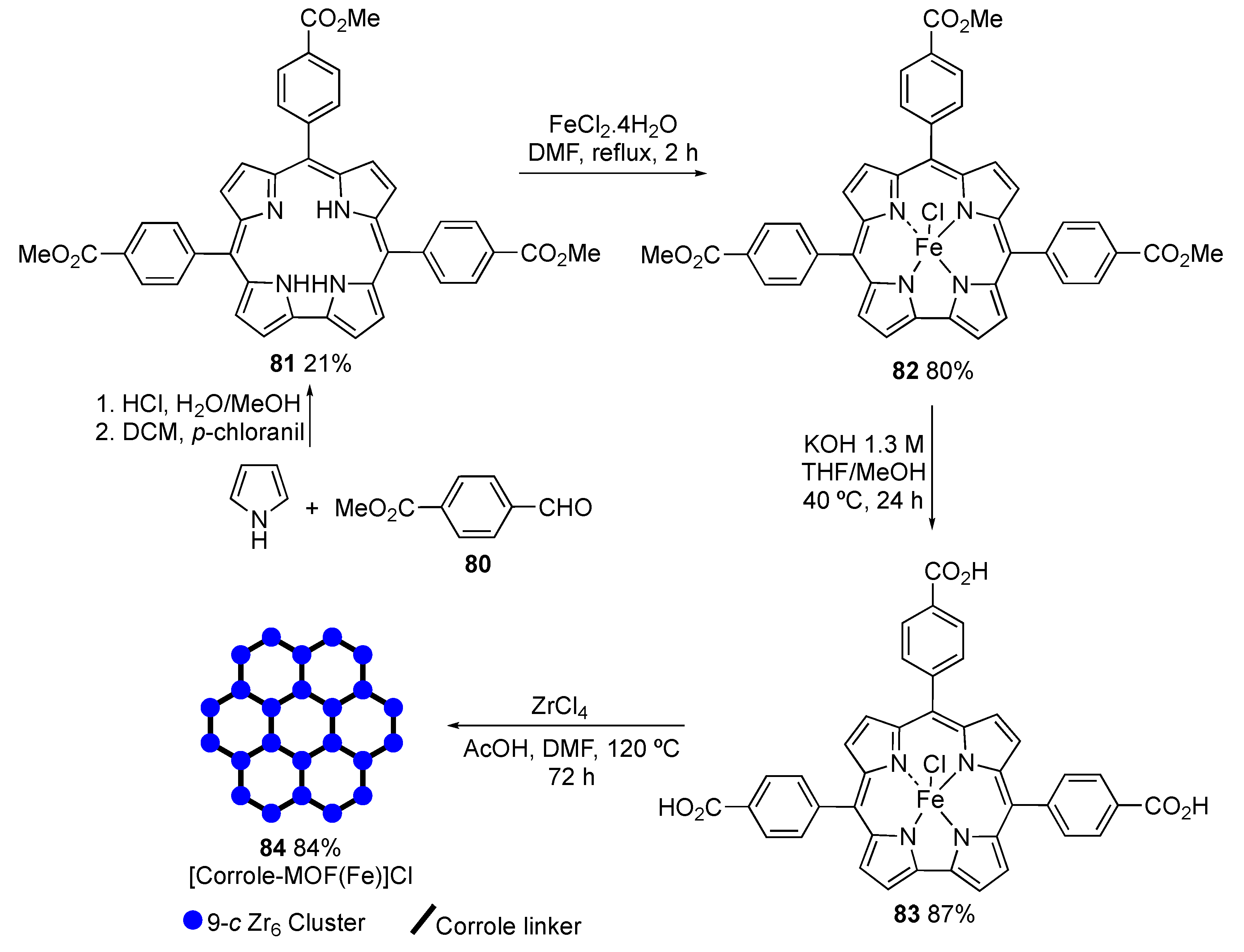
4. New Materials Containing Corroles
5. Corroles as PDT Agents in Cancer Treatment
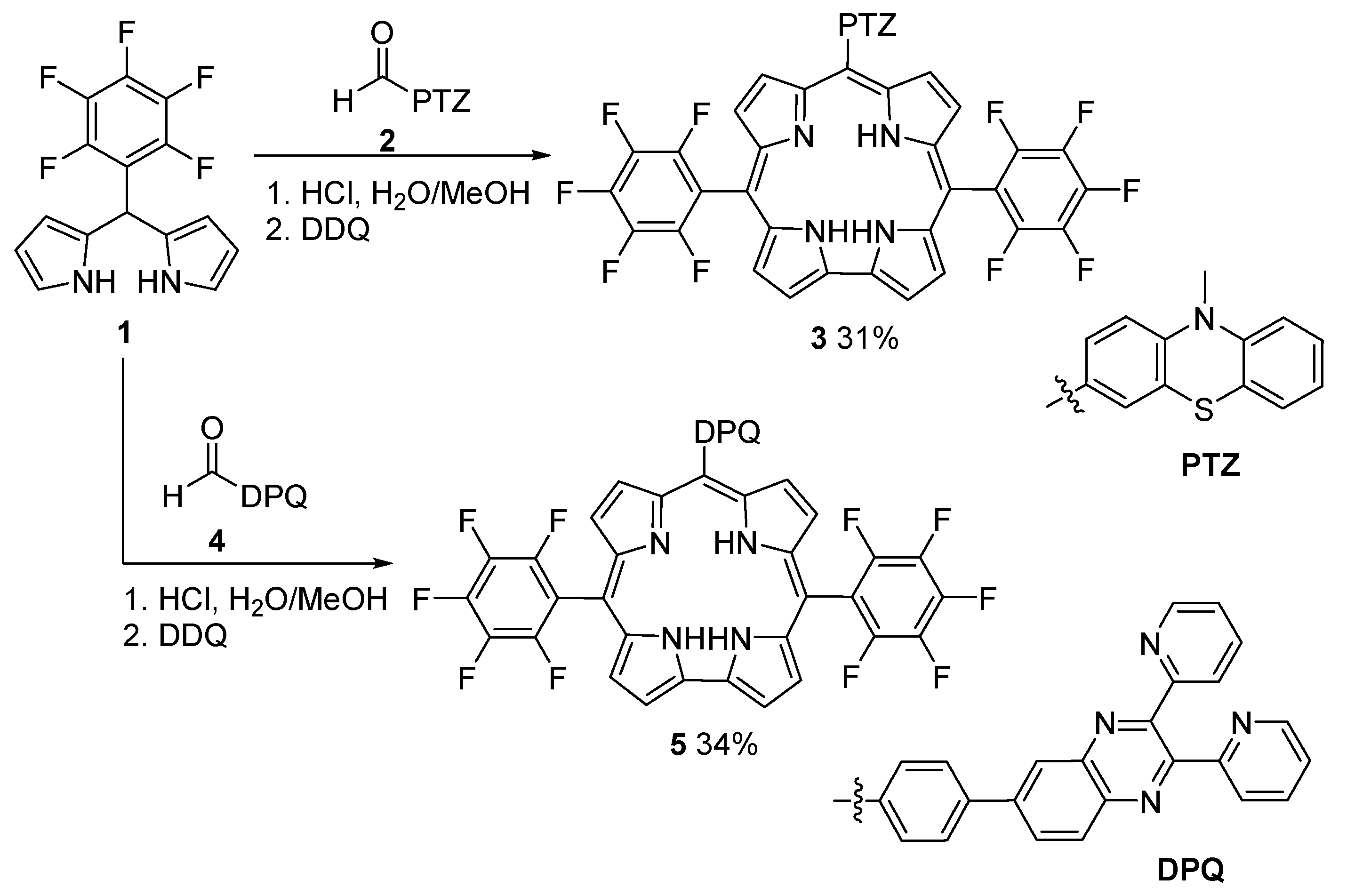
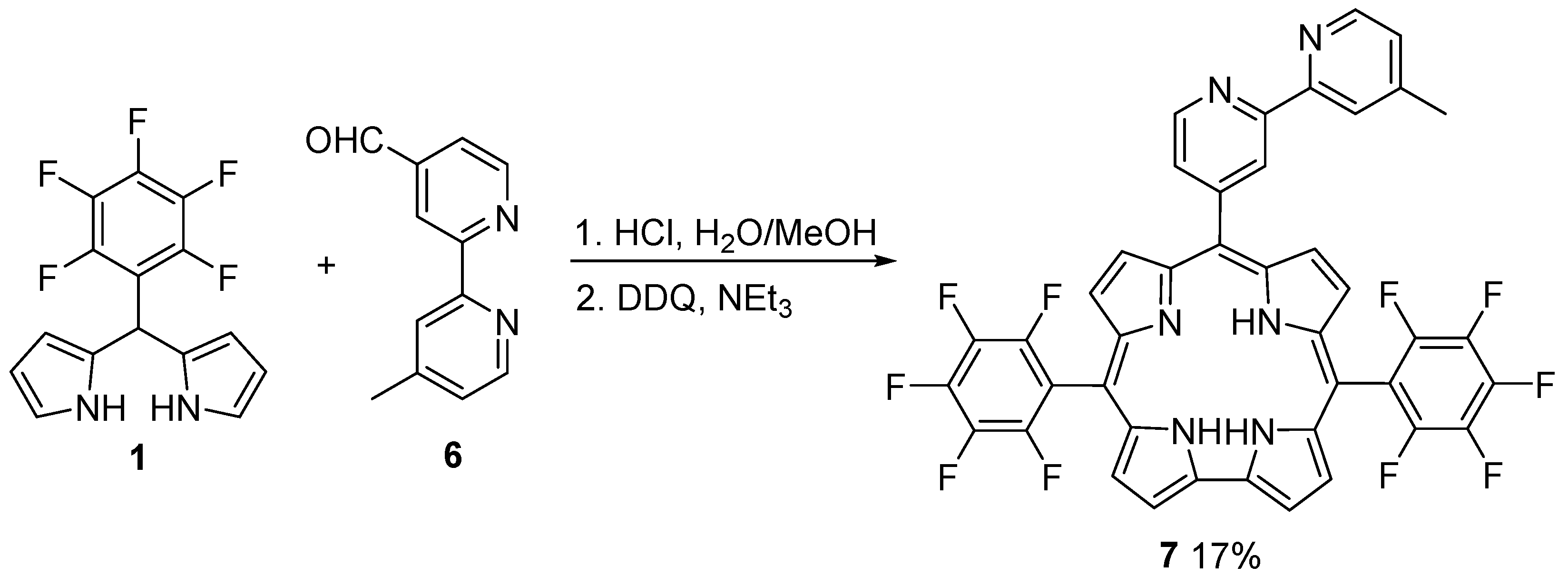
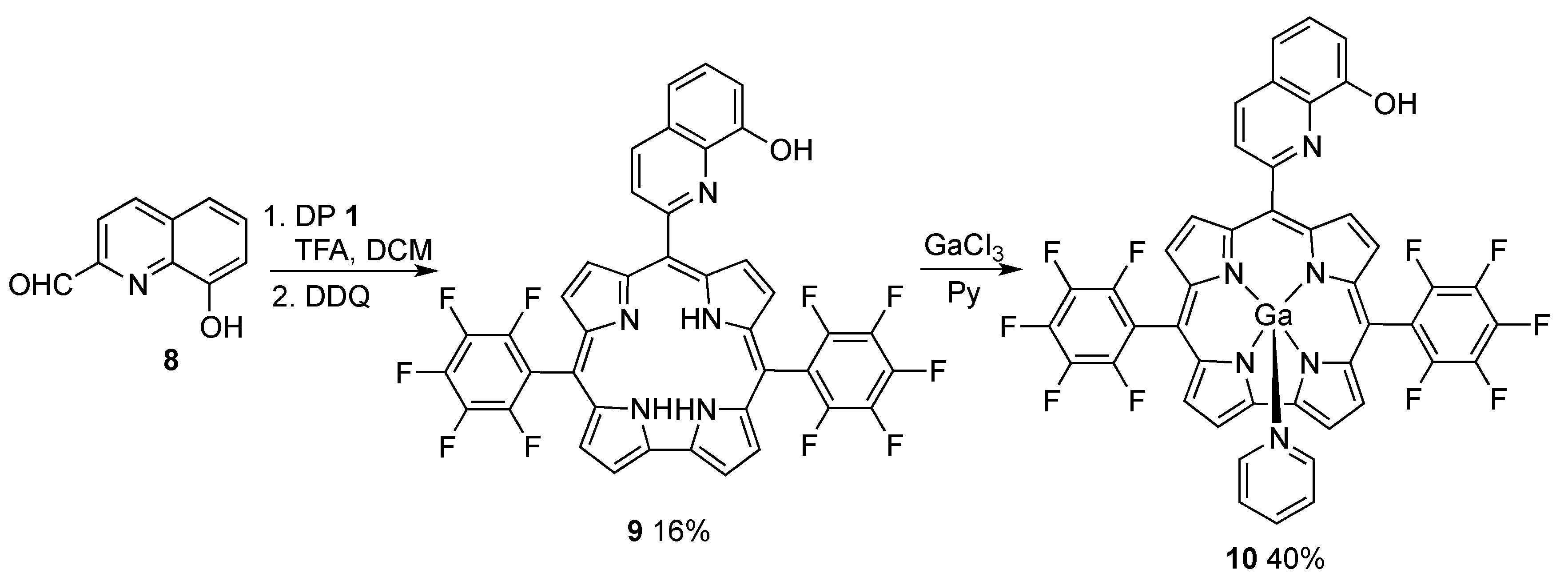
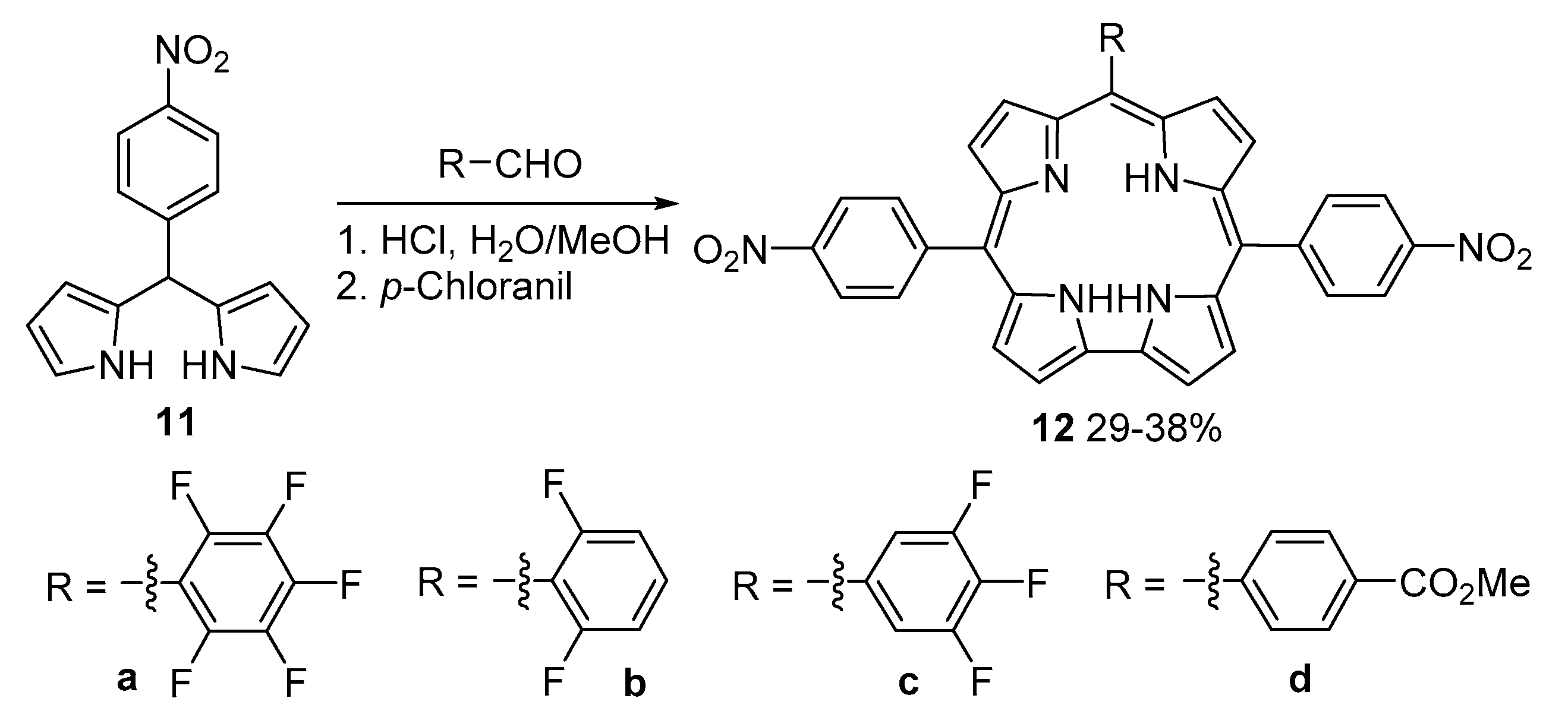
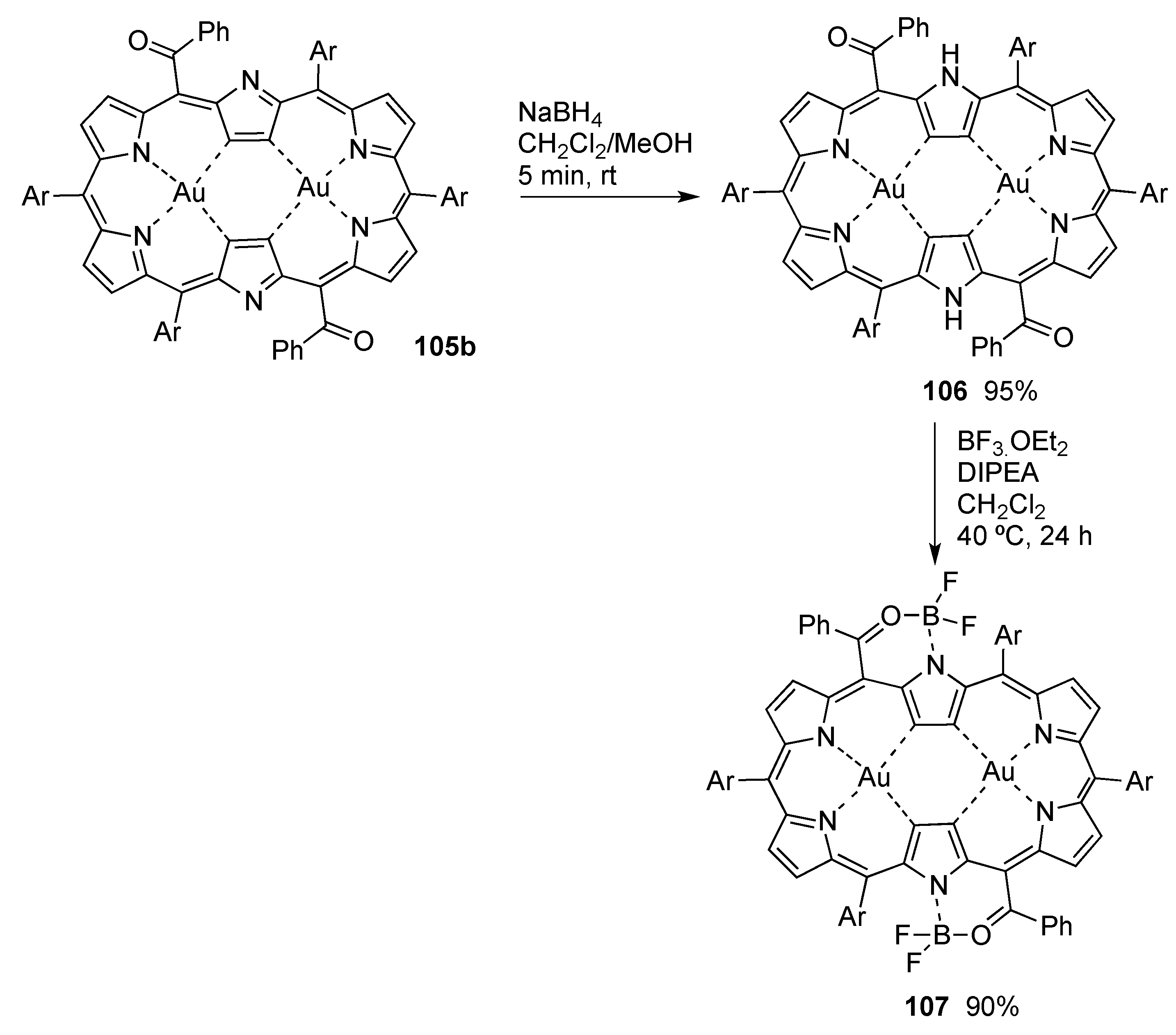
6. Hexaphyrins: Synthesis and Applications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paolesse, R.; Jaquinod, J.; Nurco, D.J.; Mini, S.; Sagone, F.; Boschi, T.; Smith, K.M. 5,10,15-Triphenylcorrole: A product from a modified Rothemund reaction. Chem. Commun. 1999, 14, 1307–1308. [Google Scholar] [CrossRef]
- Gross, Z.; Galili, N.; Saltsman, I. The First Direct Synthesis of Corroles from Pyrrole. Angew. Chem. Int. Ed. 1999, 38, 1427–1429. [Google Scholar] [CrossRef]
- Gryko, D.T.; Jadach, K. A Simple and Versatile One-Pot Synthesis of meso-Substituted trans-A2B-Corroles. J. Org. Chem. 2001, 66, 4267–4275. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S. Strategies for Corrole Functionalization. Chem. Rev. 2017, 117, 3192–3253. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A. Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations. Chem. Rev. 2017, 117, 3798–3881. [Google Scholar] [CrossRef] [PubMed]
- Orłowski, R.; Gryko, D.; Gryko, D.T. Synthesis of Corroles and Their Heteroanalogs. Chem. Rev. 2017, 117, 3102–3137. [Google Scholar] [CrossRef]
- Sarma, T.; Panda, P.K. Annulated Isomeric, Expanded, and Contracted Porphyrins. Chem. Rev. 2017, 117, 2785–2838. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, B.; Guilard, R. Some activities of PorphyChem illustrated by the applications of porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Nardis, S.; Mandoj, F.; Stefanelli, M.; Paolesse, R. Metal complexes of corrole. Coord. Chem. Rev. 2019, 388, 360–405. [Google Scholar] [CrossRef]
- Haber, A.; Angel, I.; Mahammed, A.; Gross, Z. Combating diabetes complications by 1-Fe, a corrole-based catalytic antioxidant. J. Diabetes Complicat. 2013, 27, 316–321. [Google Scholar] [CrossRef]
- Haber, A.; Gross, Z. Catalytic antioxidant therapy by metallodrugs: Lessons from metallocorroles. Chem. Commun. 2015, 51, 5812–5827. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.; Mahammed, A.; Fuhrman, B.; Volkova, N.; Coleman, R.; Hayek, T.; Aviram, M.; Gross, Z. Amphiphilic/Bipolar Metallocorroles at Catalyze the Decomposition of Reactive Oxygen and Nitrogen Species, Rescue Lipoproteins from Oxidative Damage, and Attenuate Atherosclerosis in Mice. Angew. Chem. Int. Ed. 2008, 47, 7896–7900. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Okun, Z.; Amit, T.; Mandel, S.; Saltsman, I.; Mahammed, A.; Bar-Am, O.; Gross, Z.; Youdim, M.B.H. Metallocorroles as cytoprotective agents against oxidative and nitrative stress in cellular models of neurodegeneration. J. Neurochem. 2010, 113, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Soll, M.; Bar Am, O.; Mahammed, A.; Saltsman, I.; Mandel, S.; Youdim, M.B.H.; Gross, Z. Neurorescue by a ROS Decomposition Catalyst. ACS Chem. Neurosci. 2016, 7, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Cardote, T.A.F.; Barata, J.F.B.; Amador, C.; Alves, E.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A.; Faustino, M.A.F. Evaluation of meso-substituted cationic corroles as potential antibacterial agents. An. Acad. Bras. Cienc. 2018, 90, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mack, J.; Zheng, L.-M.; Shen, Z.; Kobayashi, N. Phosphorus(V)-Corrole: Synthesis, Spectroscopic Properties, Theoretical Calculations, and Potential Utility for in Vivo Applications in Living Cells. Inorg. Chem. 2014, 53, 2797–2802. [Google Scholar] [CrossRef]
- Bornhütter, T.; Shamali, N.; Saltsman, I.; Mahammed, A.; Gross, Z.; Däschlein, G.; Röder, B. Singlet oxygen luminescence kinetics under PDI relevant conditions of pathogenic dermatophytes and molds. J. Photochem. Photobiol. B 2018, 178, 606–613. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mahammed, A.; Soll, M.; Tumanskii, B.; Gross, Z. Corroles and corrole/transferrin nanoconjugates as candidates for sonodynamic therapy. Chem. Commun. 2019, 55, 12789–12792. [Google Scholar] [CrossRef]
- Teo, R.D.; Hwang, J.Y.; Termini, J.; Gross, Z.; Gray, H.B. Fighting Cancer with Corroles. Chem. Rev. 2017, 117, 2711–2729. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, R.-X.; Liu, H.-Y.; Chang, C.K. Corrole-based photodynamic antitumor therapy. J. Chin. Chem. Soc. 2019, 66, 1090–1099. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Corroles as triplet photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. [Google Scholar] [CrossRef]
- Alka, A.; Shetti, V.S.; Ravikanth, M. Coordination chemistry of expanded porphyrins. Coord. Chem. Rev. 2019, 401, 213063. [Google Scholar] [CrossRef]
- Brewster, J.T.; Zafar, H.; Root, H.D.; Thiabaud, G.D.; Sessler, J.L. Porphyrinoid f-Element Complexes. Inorg. Chem. 2020, 59, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Jasat, A.; Dolphin, D. Expanded Porphyrins and Their Heterologs. Chem. Rev. 1997, 97, 2267–2340. [Google Scholar] [CrossRef]
- Basumatary, B.; Ramana Reddy, R.V.; Rahul Sankar, J. The Curious Case of a Parasitic Twin of the Corroles. Angew. Chem. Int. Ed. 2018, 57, 5052–5056. [Google Scholar] [CrossRef]
- Saito, S.; Osuka, A. Expanded Porphyrins: Intriguing Structures, Electronic Properties, and Reactivities. Angew. Chem. Int. Ed. 2011, 50, 4342–4373. [Google Scholar] [CrossRef]
- Inoue, M.; Osuka, A. Redox-Induced Palladium Migrations that Allow Reversible Topological Changes between Palladium(II) Complexes of Möbius Aromatic [28]Hexaphyrin and Hückel Aromatic [26]Hexaphyrin. Angew. Chem. Int. Ed. 2010, 49, 9488–9491. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kim, T.; Park, K.H.; Peng, F.; Liu, L.; Liu, Y.; Wen, B.; Liu, S.; Kirk, S.R.; Wu, L.; et al. π-Extended “Earring” Porphyrins with Multiple Cavities and Near-Infrared Absorption. Angew. Chem. Int. Ed. 2016, 55, 6438–6442. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Sprutta, N.; Latos-Grazynski, L. Figure Eights, M bius Bands, and More: Conformation and Aromaticity of Porphyrinoids. Angew. Chem. Int. Ed. 2011, 50, 4288–4340. [Google Scholar] [CrossRef]
- Szyszko, B.; Białek, M.J.; Pacholska-Dudziak, E.; Latos-Grażyński, L. Flexible Porphyrinoids. Chem. Rev. 2017, 117, 2839–2909. [Google Scholar] [CrossRef]
- Ahn, T.K.; Kwon, J.H.; Kim, D.Y.; Cho, D.W.; Jeong, D.H.; Kim, S.K.; Suzuki, M.; Shimizu, S.; Osuka, A.; Kim, D. Comparative Photophysics of [26]- and [28]Hexaphyrins(1.1.1.1.1.1): Large Two-Photon Absorption Cross Section of Aromatic [26]Hexaphyrins(1.1.1.1.1.1). J. Am. Chem. Soc. 2005, 127, 12856–12861. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef]
- Mondal, S.; Naik, P.K.; Adha, J.K.; Kar, S. Synthesis, characterization, and reactivities of high valent metal–corrole (M = Cr, Mn, and Fe) complexes. Coord. Chem. Rev. 2019, 400, 213043. [Google Scholar] [CrossRef]
- Ooi, S.; Yoneda, T.; Tanaka, T.; Osuka, A. meso-Free Corroles: Syntheses, Structures, Properties, and Chemical Reactivities. Chem. Eur. J. 2015, 21, 7772–7779. [Google Scholar] [CrossRef] [PubMed]
- Kandhadi, J.; Yan, W.C.; Cheng, F.; Wang, H.; Liu, H.Y. trans-A2B-corrole bearing 2,3-di(2-pyridyl)quinoxaline (DPQ)/phenothiazine moieties: Synthesis, characterization, electrochemistry and photophysics. New J. Chem. 2018, 42, 9987–9999. [Google Scholar] [CrossRef]
- Pivetta, R.C.; Auras, B.L.; Souza, B.d.; Neves, A.; Nunes, F.S.; Cocca, L.H.Z.; Boni, L.D.; Iglesias, B.A. Synthesis, photophysical properties and spectroelectrochemical characterization of 10-(4-methyl-bipyridyl)-5,15-(pentafluorophenyl)corrole. J. Photochem. Photobiol. A 2017, 332, 306–315. [Google Scholar] [CrossRef]
- Cai, F.; Xia, F.; Guo, Y.; Zhu, W.; Fu, B.; Liang, X.; Wang, S.; Cai, Z.; Xu, H. “Off–on–off” type of selectively pH-sensing 8-hydroxyquinoline-substituted gallium(iii) corrole. New J. Chem. 2019, 43, 18012–18017. [Google Scholar] [CrossRef]
- Yadav, O.; Varshney, A.; Kumar, A.; Ratnesh, R.K.; Mehata, M.S. A2B corroles: Fluorescence signaling systems for sensing fluoride ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 207–213. [Google Scholar] [CrossRef]
- Liang, X.; Fang, J.J.; Li, M.Z.; Chen, Q.Y.; Mack, J.; Molupe, N.; Nyokong, T.; Zhu, W.H. Push-pull type manganese(III)corroles: Synthesis, electronic structures and tunable interactions with ctDNA. J. Porphyr. Phthalocyanines 2017, 21, 751–758. [Google Scholar] [CrossRef]
- Zhao, F.; Zhan, X.; Lai, S.-H.; Zhang, L.; Liu, H.-Y. Photophysical properties and singlet oxygen generation of meso-iodinated free-base corroles. RSC Adv. 2019, 9, 12626–12634. [Google Scholar] [CrossRef]
- Ali, A.; Cheng, F.; Wen, W.-H.; Ying, X.; Kandhadi, J.; Wang, H.; Liu, H.-Y.; Chang, C.-K. Case synthesis of a β-chloro bulky bis-pocket corrole: Crystallographic characterization and photophysical properties. Chin. Chem. Lett. 2018, 29, 1888–1892. [Google Scholar] [CrossRef]
- Larsen, S.; McCormick-McPherson, L.J.; Teat, S.J.; Ghosh, A. Azulicorrole. ACS Omega 2019, 4, 6737–6745. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Pinho e Melo, T.M.V.D. Meso-Substituted Corroles from Nitrosoalkenes and Dipyrromethanes. J. Org. Chem. 2020, 85, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Teplitzky, P.; Diskin-Posner, Y.; Sundararajan, M.; Ullah, Z.; Chen, Q.C.; Shimon, L.J.W.; Saltsman, I.; Mahammed, A.; Kosa, M.; et al. Maximizing Property Tuning of Phosphorus Corrole Photocatalysts through a Trifluoromethylation Approach. Inorg. Chem. 2019, 58, 6184–6198. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zini, Y.; Fridman, N.; Chen, Q.-C.; Churchill, D.G.; Gross, Z. “Hetero-Multifunctionalization” of Gallium Corroles: Facile Synthesis, Phosphorescence, Redox Tuning, and Photooxidative Catalytic Improvement. ChemPlusChem 2020, 85, 163–168. [Google Scholar] [CrossRef]
- Mahammed, A.; Chen, K.P.; Vestfrid, J.; Zhao, J.Z.; Gross, Z. Phosphorus corrole complexes: From property tuning to applications in photocatalysis and triplet-triplet annihilation upconversion. Chem. Sci. 2019, 10, 7091–7103. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Liuzzi, S.; Marino, T.; Russo, N.; Toscano, M. Iodine substituted phosphorus corrole complexes as possible photosensitizers in photodynamic therapy: Insights from theory. J. Comp. Chem. 2020, 41, 1395–1401. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Mazzone, G.; Russo, N.; Toscano, M. Photophysical Properties of Nitrated and Halogenated Phosphorus Tritolylcorrole Complexes: Insights from Theory. Molecules 2018, 23, 2779. [Google Scholar] [CrossRef]
- Kumar, A.; Sujesh, S.; Varshney, P.; Paul, A.; Jeyaraman, S. Aminophenyl-substituted cobalt(III) corrole: A bifunctional electrocatalyst for the oxygen and hydrogen evolution reactions. Dalton Trans. 2019, 48, 11345–11351. [Google Scholar] [CrossRef]
- Stefanelli, M.; Ricci, A.; Chiarini, M.; Lo Sterzo, C.; Berionni Berna, B.; Pomarico, G.; Sabuzi, F.; Galloni, P.; Fronczek, F.R.; Smith, K.M.; et al. β-Arylethynyl substituted silver corrole complexes. Dalton Trans. 2019, 48, 13589–13598. [Google Scholar] [CrossRef]
- Che, Y.; Yuan, X.; Cai, F.; Zhao, J.; Zhao, X.; Xu, H.; Liu, L. Bodipy−Corrole dyad with truxene bridge: Photophysical Properties and Application in Triplet−Triplet Annihilation upconversion. Dyes Pigment. 2019, 171, 107756. [Google Scholar] [CrossRef]
- Larsen, S.; McCormick, L.J.; Ghosh, A. Rapid one-pot synthesis of pyrrole-appended isocorroles. Org. Biomol. Chem. 2019, 17, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Einrem, R.F.; Gagnon, K.J.; Alemayehu, A.B.; Ghosh, A. Metal–Ligand Misfits: Facile Access to Rhenium–Oxo Corroles by Oxidative Metalation. Chem. Eur. J. 2016, 22, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Einrem, R.F.; Alemayehu, A.B.; Ghosh, A. Ambient-temperature near-IR phosphorescence and potential applications of rhenium-oxo corroles. Photochem. Photobiol. Sci. 2019, 18, 1166–1170. [Google Scholar] [CrossRef]
- Lin, H.; Hossain, M.S.; Zhan, S.-Z.; Liu, H.-Y.; Si, L.-P. Electrocatalytic hydrogen evolution using triaryl corrole cobalt complex. Appl. Organomet. Chem. 2020, 34, e5583. [Google Scholar] [CrossRef]
- Mondal, S.; Garai, A.; Naik, P.K.; Adha, J.K.; Kar, S. Synthesis and characterization of antimony(V)-oxo corrole complexes. Inorg. Chim. Acta 2020, 501, 119300. [Google Scholar] [CrossRef]
- Schweyen, P.; Kleeberg, C.; Körner, D.; Thüsing, A.; Wicht, R.; Zaretzke, M.-K.; Bröring, M. Ruffling and doming: Structural and redox studies on meso-aryl and β-alkyl chromyl(V)corroles. J. Porphyr. Phthalocyanines 2020, 24, 303–313. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Xiao, Z.-Y.; Fite, S.; Mizrahi, A.; Fridman, N.; Zhan, X.; Keisar, O.; Cohen, Y.; Gross, Z. Tuning Chemical and Physical Properties of Phosphorus Corroles for Advanced Applications. Chem. Eur. J. 2019, 25, 11383–11388. [Google Scholar] [CrossRef]
- Gilhula, J.C.; Radosevich, A.T. Tetragonal phosphorus(v) cations as tunable and robust catalytic Lewis acids. Chem. Sci. 2019, 10, 7177–7182. [Google Scholar] [CrossRef]
- Caulfield, K.P.; Conradie, J.; Arman, H.D.; Ghosh, A.; Tonzetich, Z.J. Iron(II) Corrole Anions. Inorg. Chem. 2019, 58, 15225–15235. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhu, W.; Mack, J.; Soy, R.C.; Nyokong, T.; Liang, X. Meso- and axially-modified IrIIItriarylcorroles with tunable electrocatalytic properties. Dyes Pigment. 2020, 175, 108124. [Google Scholar] [CrossRef]
- Thomassen, I.K.; McCormick-McPherson, L.J.; Borisov, S.M.; Ghosh, A. Iridium Corroles Exhibit Weak Near-Infrared Phosphorescence but Efficiently Sensitize Singlet Oxygen Formation. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-H.; Wang, L.-L.; Wan, B.; Lu, A.-W.; Wang, H.; Liu, H.-Y. Photophysical properties, singlet oxygen generation and DNA binding affinity of Tris(4-pyridyl)corrole and its phosphorous, gallium and tin complexes. J. Photochem. Photobiol. A 2020, 390, 112283. [Google Scholar] [CrossRef]
- Lu, G.; Sun, Q.; Zhang, T.; Kong, X.; Wang, Q.; He, C. Preparation of new semiconducting corrole nanostructures and their application as gas sensor. Synth. Met. 2019, 252, 69–75. [Google Scholar] [CrossRef]
- Oppenheim, J.; Gray, M.H.B.; Di Bilio, A.J.; Brennan, B.J.; Henling, L.M.; Lim, P.; Soll, M.; Termini, J.; Virgil, S.C.; Gross, Z.; et al. Structures and Spectroscopic Properties of Metallocorrole Nanoparticles. Inorg. Chem. 2019, 58, 10287–10294. [Google Scholar] [CrossRef] [PubMed]
- Agadjanian, H.; Ma, J.; Rentsendorj, A.; Valluripalli, V.; Hwang, J.Y.; Mahammed, A.; Farkas, D.L.; Gray, H.B.; Gross, Z.; Medina-Kauwe, L.K. Tumor Detection and Elimination by a Targeted Gallium Corrole. Proc. Natl. Acad. Sci. USA 2009, 106, 6105–6110. [Google Scholar] [CrossRef] [PubMed]
- Soll, M.; Goswami, T.K.; Chen, Q.C.; Saltsman, I.; Teo, R.D.; Shahgholi, M.; Lim, P.; Di Bilio, A.J.; Cohen, S.; Termini, J.; et al. Cell-Penetrating Protein/Corrole Nanoparticles. Sci. Rep. 2019, 9, 2294. [Google Scholar] [CrossRef]
- Berionni Berna, B.; Savoldelli, A.; Pomarico, G.; Zurlo, F.; Magna, G.; Paolesse, R.; Fronczek, F.R.; Smith, K.M.; Nardis, S. Grafting Copper and Gallium Corroles onto Zinc Oxide Nanoparticles. ChemPlusChem 2019, 84, 154–160. [Google Scholar] [CrossRef]
- Tang, J.; Chen, B.; Zhang, Y.; Lu, J.; Zhang, T.; Guo, Q.; Zhang, J. Synthesis and gas sensitivity properties of novel metallocorroles and functionalized graphene oxide. Funct. Mater. Lett. 2019, 12, 1940001. [Google Scholar] [CrossRef]
- Sahu, K.; Mondal, S.; Patra, B.; Pain, T.; Patra, S.K.; Dosche, C.; Kar, S. Regioselective thiocyanation of corroles and the synthesis of gold nanoparticle–corrole assemblies. Nanoscale Adv. 2020, 2, 166–170. [Google Scholar] [CrossRef]
- Lu, G.; Bao, L.; Hu, X.; Liu, X.; Zhu, W. Synthesis, spectroscopic characterization and photocatalytic properties of corrole modified GPTMS/TiO2 nanoparticles. Inorg. Chem. Commun. 2018, 98, 165–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, S.; Niu, Z.; Peng, Y.; Shan, C.; Verma, G.; Wojtas, L.; Zhang, Z.; Zhang, B.; Feng, Y.; et al. Robust Corrole-Based Metal—Organic Frameworks with Rare 9-Connected Zr/Hf-Oxo Clusters. J. Am. Chem. Soc. 2019, 141, 14443–14450. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Brandès, S.; Quesneau, V.; Fonquernie, O.; Desbois, N.; Blondeau-Patissier, V.; Gros, C.P. Porous organic polymers based on cobalt corroles for carbon monoxide binding. Dalton Trans. 2019, 48, 11651–11662. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Neves, M.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef]
- Babu, B.; Prinsloo, E.; Mack, J.; Nyokong, T. Synthesis, characterization and photodynamic activity of Sn(iv) triarylcorroles with red-shifted Q bands. New J. Chem. 2019, 43, 18805–18812. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Akram, W.; Cheng, F.; Liu, Z.-Y.; Liao, Y.-H.; Ye, Y.; Liu, H.-Y. DNA interaction and photodynamic antitumor activity of transition metal mono-hydroxyl corrole. Bioorg. Chem. 2019, 90, 103085. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.H.; Akram, W.; Sun, Y.M.; Liao, Y.H.; Si, L.P.; Liu, H.Y.; Chang, C.K. Tri-hydroxyl Corrole and Its Gallium(III) Complex: DNA-Binding, Photocleavage and in Vitro Photodynamic Antitumor Activities. Chin. J. Inorg. Chem. 2019, 35, 1687–1697. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.J.; Huang, H.; Wan, B.; Wu, S.; Liu, H.Y.; Zhang, H.T. The photocytotoxicity effect of cationic sulfonated corrole towards lung cancer cells: In vitro and in vivo study. Lasers Med. Sci. 2019, 34, 1353–1363. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Zamarrón, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Röder, B.; Juarranz, Á.; Sanz-Rodríguez, F. Photodynamic effects induced by meso-tris(pentafluorophenyl)corrole and its cyclodextrin conjugates on cytoskeletal components of HeLa cells. Eur. J. Med. Chem. 2015, 92, 135–144. [Google Scholar] [CrossRef]
- Einrem, R.F.; Alemayehu, A.B.; Borisov, S.M.; Ghosh, A.; Gederaas, O.A. Amphiphilic Rhenium-Oxo Corroles as a New Class of Sensitizers for Photodynamic Therapy. ACS Omega 2020, 5, 10596–10601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dai, W.; Peng, Y.; Niu, Z.; Sun, Q.; Shan, C.; Yang, H.; Verma, G.; Wojtas, L.; Yuan, D.; et al. A Corrole-Based Covalent Organic Framework Featuring Desymmetrized Topology. Angew. Chem. Int. Ed. 2020, 59, 4354–4359. [Google Scholar] [CrossRef] [PubMed]
- Carbon-linked HexapyrrolicSystems and Heteroatom Analogs. In Expanded, Contracted & Isomeric Porphyrins; Jonathan, L., Sessler, S.J.W., Eds.; Tetrahedron Organic Chemistry Series; Elsevier: Amsterdam, The Netherlands, 1997; volume 15, pp. 329–367. [Google Scholar]
- Neves, M.G.P.M.S.; Martins, R.M.; Tomé, A.C.; Silvestre, A.J.D.; Silva, A.M.S.; Félix, V.; Drew, M.G.B.; Cavaleiro, J.A.S. meso-Substituted expanded porphyrins: New and stable hexaphyrins. Chem. Commun. 1999, 385–386. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Furuta, H.; Yoza, K.; Igarashi, S.; Osuka, A. meso-Aryl-Substituted Expanded Porphyrins. J. Am. Chem. Soc. 2001, 123, 7190–7191. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, R.; Shimizu, S.; Suzuki, M.; Shin, J.-Y.; Furuta, H.; Osuka, A. Ring size selective synthesis of meso-aryl expanded porphyrins. Tetrahedron Lett. 2003, 44, 2505–2507. [Google Scholar] [CrossRef]
- Suzuki, M.; Osuka, A. Improved Synthesis of meso-Aryl-Substituted [26]Hexaphyrins. Org. Lett. 2003, 5, 3943–3946. [Google Scholar] [CrossRef]
- Ishida, S.-I.; Soya, T.; Osuka, A. A Stable Antiaromatic 5,20-Dibenzoyl [28]Hexaphyrin(1.1.1.1.1.1): Core AuIII Metalation and Subsequent Peripheral BIII Metalation. Angew. Chem. Int. Ed. 2018, 57, 13640–13643. [Google Scholar] [CrossRef]
- Nakai, A.; Kim, J.; Kim, D.; Osuka, A. 5,20-Bis(ethoxycarbonyl)-Substituted Antiaromatic [28]Hexaphyrin and Its Bis-NiII and Bis-CuII Complexes. Chem. Asian J. 2019, 14, 968–971. [Google Scholar] [CrossRef]
- Nakai, A.; Ishida, S.-I.; Soya, T.; Osuka, A. π-Ruthenium Complexes of Hexaphyrins(1.1.1.1.1.1): A Triple-Decker Complex Bearing Two Ruthenoarene Units. Angew. Chem. Int. Ed. 2019, 58, 8197–8200. [Google Scholar] [CrossRef]
- Yoneda, T.; Osuka, A. Synthesis of a [26]Hexaphyrin Bis-PdII Complex with a Characteristic Aromatic Circuit. Chem. Eur. J. 2013, 19, 7314–7318. [Google Scholar] [CrossRef]
- Yoneda, T.; Kim, T.; Soya, T.; Neya, S.; Oh, J.; Kim, D.; Osuka, A. Conformational Fixation of a Rectangular Antiaromatic [28]Hexaphyrin Using Rationally Installed Peripheral Straps. Chem. Eur. J. 2016, 22, 4413–4417. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Yoneda, T.; Ishida, S.-i.; Kato, K.; Osuka, A. Aromatic and Antiaromatic Cyclophane-type Hexaphyrin Dimers. Chem. Asian J. 2019, 14, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Suzuki, M.; Shimizu, S.; Shin, J.-Y.; Osuka, A. Regioselective nucleophilic substitution reaction of meso-hexakis(pentafluorophenyl) substituted [26]hexaphyrin. Tetrahedron Lett. 2003, 44, 4597–4601. [Google Scholar] [CrossRef]
- Klingenburg, R.; Stark, C.B.W.; Wiehe, A. Nucleophilic Thioglycosylation of Pentafluorophenyl-Substituted Porphyrinoids: Synthesis of Glycosylated Calix[n]phyrin and [28]Hexaphyrin Systems. Org. Lett. 2019, 21, 5417–5420. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.T.; Root, H.D.; Mangel, D.; Samia, A.; Zafar, H.; Sedgwick, A.C.; Lynch, V.M.; Sessler, J.L. UO22+-Mediated ring contraction of pyrihexaphyrin: Synthesis of a contracted expanded porphyrin-uranyl complex. Chem. Sci. 2019, 10, 5596–5602. [Google Scholar] [CrossRef]
- Samala, S.; Dutta, R.; He, Q.; Park, Y.; Chandra, B.; Lynch, V.M.; Jung, Y.M.; Sessler, J.L.; Lee, C.-H. A robust bis-rhodium(I) complex of π-extended planar, anti-aromatic hexaphyrin[1.0.1.0.1.0]. Chem. Commun. 2020, 56, 758–761. [Google Scholar] [CrossRef]
- Shimomura, K.; Kai, H.; Nakamura, Y.; Hong, Y.; Mori, S.; Miki, K.; Ohe, K.; Notsuka, Y.; Yamaoka, Y.; Ishida, M.; et al. Bis-Metal Complexes of Doubly N-Confused Dioxohexaphyrins as Potential Near-Infrared-II Photoacoustic Dyes. J. Am. Chem. Soc. 2020, 142, 4429–4437. [Google Scholar] [CrossRef]
- Xue, S.; Kuzuhara, D.; Aratani, N.; Yamada, H. Control of Aromaticity and cis-/trans-Isomeric Structure of Non-Planar Hexaphyrin(2.1.2.1.2.1) and Metal Complexes. Angew. Chem. Int. Ed. 2019, 58, 12524–12528. [Google Scholar] [CrossRef]
- Xue, S.; Kuzuhara, D.; Aratani, N.; Yamada, H. [30]Hexaphyrin(2.1.2.1.2.1) as Aromatic Planar Ligand and Its Trinuclear Rhodium(I) Complex. Inorg. Chem. 2018, 57, 9902–9906. [Google Scholar] [CrossRef]
- Laidig, K.E.; Speers, P.; Streiwieser, A. Origin of depressed dipole moments in fivemembered, unsaturated heterocycles. Can. J. Chem. 1996, 74, 1215–1220. [Google Scholar] [CrossRef]
- Ajay, J.; Shirisha, S.; Ishida, M.; Ito, K.; Mori, S.; Furuta, H.; Gokulnath, S. Planar Antiaromatic Core-Modified 24π Hexaphyrin(1.0.1.0.1.0) and 32π Octaphyrin(1.0.1.0.1.0.1.0) Bearing Alternate Hybrid Diheterole Units. Chem. Eur. J. 2019, 25, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Abdisa Kerayu, B.; Ching, W.-M.; dela Cruz, J.-a.; Hung, C.-H. 2,5-Thienylene-Strapped Bicyclic and Tricyclic Expanded Porphyrins. ChemPlusChem 2019, 84, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Ambhore, M.D.; Basavarajappa, A.; Anand, V.G. A wide-range of redox states of core-modified expanded porphyrinoids. Chem. Commun. 2019, 55, 6763–6766. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Ghosh, A.; Srinivasan, A.; Suresh, C.H.; Chandrashekar, T.K. Protonation-Triggered Hückel and Möbius Aromatic Transformations in Nonaromatic Core-Modified [30]Hexaphyrin(2.1.1.2.1.1) and Annulated [28]Hexaphyrin(2.1.1.0.1.1). Org. Lett. 2019, 21, 9637–9641. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Ambhore, M.D.; Anand, V.G. Non-planar core-modified dibenzi expanded isophlorin. J. Porphyr. Phthalocyan. 2020, 24, 298–302. [Google Scholar] [CrossRef]
- Kishore, M.V.N.; Panda, P.K. Revisiting the intense NIR active bronzaphyrin, a 26-π aromatic expanded porphyrin: Synthesis and structural analysis. Chem. Commun. 2018, 54, 13135–13138. [Google Scholar] [CrossRef]
- Tian, J.; Ding, L.; Xu, H.-J.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J.-S. Cell-Specific and pH-Activatable Rubyrin-Loaded Nanoparticles for Highly Selective Near-Infrared Photodynamic Therapy against Cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [Google Scholar] [CrossRef]



































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, S.M.M.; Pineiro, M.; Pinho e Melo, T.M.V.D. Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy. Molecules 2020, 25, 3450. https://doi.org/10.3390/molecules25153450
Lopes SMM, Pineiro M, Pinho e Melo TMVD. Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy. Molecules. 2020; 25(15):3450. https://doi.org/10.3390/molecules25153450
Chicago/Turabian StyleLopes, Susana M. M., Marta Pineiro, and Teresa M. V. D. Pinho e Melo. 2020. "Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy" Molecules 25, no. 15: 3450. https://doi.org/10.3390/molecules25153450
APA StyleLopes, S. M. M., Pineiro, M., & Pinho e Melo, T. M. V. D. (2020). Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy. Molecules, 25(15), 3450. https://doi.org/10.3390/molecules25153450