Facile Fabrication of Natural Polyelectrolyte-Nanoclay Composites: Halloysite Nanotubes, Nucleotides and DNA Study
Abstract
:1. Introduction
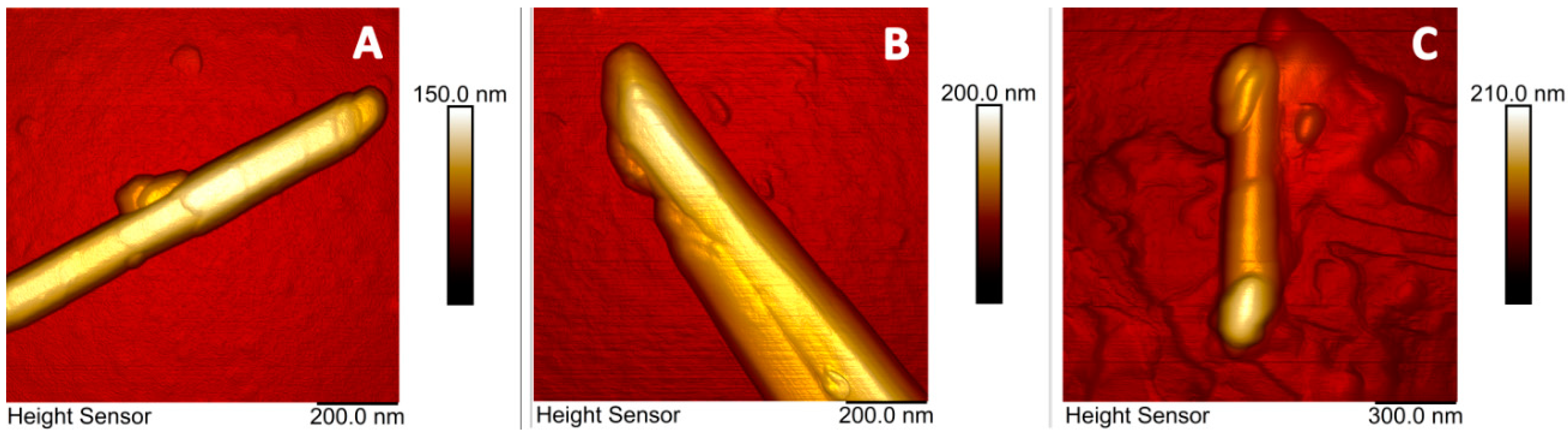
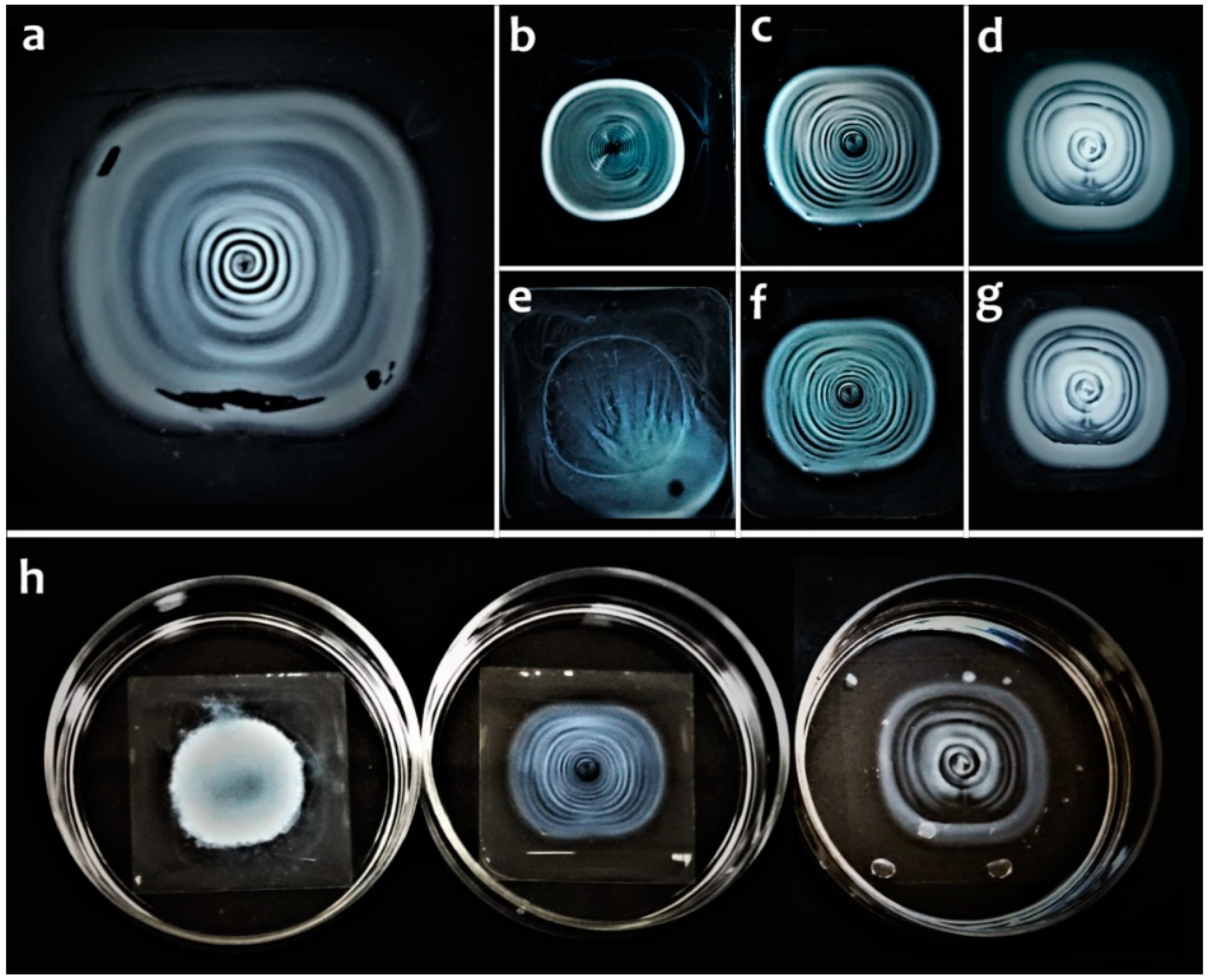
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Binding and Release of DNA, Nucleotide Phosphates or Uridine
4.2.2. Binding and Release of Fragmented DNA
4.2.3. Thermogravimetric Analysis
4.2.4. Microscopy
4.2.5. Halloysite Internalization by Cells
4.2.6. Fabrication of Concentric Rings from DNA-Modified Halloysite Nanotubes on a Solid Substrate
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lvov, Y.; Panchal, A.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial self-assembly in halloysite nanotube composites. Langmuir 2019, 35, 8646–8657. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef]
- Rozhina, E.; Ishmukhametov, I.; Batasheva, S.; Akhatova, F.; Fakhrullin, R. Nanoarchitectonics meets cell surface engineering: Shape recognition of human cells by halloysite-doped silica cell imprints. Beilstein J. Nanotechnol. 2019, 10, 1818–1825. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/keratin nanocomposite for human hair photoprotection coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef]
- Miyata, K.; Kakizawa, Y.; Nishiyama, N.; Harada, A.; Yamasaki, Y.; Koyama, H.; Kataoka, K. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J. Am. Chem. Soc. 2004, 126, 2355–2361. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Q.-Y.; Zhang, X.-W. Interaction of DNA with clay minerals and soil colloidal particles and protection against degradation by DNase. Environ. Sci. Technol. 2006, 40, 2971–2976. [Google Scholar] [CrossRef]
- Khanna, M.; Stotzky, G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Appl. Environ. Microbiol. 1992, 58, 1930–1939. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; Huang, Q.-Y.; Zhang, X.-W.; Chen, H. Binding and transformation of extracellular DNA in soil. Pedosphere 2005, 15, 16–23. [Google Scholar]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Biomedical applications of cationic clay minerals. RSC Adv. 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.X. Adsorption of proteins and nucleic acids on clay minerals and their interaction: A review. Appl. Clay Sci. 2013, 80, 443–452. [Google Scholar] [CrossRef]
- Kawase, M.; Hayashi, Y.; Kinoshita, F.; Yamato, E.; Miyazaki, J.; Yamakawa, J.; Ishida, T.; Tamura, M.; Yagi, K. Protective effect of montmorillonite on plasmid DNA in oral gene delivery into small intestine. Biol. Pharm. Bull. 2004, 27, 2049–2051. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.J. Use of natural halloysite as a functional cosmetics carrier. Econ. Environ. Geol. 2015, 48, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.; Lvov, Y. Self-assembly of clay nanotubes on hair surface for medical and cosmetic formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef]
- Naumenko, E.; Fakhrullin, R. Halloysite nanoclay/biopolymer composite materials in tissue engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Green and functional polymer-clay nanotube composites withsustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef] [Green Version]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakrullin, R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 5, 10560–10571. [Google Scholar] [CrossRef] [PubMed]
- Yendluri, R.; Lvov, Y.; de Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel encapsulated in halloysite clay nanotubes for intestinal and intracellular delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullina, G.I.; Khakimova, E.; Akhatova, F.S.; Lazzara, G.; Parisi, F.; Fakhrullin, R.F. Selective antimicrobial effects of curcumn@halloysite nanoformulation: A Caenorhabditis elegans study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial applications of clay nanotube-based composites. Nanomaterials 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Micó-Vicent, B.; Martínez-Verdú, F.M.; Novikov, A.; Stavitskaya, A.; Vinokurov, V.; Rozhina, E.; Fakhrullin, R.; Yendluri, R.; Lvov, Y. Stabilized dye-pigment formulations with platy and tubular nanoclays. Adv. Funct. Mater. 2017, 28, 1703553. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Barone, G.; Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Nicotra, G.; Spinella, C.; Spinelli, G.; Riela, S. Halloysite nanotubes-carbon dots hybrids multifunctional nanocarrier with positive cell target ability as a potential non-viral vector for oral gene therapy. J. Colloid. Interface Sci. 2019, 552, 236–246. [Google Scholar] [CrossRef]
- Shamsi, M.H.; Geckeler, K.E. The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology 2008, 19, 075604. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, G.-E.; Cho, S.J.; Geckeler, K.E.; Fuchs, H. Cellular interaction of doxorubicin-loaded DNA-modified halloysite nanotubes. Nanoscale 2013, 5, 8577–8585. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, J.; Shen, Y.; Zhou, C.; Liu, M. Polyethyleneimine grafted short halloysite nanotubes for gene delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 224–235. [Google Scholar] [CrossRef]
- Shi, Y.-F.; Tian, Z.; Zhang, Y.; Shen, H.-B.; Jia, N.-Q. Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Res. Lett. 2011, 6, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Z.; Wu, Y.-P.; Gao, H.-Y.; Li, Y.-F.; He, R.-R.; Liu, M. Functionalization of halloysite nanotubes via grafting of dendrimer for efficient intracellular delivery of siRNA. Bioconjug. Chem. 2018, 29, 2606–2618. [Google Scholar] [CrossRef] [PubMed]
- Beall, G.W.; Sowersby, D.S.; Roberts, R.D.; Robson, M.H.; Lewis, L.K. Analysis of oligonucleotide DNA binding and sedimentation properties of montmorillonite clay using ultraviolet light spectroscopy. Biomacromolecules 2009, 10, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; Huang, Q.; Li, M.; Liang, W. Binding and degradation of DNA on montmorillonite coated by hydroxyl aluminum species. Colloid Surf. B 2008, 62, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, P.; Zhu, N. The protective effect of clay minerals against damage to adsorbed DNA induced by cadmium and mercury. Chemoshere 2014, 95, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Yoder, M.; Calamai, L.; Stotzky, G. X-ray diffractometry and electron microscopy of DNA from Bacillus subtilis bound on clay minerals. Sci. Soils 1998, 3, 1–12. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Livolant, F. Ordered phases of DNA in vivo and vitro. Physica A 1991, 176, 117–137. [Google Scholar] [CrossRef]
- He, R.; Liu, M.; Shen, Y.; Long, Z.; Zhou, C. Large-area assembly of halloysite nanotubes for enhancing the capture of tumor cells. J. Mater. Chem. B 2017, 5, 1712–1723. [Google Scholar] [CrossRef]
- Liu, M.; He, R.; Yang, J.; Zhao, W.; Zhou, C. Stripe-like clay nanotubes patterns in glass capillary tubes for capture of tumor cells. ACS Appl. Mater. Interfaces 2016, 8, 7709–7719. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, C.; Lvov, Y.; Liu, M. Clay nanotubes aligned with shear forces for mesenchymal stem cell patterning. Small 2019, 15, 1900357. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huo, Z.; Liu, T.; Shen, Y.; He, R.; Zhou, C. Self-assembling halloysite nanotubes into concentric ring patterns in a sphere-on-flat geometry. Langmuir 2017, 33, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cavallaro, G.; Lvov, Y. Orientation of charged clay nanotubes in evaporating droplet meniscus. J. Colloid Interface Sci. 2015, 440, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryuchkova, M.; Batasheva, S.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Panchal, A.; Lvov, Y.; Fakhrullin, R. Self-assembly of concentric microrings of tubule and platy nanoclays for cell patterning and capturing. Appl. Clay Sci. 2020, 195, 105707. [Google Scholar] [CrossRef]
- Hashizume, H.; van der Gaast, S.; Theng, B.K.G. Adsorption of adenine, cytosine, uracil, ribose, and phosphate by Mg-exchanged montmorillonite. Clay Miner. 2010, 45, 469–475. [Google Scholar] [CrossRef]
- Ferris, J.P. Mineral catalysis and prebiotic synthesis: Montmorillonite-catalyzed formation of RNA. Elements 2005, 1, 145–149. [Google Scholar] [CrossRef]
- Vettori, C.; Paffetti, D.; Pietramellara, G.; Stotzky, G.; Gallori, E. Amplification of bacterial DNA bound on clay minerals by the random amplified polymorphic DNA (RAPD) technique. FEMS Microbiol. Ecol. 1996, 20, 251–260. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Khanna, M.; Toranzos, G.A.; Stotzky, G. Amplification of DNA bound on clay minerals. Mol. Ecol. 1998, 7, 775–778. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvauz, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Theng, B.K.G.; Russell, M.; Churchman, C.J.; Parfitt, R.L. Surface properties of allophone, halloysite and imogolite. Clays Clay Min. 1982, 30, 143–149. [Google Scholar] [CrossRef]
- Lun, H.; Ouyang, J.; Yang, H. Enhancing dispersion of halloysite nanotubes via chemical modification. Phys. Chem. Miner. 2014, 41, 281–288. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.Y.; Miller, K.; Welbourn, R.; Stocker, I.; Clarke, S.; Casford, M.; Gutfreund, P.; Skoda, M.W.A. Cation bridging studied by specular neutron reflection. Langmuir 2013, 29, 5520–5527. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Qin, C.; Yang, B.; Hu, X.; Liu, C.; Waigi, G.; Li, X.; Ling, W. Metal cation saturation on montmorillonites facilitates the adsorption of DNA via cation bridging. Chemosphere 2019, 235, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Grillo, I.; Gradzielski, M.; Lazzara, G. Structure of hybrid materials based on halloysite nanotubes filled with anionic surfactants. J. Phys. Chem. C 2016, 120, 13492–13502. [Google Scholar] [CrossRef]
- Cavallaro, G.; Chiappisi, L.; Gradzielski, M.; Lazzara, G. Effect of the supramolecular interactions on the nanostructure of halloysite/biopolymer hybrids: A comprehensive study by SANS, fluorescence correlation spectroscopy and electric birefringence. Phys. Chem. Chem. Phys. 2020, 22, 8193–8202. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Wolff, J.A.; Budker, V. The mechanism of naked DNA uptake and expression. Adv. Genet. 2005, 54, 1–20. [Google Scholar]
- Loyter, A.; Scangos, G.A.; Ruddle, F.H. Mechanisms of DNA uptake by mammalian cells: Fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc. Natl. Acad. Sci. USA 1982, 79, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Sellers, D.L.; Tan, J.-K.Y.; Peeler, D.J.; Horner, P.J.; Pun, S.H. Development of switchable polymers to address the dilemma of stability and cargo release in polycationic nucleic acid carriers. Biomaterials 2017, 127, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Durán, M.C.; Willenbrock, S.; Barchanski, A.; Müller, J.-M.V.; Maiolini, A.; Soller, J.T.; Barcikowski, S.; Nolte, I.; Feige, K.; Escobar, H.M. Comparison of nanoparticle-mediated transfection methods for DNA expression plasmids: Efficiency and cytotoxicity. J. Nanobiotechnol. 2011, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, C.L.; Leong, K.W. Balancing protection and release of DNA: Tools to address a bottleneck of non-viral gene delivery. J. R. Soc. Interface 2010, 7, S67–S82. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Benetoli, L.O.D.B.; de Santana, H.; Zaia, C.T.B.V.; Zaia, D.A.M. Adsorption of nucleic acid bases on clays: An investigation using Langmuir and Freundlich isotherms and FT-IR spectroscopy. Mon. Chem. Chem. Mon. 2008, 139, 753–761. [Google Scholar] [CrossRef]
- Castro-Smirnov, F.A.; Piétrement, O.; Aranda, P.; Bertrand, J.-R.; Ayache, J.; Le Cam, E.; Ruiz-Hitzky, E.; Lopez, B.S. Physical interactions between DNA and sepiolite nanofibers, and potential application for DNA transfer into mammalian cells. Sci. Rep. 2016, 6, 36341. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Elimelech, M. Plasmid DNA adsorption on silica: Kinetics and conformational changes in monovalent and divalent salts. Biomacromolecules 2007, 8, 24–32. [Google Scholar] [CrossRef]
- Roth, G.A.; Tahiliani, S.; Neu-Baker, N.M.; Brenner, S.A. Hyperspectral microscopy as an analytical tool for nanomaterials. WIREs Nanomed. Nanobiotechnol. 2015, 7, 565–579. [Google Scholar] [CrossRef]
- Zamora-Perez, P.; Tsoutsi, D.; Xu, R.; Rivera_Gil, P. Hyperspectral-ehnanced dark field microscopy for single and collective nanoparticle characterization in biological environments. Materials 2018, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Piana, S.; Colletti, C.G.; Noto, R.; Riela, S.; Baiamonte, C.; Giordano, C.; Pizzolanti, G.; Cavallaro, G.; Milioto, S.; et al. Multicavity halloysite-amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cell. J. Mater. Chem. B 2015, 3, 4074–4081. [Google Scholar] [CrossRef] [Green Version]
- Kamalieva, R.F.; Ishmukhametov, I.R.; Batasheva, S.N.; Rozhina, E.V.; Fakhrullin, R.F. Uptake of halloysite clay nanotubes by human cells: Colourimetric viability tests and microscopy study. Nano Struct. Nano Objects 2018, 15, 54–60. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Akhatova, F.; Lazzara, G.; Cavallaro, G.; Nigamatzyanova, L.; Fakhrullin, R. Selective cytotoxic activity of prodigiosin@halloysite nanoformulation. Front. Bioeng. Biotechnol. 2020, 8, 424. [Google Scholar] [CrossRef]
- Rozhina, E.; Panchal, A.; Akhatova, F.; Lvov, Y.; Fakhrullin, R. Cytocompatibility and cellular uptake of alkylsilane-modified hydrophobic halloysite nanotubes. Appl. Clay Sci. 2020, 185, 105371. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Vikuliva, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally derived nano- and micro-drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020, 4, 5638–5655. [Google Scholar] [CrossRef] [Green Version]
- Rong, R.; Xu, X.; Zhu, S.; Li, B.; Wang, X.; Tang, K. Facile preaparation of homogeneous and length controllable halloysite nanotubes by ultrasonic scission and uniform viscosity centrifugation. Chem. Eng. J. 2016, 291, 20–29. [Google Scholar] [CrossRef]
- Fukui, Y.; Fujimoto, K. The preparation of sugar polymer-coated nanocapsules by the layer-by-layer deposition on the liposome. Langmuir 2009, 25, 10020–10025. [Google Scholar] [CrossRef]
- Cavalieri, F.; Postma, A.; Lee, L.; Caruso, F. Assembly and functionalization of DNA#polymer microcapsules. ACS Nano 2009, 3, 234–240. [Google Scholar]
- Goda, E.S.; Yoon, K.R.; El-sayed, S.H.; Hong, S.E. Halloysite nanotubes as smart flame retardant and economic reinforcing materials: A review. Thermochim. Acta 2018, 669, 173–184. [Google Scholar] [CrossRef]
- Kwon, Y.-W.; Lee, C.H.; Choi, D.H.; Jin, J.-I. Materials science of DNA. J. Mater. Chem. 2009, 19, 1353–1380. [Google Scholar] [CrossRef]
- Lin, Z.; Granick, S. Patterns formed by droplet evaporation from a restricted geometry. J. Am. Chem. Soc. 2005, 127, 2816–2817. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xia, J.; Hong, S.W.; Lin, Z.; Qiu, F.; Yang, Y. Self-assembly of gradient concentric rings via solvent evaporation from a capillary bridge. Phys. Rev. Lett. 2006, 96, 066104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.; Li, B.; Lin, Z. Drying-mediated assembly of colloidal nanoparticles into large-scale microchannels. ACS Nano 2013, 7, 6079–6085. [Google Scholar] [CrossRef]
- Zhang, S.; Luan, W.; Zhong, Q.; Yina, S.; Yang, F. Evaporation-induced self-assembly of quantum dots-based concentric rings on polymer-based nanocomposite films. Soft Matter 2016, 12, 8285–8296. [Google Scholar] [CrossRef]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. Effect of particle geometry on triple line motion of nano-fluid drops and deposit nano-structuring. Adv. Colloid Interface Sci. 2015, 222, 44–57. [Google Scholar] [CrossRef]
- Han, W.; Lin, Z. Learning from “coffee rings”: Ordered structures enabled by controlled evaporative self-assembly. Angew. Chem. Int. Ed. 2012, 51, 1534–1546. [Google Scholar] [CrossRef]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay nanotube encapsulation for functional biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide in vivo: A Paramecium caudatum study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef] [Green Version]
- von Klitzing, R.; Stehl, D.; Pogrzeba, T.; Schomäcker, R.; Minullina, R.; Panchal, A.; Konnova, S.; Fakhrullin, R.; Koetz, J.; Möhwald, H.; et al. Halloysite stabilized emulsions for hydroformylation of long chain olefins. Adv. Mater. Interfaces 2017, 4, 1600435. [Google Scholar] [CrossRef]
- Panchal, A.; Rahman, N.; Konnova, S.; Fakhrullin, R.; Zhang, D.; Blake, D.; John, V.; Ivanov, E.; Lvov, Y. Clay nanotube liquid marbles enhanced with inner biofilm formation for the encapsulation and storage of bacteria at room temperature. ACS Appl. Nano Mater. 2020, 3, 1263–1271. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 209–214. [Google Scholar]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chem. Eur. J. 2013, 19, 1778–1783. [Google Scholar] [CrossRef]
- Saengkrit, N.; Saesso, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloid Surf. B 2014, 114, 349–356. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, NY, USA, 1997; 378p. [Google Scholar]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |






| Substance | (Poly)nucleotide ζ-Potential, mV | HNTs with a Substance Bound | HNTs with a Substance Bound in the Presence of MgCl2 | ||
|---|---|---|---|---|---|
| ζ-Potential, mV | Size, nm | ζ-Potential, mV | Size, nm | ||
| Pure halloysite nanotubes (HNTs) | −25.2 ± 0.2 | 2283 ± 53.84 | −18.9 ± 0.3 | 4839 ± 20.22 | |
| UMP Na2 | −20.3 ± 0.9 | −29.9 ± 0.6 | 2242 ± 95.77 | −23.5 ± 0.4 | 1251 ± 46.87 |
| ADP Na3 | −21.2 ± 1.3 | −34.4 ± 1.1 | 601.4 ± 18.73 | −20.6 ± 0.15 | 897.0 ± 39.83 |
| dATP Na | −19.6 ± 1.8 | −44.6 ± 0.11 | 557.2 ± 10.53 | −26.2 ± 0.4 | 512.2 ± 4.5 |
| AMP | −11.9 ± 0.8 | −37.7 ± 0.6 | 1296 ± 48.57 | −26.4 ± 0.6 | 958.6 ± 38.39 |
| Uridine | −21.0 ± 1.2 | −28.4 ± 0.3 | 5351 ± 256,0 | −21.3 ± 0.15 | 2026 ± 72.9 |
| polyAU | −39.7 ± 0.9 | −40.2 ± 0.4 | 803.0 ± 35.66 | −27.7 ± 0.4 | 644.8 ± 12.2 |
| ATP Mg | −10.6 ± 4 | −37.3 ± 0.4 | 561.7 ± 8.843 | −31.0 ± 0.6 | 468.6 ± 5.546 |
| Chicken erythrocyte DNA | −73.6 ± 2 | - | - | - | - |
| Ultrasonicated DNA | −56,4 ± 11,6 | −34,4 ± 1,1 | 708.2 ± 11.47 | −30,8 ± 0,61 | 936.6 ± 25.32 |
| Sample | Amount of (Poly)nucleotide Loaded to Halloysite, wt% |
|---|---|
| HNT-usDNA | 0 |
| HNT-usDNA+MgCl2 | 11.1 |
| HNT-dATPNa | 0 |
| HNT-dATPNa+MgCl2 | 0 |
| HNT-ATPMg | 8.0 |
| HNT-polyAU | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batasheva, S.; Kryuchkova, M.; Fakhrullin, R.; Cavallaro, G.; Lazzara, G.; Akhatova, F.; Nigamatzyanova, L.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Facile Fabrication of Natural Polyelectrolyte-Nanoclay Composites: Halloysite Nanotubes, Nucleotides and DNA Study. Molecules 2020, 25, 3557. https://doi.org/10.3390/molecules25153557
Batasheva S, Kryuchkova M, Fakhrullin R, Cavallaro G, Lazzara G, Akhatova F, Nigamatzyanova L, Evtugyn V, Rozhina E, Fakhrullin R. Facile Fabrication of Natural Polyelectrolyte-Nanoclay Composites: Halloysite Nanotubes, Nucleotides and DNA Study. Molecules. 2020; 25(15):3557. https://doi.org/10.3390/molecules25153557
Chicago/Turabian StyleBatasheva, Svetlana, Marina Kryuchkova, Ramil Fakhrullin, Giuseppe Cavallaro, Giuseppe Lazzara, Farida Akhatova, Läysän Nigamatzyanova, Vladimir Evtugyn, Elvira Rozhina, and Rawil Fakhrullin. 2020. "Facile Fabrication of Natural Polyelectrolyte-Nanoclay Composites: Halloysite Nanotubes, Nucleotides and DNA Study" Molecules 25, no. 15: 3557. https://doi.org/10.3390/molecules25153557
APA StyleBatasheva, S., Kryuchkova, M., Fakhrullin, R., Cavallaro, G., Lazzara, G., Akhatova, F., Nigamatzyanova, L., Evtugyn, V., Rozhina, E., & Fakhrullin, R. (2020). Facile Fabrication of Natural Polyelectrolyte-Nanoclay Composites: Halloysite Nanotubes, Nucleotides and DNA Study. Molecules, 25(15), 3557. https://doi.org/10.3390/molecules25153557










