In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, ?-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
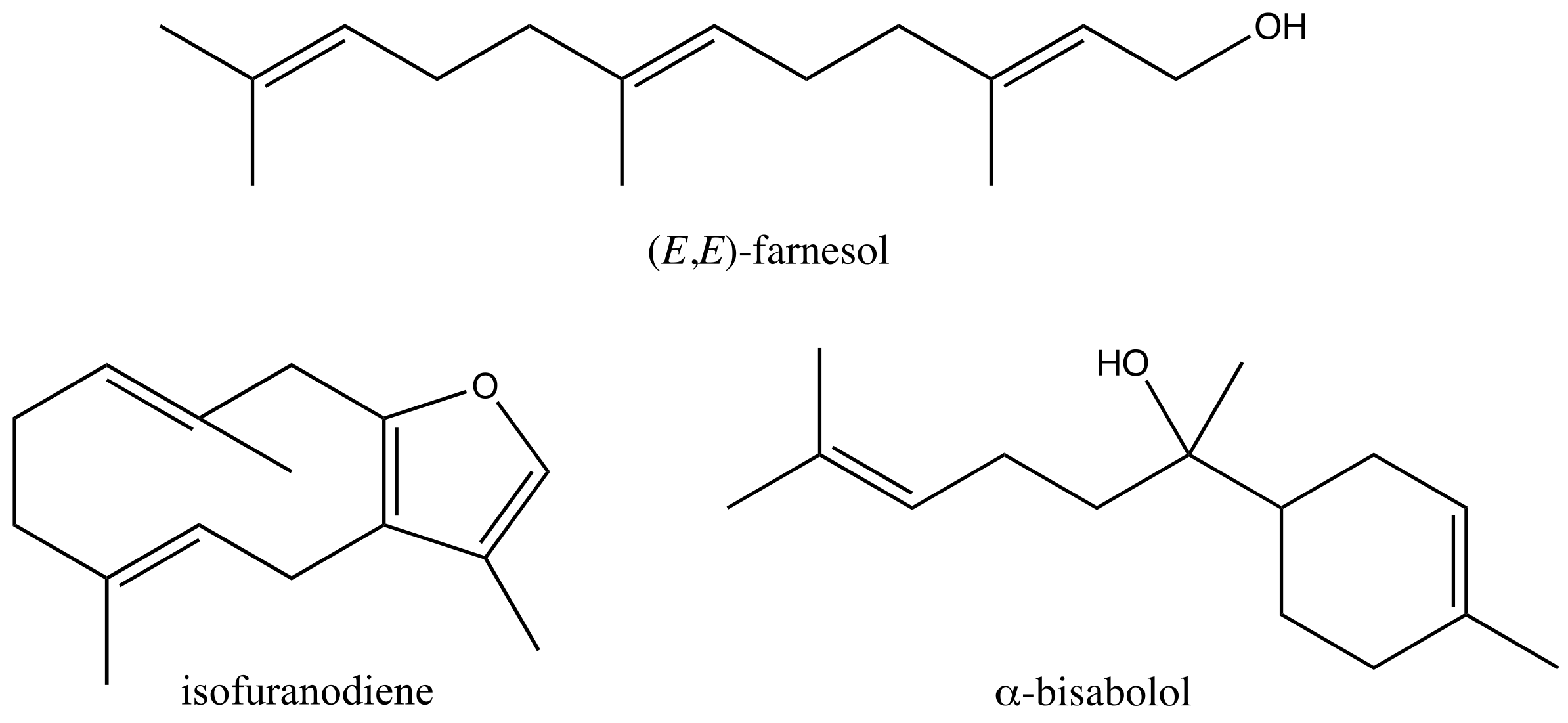
4.1. Isolation and Crystallization of Isofuranodiene
4.2. Collection of Protoscoleces
4.3. Protoscoleces Viability Test
4.4. In Vitro Scolicidal Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Youssefi, M.R.; Mirshafiei, S.; Moshfegh, Z.; Soleymani, N.; Rahimi, M.T. Cystic echinococcosis is an occupational disease? J. Parasit. Dis. 2016, 40, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohansal, M.H.; Nourian, A.; Rahimi, M.T.; Daryani, A.; Spotin, A.; Ahmadpour, E. Natural products applied against hydatid cyst protoscoleces: a review of past to present. Acta Trop. 2017, 176, 385–394. [Google Scholar] [CrossRef]
- Rahimi-Esboei, B.; Ebrahimzadeh, M.; Fathi, H.; Rezaei Anzahaei, F. Scolicidal effect of Allium sativum flowers on hydatid cyst protoscoleces. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 129–132. [Google Scholar] [PubMed]
- Niazi, M.; Saki, M.; Sepahvand, M.; Jahanbakhsh, S.; Khatami, M.; Beyranvand, M. In vitro and ex vivo scolicidal effects of Olea europaea L. to inactivate the protoscolecs during hydatid cyst surgery. Ann. Med. Surg. 2019, 42, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.H.; Wani, N.A.; Zargar, S.A.; Wani, M.A.; Tabassum, R.; Hussain, Z.; Baba, A.A.; Lone, R.A. Albendazole as an adjuvant to the standard surgical management of hydatid cyst liver. Int. J. Surg. 2008, 6, 448–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Bruno, M.; Benelli, G. Larvicidal activity of essential oils of five Apiaceae taxa and some of their main constituents against Culex quinquefasciatus. Chem. Biodivers. 2018, 15, e1700382. [Google Scholar] [CrossRef]
- Fakhar, M.; Chabra, A.; Rahimi-Esboei, B.; Rezaei, F. In vitro protoscolicidal effects of fungal chitosan isolated from Penicillium waksmanii and Penicillium citrinum. J. Paras. Dis. 2015, 39, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Posadzki, P.; Watson, L.; Ernst, E. Herb–drug interactions: an overview of systematic reviews. Br. J. Clin. Pharmacol. 2013, 75, 603–618. [Google Scholar] [CrossRef] [Green Version]
- Dewick, P.M. The biosynthesis of C 5–C 25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Nicoletti, M.; Baldan, V.; Dall’Acqua, S. New drugs from old natural compounds: scarcely investigated sesquiterpenes as new possible therapeutic agents. Curr. Med. Chem. 2018, 25, 1241–1258. [Google Scholar] [CrossRef]
- Maggi, F.; Papa, F.; Giuliani, C.; Maleci Bini, L.; Venditti, A.; Bianco, A.; Nicoletti, M.; Iannarelli, R.; Caprioli, G.; Sagratini, G.; et al. Essential oil chemotypification and secretory structures of the neglected vegetable Smyrnium olusatrum L. (Apiaceae) growing in central Italy. Flavour Frag. J. 2015, 30, 139–159. [Google Scholar] [CrossRef]
- Quassinti, L.; Maggi, F.; Barboni, L.; Ricciutelli, M.; Cortese, M.; Papa, F.; Garulli, C.; Kalogris, C.; Vittori, S.; Bramucci, M. Wild celery (Smyrnium olusatrum L.) oil and isofuranodiene induce apoptosis in human colon carcinoma cells. Fitoterapia 2014, 97, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, R.; Ranjbarian, F.; Dall’Acqua, S.; Papa, F.; Iannarelli, R.; Kamte, S.L.N.; Vittori, S.; Benelli, G.; Maggi, F.; Hofer, A.; et al. An overlooked horticultural crop, Smyrnium olusatrum, as a potential source of compounds effective against African trypanosomiasis. Parasitol. Int. 2017, 66, 146–151. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Nicoletti, M.; Petrelli, R.; Cappellacci, L.; Galassi, R.; Maggi, F. Isofuranodiene and germacrone from Smyrnium olusatrum essential oil as acaricides and oviposition inhibitors against Tetranychus urticae: impact of chemical stabilization of isofuranodiene by interaction with silver triflate. J. Pest. Sci. 2017, 90, 693–699. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crop. Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, J.; Papa, F.; Maggi, F.; Chen, X. Isofuranodiene, the main volatile constituent of wild celery (Smyrnium olusatrum L.), protects d-galactosamin/lipopolysacchride-induced liver injury in rats. Nat. Prod. Res. 2016, 30, 1162–1165. [Google Scholar] [CrossRef]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Kavallieratos, N.G.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Benelli, G. Rationale for developing novel mosquito larvicides based on isofuranodiene microemulsions. J. Pest. Sci. 2019, 92, 909–921. [Google Scholar] [CrossRef]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical composition and antibacterial activity of seven uncommon essential oils. J. Essent. Oil Res. 2018, 30, 233–243. [Google Scholar] [CrossRef]
- Maggi, F.; Papa, F.; Pucciarelli, S.; Bramucci, M.; Quassinti, L.; Barboni, L.; Dal Ben, D.; Ramadori, A.T.; Graiff, C.; Galassi, R. Stabilization of the cyclodecadiene derivative isofuranodiene by silver (I) coordination. Mechanistic and biological aspects. Fitoterapia 2017, 117, 52–60. [Google Scholar] [CrossRef]
- Brunetti, A.; Marinelli, O.; Morelli, M.B.; Iannarelli, R.; Amantini, C.; Russotti, D.; Santoni, G.; Maggi, F.; Nabissi, M. Isofuranodiene synergizes with temozolomide in inducing glioma cells death. Phytomedicine 2019, 52, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Barboni, L.; Papa, F.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. A forgotten vegetable (Smyrnium olusatrum L., Apiaceae) as a rich source of isofuranodiene. Food Chem. 2012, 135, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Pisani, M.; Quassinti, L.; Bramucci, M.; Galassi, R.; Maggi, F.; Rossi, B.; Damin, A.; Carloni, P.; Astolfi, P. Nanostructured Liquid Crystalline Particles as Delivery Vectors for Isofuranodiene: Characterization and In-vitro Anticancer Activity. Colloid. Surf. B 2020, 192, 111050. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264. 7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Tabari, M.A.; Tehrani, M.A.B. Evidence for the involvement of the GABAergic, but not serotonergic transmission in the anxiolytic-like effect of bisabolol in the mouse elevated plus maze. N-S Arch. Pharmacol. 2017, 390, 1041–1046. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on alpha-bisabolol. Food Chem. Toxicol. 2008, 46, S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Miwa, T.; Shibuya, M.; Yamamoto, Y.; Nagino, M. The anticancer effects of novel α-bisabolol derivatives against pancreatic cancer. Anticancer Res. 2017, 37, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalieri, E.; Rigo, A.; Bonifacio, M.; de Prati, A.C.; Guardalben, E.; Bergamini, C.; Fato, R.; Pizzolo, G.; Suzuki, H.; Vinante, F. Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells. J. Transl. Med. 2011, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Rigo, A.; Vinante, F. The antineoplastic agent α-bisabolol promotes cell death by inducing pores in mitochondria and lysosomes. Apoptosis 2016, 21, 917–927. [Google Scholar] [CrossRef]
- Bockman, M.R.; Kalinda, A.S.; Petrelli, R.; De La Mora-Rey, T.; Tiwari, D.; Liu, F.; Dawadi, S.; Nandakumar, M.; Rhee, K.Y.; Schnappinger, D.; et al. Targeting Mycobacterium tuberculosis Biotin Protein Ligase (MtBPL) with Nucleoside-Based Bisubstrate Adenylation Inhibitors. J. Med. Chem. 2015, 58, 7349–7369. [Google Scholar]
- Rigo, A.; Ferrarini, I.; Bonalumi, A.; Tecchio, C.; Montresor, A.; Laudanna, C.; Vinante, F. Efficient lysis of B-chronic lymphocytic leukemia cells by the plant-derived sesquiterpene alcohol α-bisabolol, a dual proapoptotic and antiautophagic agent. Oncotarget 2018, 9, 25877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; de Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Bioph. Res. Co. 2004, 315, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Sultana, S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation, and apoptotic responses in the colon of Wistar rats. Chem. Biol. Interact. 2011, 192, 193–200. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Delmondes, G.; Bezerra, D.S.; de Queiroz Dias, D.; de Souza Borges, A.; Araújo, I.M.; da Cunha, G.L.; Bandeira, F.R.; Barbosa, R.; Bezerra Felipe, C.F.; Melo Coutinho, H.D.; et al. Toxicological and pharmacologic effects of farnesol (C15H26O): a descriptive systematic review. Food Chem. Toxicol. 2019, 129, 169–200. [Google Scholar] [CrossRef] [PubMed]
- Lapczynski, A.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on farnesol. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Shahnouri, M.; Tabari, M.A.; Araghi, A. Neuropharmacological properties of farnesol in Murine model. Iran J. Vet. Res. 2016, 17, 259. [Google Scholar]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [Green Version]
- Lorek, J.; Pöggeler, S.; Weide, M.R.; Breves, R.; Bockmühl, D.P. Influence of farnesol on the morphogenesis of Aspergillus niger. J. Basic Microbiol. 2008, 48, 99–103. [Google Scholar] [CrossRef]
- Shea, J.M.; Del Poeta, M. Lipid signaling in pathogenic fungi. Curr. Opin. Microbiol. 2006, 9, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential anti-inflammatory and anticancer properties of farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, C.; Kim, S.-H.; Sethi, G.; Ahn, K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015, 360, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D. Mycotoxins in spices and herbs–An update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Nasiri, M.; Hamidi, M.; Kiani, K.; Alian Samakkhah, S.; Maggi, F. Towards green drugs against cestodes: Effectiveness of Pelargonium roseum and Ferula gummosa essential oils and their main component on Echinococcus granulosus protoscoleces. Vet. Parasitol. 2019, 266, 84–87. [Google Scholar] [CrossRef]
- Fabbri, J.; Maggiore, M.A.; Pensel, P.E.; Albani, C.M.; Denegri, G.M.; Elissondo, M.C. Could beta-myrcene be an alternative to albendazole for the treatment of experimental cystic echinococcosis? Acta Trop. 2018, 187, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Kamte, S.L.N.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10515–10525. [Google Scholar] [CrossRef]
- Elissondo, M.C.; Albani, C.M.; Gende, L.; Eguaras, M.; Denegri, G. Efficacy of thymol against Echinococcus granulosus protoscoleces. Parasitol. Int. 2008, 57, 185–190. [Google Scholar] [CrossRef]
- Elissondo, M.C.; Pensel, P.E.; Denegri, G.M. Could thymol have effectiveness on scolices and germinal layer of hydatid cysts? Acta Trop. 2013, 125, 251–257. [Google Scholar] [CrossRef]
- Fabbri, J.; Maggiore, M.A.; Pensel, P.E.; Denegri, G.M.; Gende, L.B.; Elissondo, M.C. In vitro and in vivo efficacy of carvacrol against Echinococcus granulosus. Acta Trop. 2016, 164, 272–279. [Google Scholar] [CrossRef]
- Hosseini, M.; Yousefi, M.; Abouhosseini, M. Comparison of the Effect of Artemisia Sieberi Essential Oil and Albendazole Drug on Protoscoleces of Hydatid Cyst under in Vitro Conditions. J. Babol Univ. Med. Sci. 2017, 19, 63–68. [Google Scholar]
- Su, V.; King, D.; Woodrow, I.; McFadden, G.; Gleadow, R. Plasmodium falciparum growth is arrested by monoterpenes from eucalyptus oil. Flavour Frag. J. 2008, 23, 315–318. [Google Scholar] [CrossRef]
- Arruda, D.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Katzin, A.M.; Uliana, S.R. Inhibitory activity of limonene against Leishmania parasites in vitro and in vivo. Biomed. Pharmacother. 2009, 63, 643–649. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Tested Compound | Concentration (µg/mL) | 1 h Mortality (%) ± SE a | LC50 (µg/mL) (LCL-UCL) | LC90 (µg/mL) (LCL-UCL) | χ2 (df) b |
|---|---|---|---|---|---|
| Isofuranodiene | 1 | 28.33 ± 1.20 | 8.87 (5.76–11.87) | 25.48 (19.75–31.96) | 9.846 (4) n.s. |
| 2.5 | 32.66 ± 0.88 | ||||
| 5 | 37.00 ± 0.85 | ||||
| 10 | 41.00 ± 1.10 | ||||
| 25 | 95.66 ± 0.61 | ||||
| 50 | 100.00 ± 0.00 | ||||
| 100 | 100.00 ± 0.00 | ||||
| 200 | 100.00 ± 0.00 | ||||
| α-Bisabolol | 1 | 17.60 ± 0.53 | 103.20 (84.62–127.16) | 341.47 (288.1–404.86) | 2.130 (5) n.s. |
| 2.5 | 20.74 ± 1.14 | ||||
| 5 | 22.00 ± 0.47 | ||||
| 10 | 24.55 ± 0.76 | ||||
| 25 | 31.66 ± 0.46 | ||||
| 50 | 37.88 ± 0.41 | ||||
| 100 | 50.06 ± 1.02 | ||||
| 200 | 59.55 ± 0.46 | ||||
| Farnesol | 1 | 19.11 ± 0.90 | 113.68 (97.89–168.31) | 386.78 (311.98–451.59) | 7.494 (5) n.s. |
| 2.5 | 20.02 ± 0.02 | ||||
| 5 | 23.29 ± 0.20 | ||||
| 10 | 24.49 ± 0.69 | ||||
| 25 | 34.21 ± 0.12 | ||||
| 50 | 39.66 ± 1.33 | ||||
| 100 | 49.89 ± 0.54 | ||||
| 200 | 58.65 ± 1.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssefi, M.R.; Nikpay, A.; Hassanpour, N.; Mirzapour, A.; Tabari, P.S.; Pavela, R.; Maggi, F.; Petrelli, R. In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, ?-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces. Molecules 2020, 25, 3593. https://doi.org/10.3390/molecules25163593
Youssefi MR, Nikpay A, Hassanpour N, Mirzapour A, Tabari PS, Pavela R, Maggi F, Petrelli R. In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, ?-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces. Molecules. 2020; 25(16):3593. https://doi.org/10.3390/molecules25163593
Chicago/Turabian StyleYoussefi, Mohammad Reza, Ali Nikpay, Niloufar Hassanpour, Aida Mirzapour, Parisa Saleh Tabari, Roman Pavela, Filippo Maggi, and Riccardo Petrelli. 2020. "In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, ?-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces" Molecules 25, no. 16: 3593. https://doi.org/10.3390/molecules25163593
APA StyleYoussefi, M. R., Nikpay, A., Hassanpour, N., Mirzapour, A., Tabari, P. S., Pavela, R., Maggi, F., & Petrelli, R. (2020). In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, ?-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces. Molecules, 25(16), 3593. https://doi.org/10.3390/molecules25163593









