Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids
Abstract
1. Introduction
2. Results
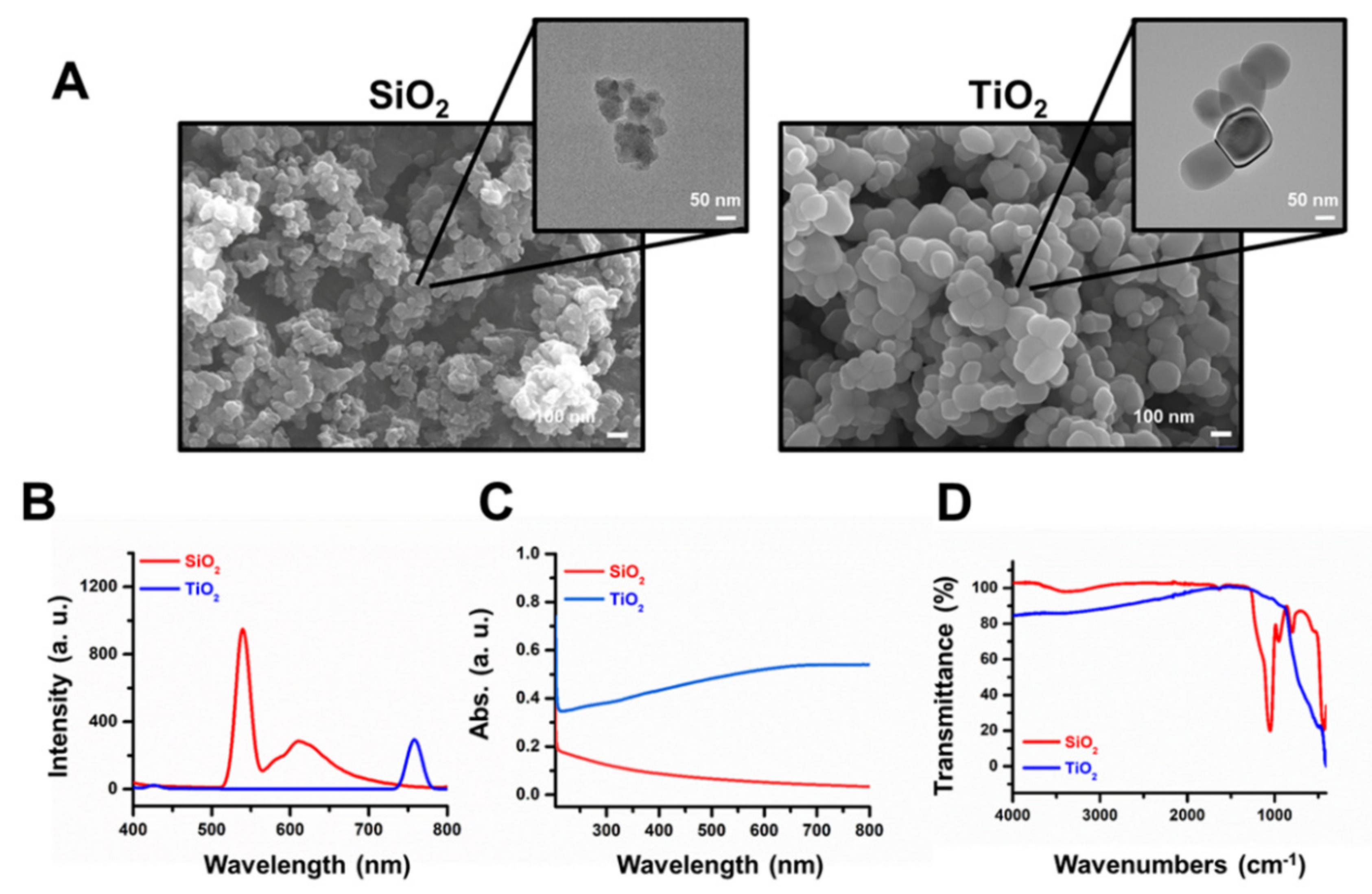
2.1. Characterization of SiO2 and TiO2 Nanoparticles
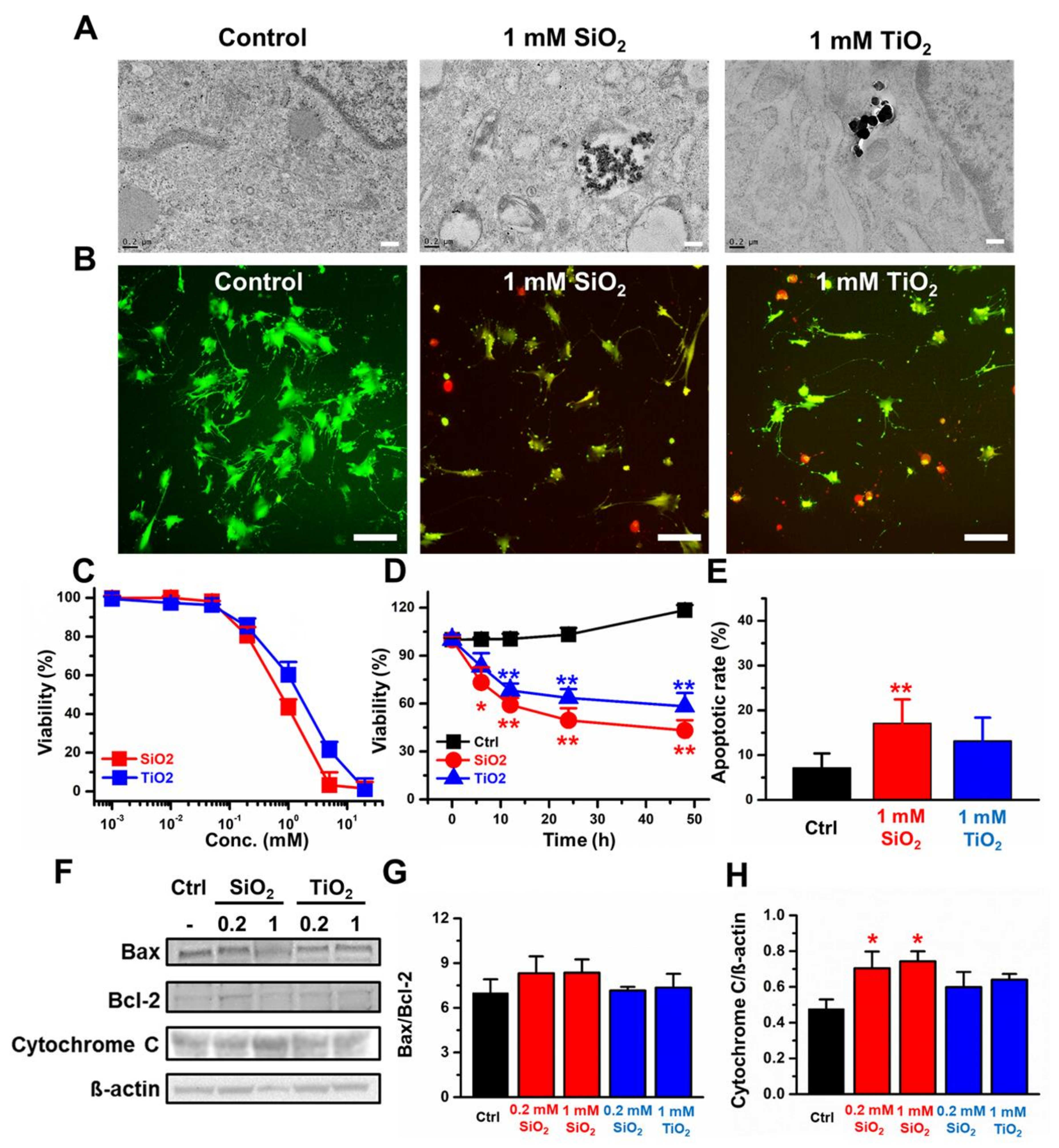
2.2. Toxicity in Two-Dimensional (2D) CCD-18Co Cells
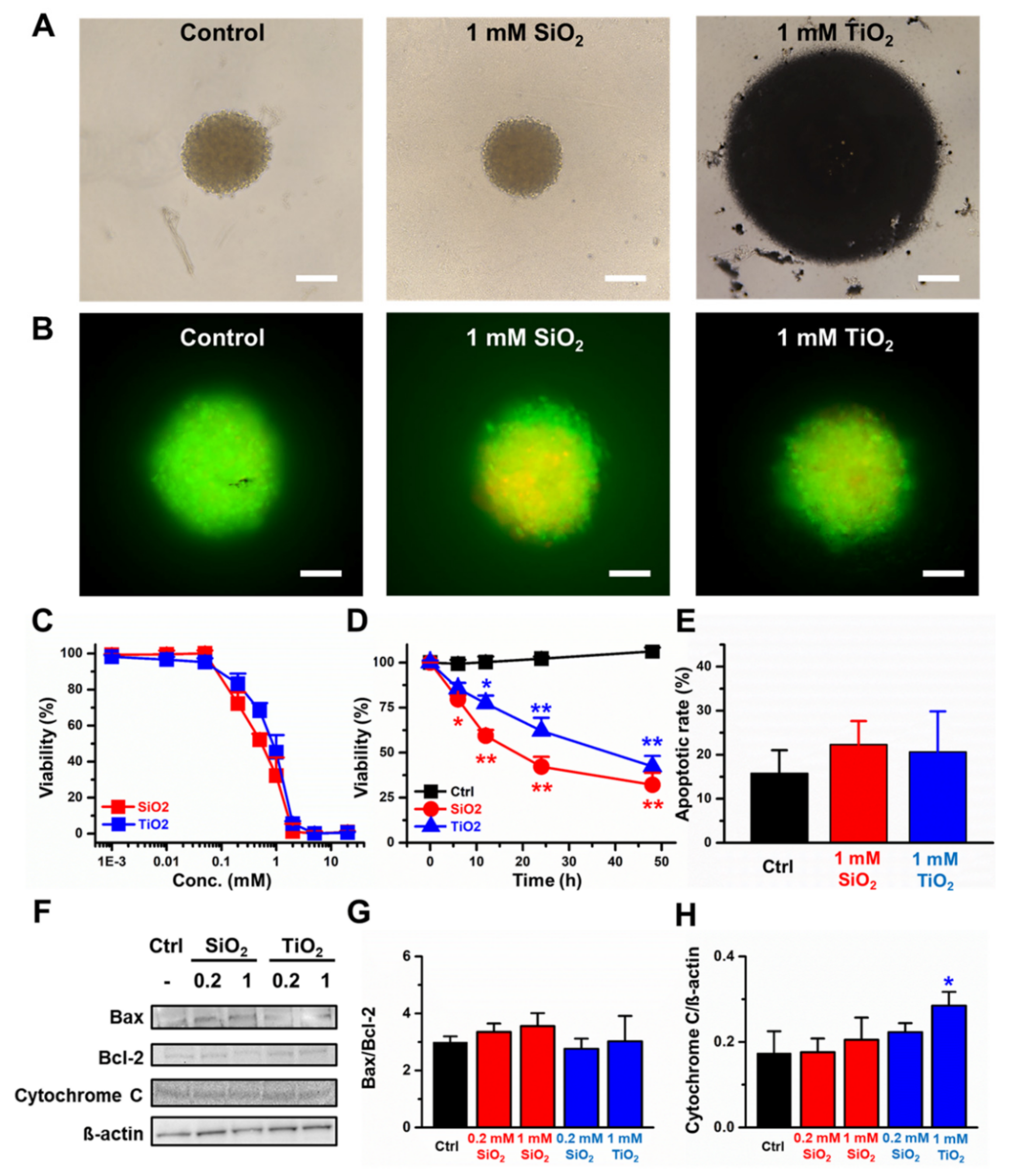
2.3. Toxicity of the CCD-18Co 3D Spheroids
2.4. In Vivo Toxicities of the SiO2 and TiO2 Nanoparticles
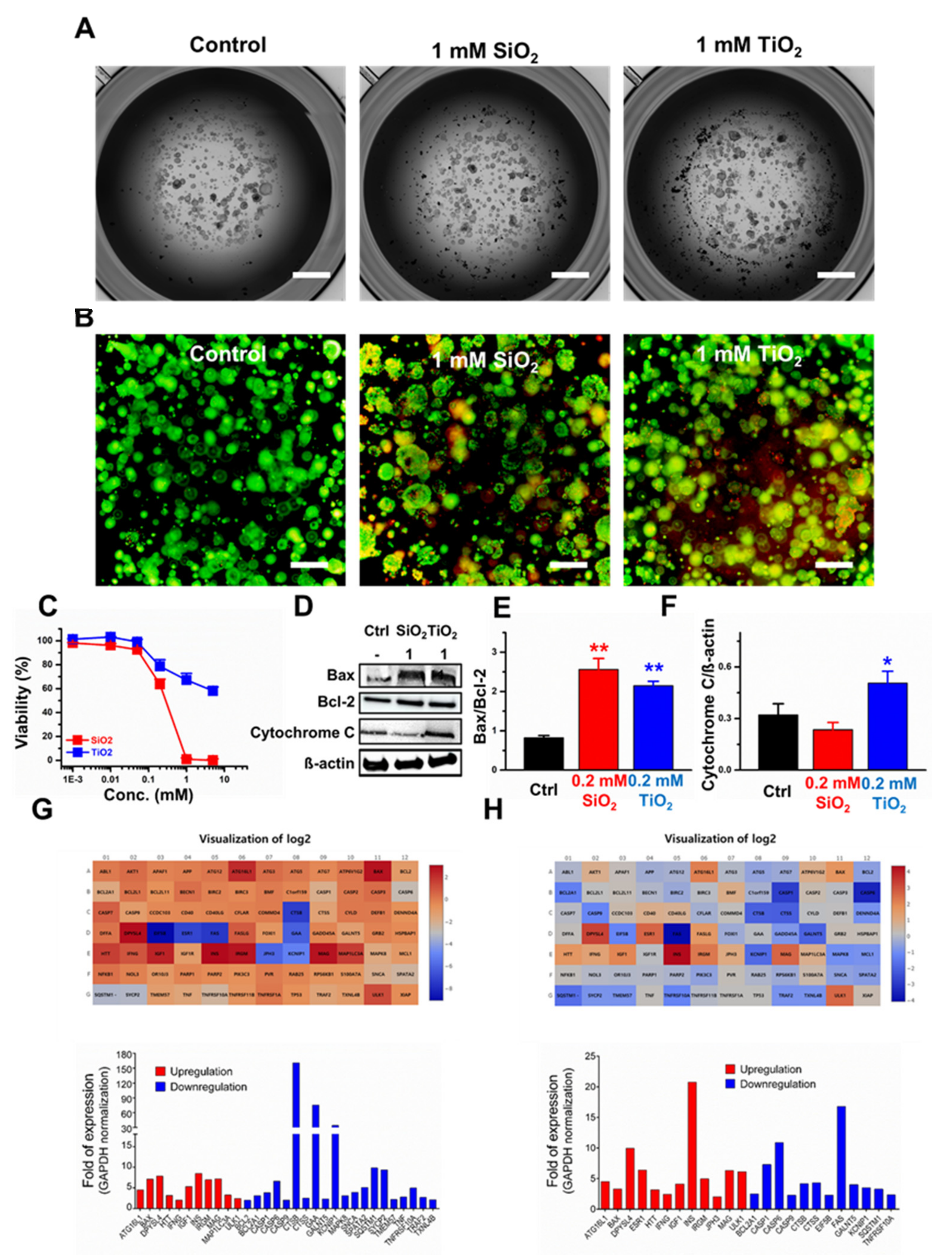
2.5. Toxicity Assessment of the SiO2 and TiO2 Nanoparticles in Human Colon Organoids
3. Discussion
4. Materials and Methods
4.1. Experimental Section
Materials
4.2. SiO2 and TiO2 Nanoparticle Preparation and Characterization
4.3. Incubation of 2D and 3D CCD-18Co and Human Colon Organoids with SiO2 and TiO2 Nanoparticles and Assessment of Cell Viability
4.4. The 2D/3D CCD-18Co and Human Colon Organoid Imaging
4.5. PCR Arrays
4.6. Western Blot
4.7. In Vivo ICR Mice Toxicity Study
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jansen, T.; Claassen, L.; van Kamp, I.; Timmermans, D.R.M. ‘All chemical substances are harmful.’ public appraisal of uncertain risks of food additives and contaminants. Food Chem. Toxicol. 2020, 136, 110959. [Google Scholar] [CrossRef] [PubMed]
- Phue, W.H.; Liu, M.; Xu, K.; Srinivasan, D.; Ismail, A.; George, S. A comparative analysis of different grades of silica particles and temperature variants of food-grade silica nanoparticles for their physicochemical properties and effect on trypsin. J. Agric. Food Chem. 2019, 67, 12264–12272. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; DeLoid, G.M.; Bitounis, D.; Torre-Roche, R.D.L.; White, J.C.; Zhang, Z.; Ho, C.G.; Ng, K.W.; Eitzer, B.D.; Demokritou, P. Co-exposure to the food additives SiO2 (E551) or TiO2 (E171) and the pesticide boscalid increases cytotoxicity and bioavailability of the pesticide in a tri-culture small intestinal epithelium model: Potential health implications. Environ. Sci. Nano 2019, 6, 2786–2800. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Kramer, E.; Oomen, A.G.; Rivera, Z.E.H.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Mutsuga, M.; Sato, K.; Hirahara, Y.; Kawamura, Y. Analytical methods for SiO2 and other inorganic oxides in titanium dioxide or certain silicates for food additive specifications. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2011, 28, 423–427. [Google Scholar] [CrossRef]
- Winter, M.; Beer, H.-D.; Hornung, V.; Krämer, U.; Schins, R.P.F.; Förster, I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology 2011, 5, 326–340. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the food additive Titanium Dioxide (E171) on gut microbiota-host interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Jovanović, B.; Jovanović, N.; Cvetković, V.J.; Matić, S.; Stanić, S.; Whitley, E.M.; Mitrović, T.L. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster-a 20 generation dietary exposure experiment. Sci. Rep. 2018, 8, 17922. [Google Scholar] [CrossRef]
- Lim, J.-H.; Bae, D.; Fong, A. Titanium dioxide in food products: Quantitative analysis using ICP-MS and Raman spectroscopy. J. Agric. Food Chem. 2018, 66, 13533–13540. [Google Scholar] [CrossRef]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172&showFR=1&subpartNode=21:3.0.1.1.3.5 (accessed on 23 June 2020).
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.3126&SearchTerm=titanium%20dioxide (accessed on 23 June 2020).
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef]
- Lozano, O.; Silva-Platas, C.; Chapoy-Villanueva, H.; Pérez, B.E.; Lees, J.G.; Ramachandra, C.J.A.; Contreras-Torres, F.F.; Lázaro-Alfaro, A.; Luna-Figueroa, E.; Bernal-Ramírez, J.; et al. Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes. Part Fibre Toxicol. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; You, X.; Zhu, T.; Gao, S.; Wang, Y.; Wang, R.; Yu, H.; Qian, B. Silica nanoparticles enhance germ cell apoptosis by inducing reactive oxygen species (ROS) formation in Caenorhabditis elegans. J. Toxicol. Sci. 2020, 45, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lojk, J.; Repas, J.; Veranič, P.; Bregar, V.B.; Pavlin, M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology 2020, 432, 152364. [Google Scholar] [CrossRef]
- Han, S.; Chen, Z.J.; Zhou, D.; Zheng, P.; Zhang, J.H.; Jia, G. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days. J. Peking Univ. Health Sci. 2020, 52, 457–463. [Google Scholar] [CrossRef]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne Titanium dioxide nanoparticles induce stronger adverse effects in obese mice than non-obese mice: Gut microbiota dysbiosis, colonic inflammation, and proteome alterations. Small 2020, e2001858. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sui, J.; Fu, Y.; Wu, W.; Liu, T.; Yang, S.; Liang, G. Titanium dioxide nanoparticles induced the apoptosis of RAW264.7 macrophages through miR-29b-3p/NFAT5 pathway. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Rios, A.C.; Clevers, H. Imaging organoids: A bright future ahead. Nat. Methods 2018, 15, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, M.; Kriegstein, A.R. Cerebral organoids in a dish: Progress and prospects. Cell 2013, 155, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.I.; Jones, C.F.; Thiagarajan, G.; Kurtzeborn, K.; Ghandehari, H.; Brooks, B.D.; Grainger, D.W. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials 2014, 35, 6323–6331. [Google Scholar] [CrossRef] [PubMed]
- Belair, D.G.; Wolf, C.J.; Moorefield, S.D.; Wood, C.; Becker, C.; Abbott, B.D. A three-dimensional organoid culture model to assess the influence of chemicals on morphogenetic fusion. Toxicol. Sci. 2018, 166, 394–408. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Sun, B.; Xia, T.; Hu, S. Predictive metabolomic signatures for safety assessment of metal oxide nanoparticles. Acs Nano 2019, 13, 13065–13082. [Google Scholar] [CrossRef]
- Bengalli, R.; Ortelli, S.; Blosi, M.; Costa, A.; Mantecca, P.; Fiandra, L. In vitro toxicity of TiO2:SiO2 nanocomposites with different photocatalytic properties. Nanomaterials 2019, 9, 1041. [Google Scholar] [CrossRef]
- Kitchin, K.T.; Richards, J.A.; Robinette, B.L.; Wallace, K.A.; Coates, N.H.; Castellon, B.T.; Grulke, E.A. Biochemical effects of some CeO2, SiO2, and TiO2 nanomaterials in HepG2 cells. Cell Biol. Toxicol. 2019, 35, 129–145. [Google Scholar] [CrossRef]
- Ramalla, I.; Gupta, R.K.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509–519. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Themochemical Tables fourth Edition. J. Phys. Chem. Ref. Data Monogr. 1998, 9, 1–1951. [Google Scholar]
- Titanium Dioxide (Anatase). Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C13463677&Type=IR-SPEC&Index=0 (accessed on 23 June 2020).
- Van der Zande, M.; Vandebriel, R.J.; Groot, M.J.; Kramer, E.; Herrera Rivera, Z.E.; Rasmussen, K.; Ossenkoppele, J.S.; Tromp, P.; Gremmer, E.R.; Peters, R.J.; et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part. Fibre Toxicol. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Fritsch-Decker, S.; An, Z.; Yan, J.; Hansjosten, I.; Al-Rawi, M.; Peravali, R.; Diabaté, S.; Weiss, C. Silica Nanoparticles provoke cell death independent of p53 and BAX in human colon cancer cells. Nanomaterials 2019, 9, 1172. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.B.; Jung, W.H.; Kim, K.Y.; Koh, B. Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids. Molecules 2020, 25, 3594. https://doi.org/10.3390/molecules25163594
Park SB, Jung WH, Kim KY, Koh B. Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids. Molecules. 2020; 25(16):3594. https://doi.org/10.3390/molecules25163594
Chicago/Turabian StylePark, Sung Bum, Won Hoon Jung, Ki Young Kim, and Byumseok Koh. 2020. "Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids" Molecules 25, no. 16: 3594. https://doi.org/10.3390/molecules25163594
APA StylePark, S. B., Jung, W. H., Kim, K. Y., & Koh, B. (2020). Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids. Molecules, 25(16), 3594. https://doi.org/10.3390/molecules25163594





