Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells
Abstract
:1. Introduction
2. Structure of Tubulin: Molecular Basis of MT Dynamics
3. Intrinsic Factors Affecting Microtubule Dynamics
3.1. Tubulin Isotypes and Microtubule Dynamics
| Tubulin Isotype | Gene Name | Expression | Cell Type-Specific Functions | Sequence of C-Terminus * | Reference |
|---|---|---|---|---|---|
| αIa | TUBA1A | ubiquitous | neuron migration | VEGEGEEEGEEY | [40,51] |
| αIb | TUBA1B | ubiquitous | VEGEGEEEGEEY | [52] | |
| αIc | TUBA1C | ubiquitous | ADGEDEGEEY | [53] | |
| αIIIc | TUBA3C | testis | VEAEAEEGEEY | [54] | |
| αIIIe | TUBA3E | testis | VEAEAEEGEAY | [55] | |
| αIVa | TUBA4A | ubiquitous | platelet biogenesis | YEDEDEGEE | [41,56] |
| αVIII | TUBA8 | high: heart and skeletal muscle moderate: brain, testis, and thyroid very low: all other tissues | spermatogenesis | FEEENEGEEF | [35,42,57] |
| βI | TUBB | ubiquitous | survival of differentiated neuroblastoma | EEEEDFGEEAEEEA | [43,44,58] |
| βIIa | TUBB2A | high: brain very low: all other tissues | neurite outgrowth | DEQGEFEEEEGEDEA | [43,44,59] |
| βIIb | TUBB2B | high: brain very low: all other tissues | neurite outgrowth | DEQGEFEEEEGEDEA | [43,44,60] |
| βIII | TUBB3 | moderate: brain low: testis | oxidative stress axon and nerve regeneration | EEEGEMYEDDEEEESEAQGPK | [43,44,48,61] |
| βIVa | TUBB4A | high: brain moderate/low: other tissues | neurons and oligodendrocyte function | EEGEFEEEAEEEVA | [43,49,62] |
| βIVb | TUBB4B/ TUBB2C | ubiquitously expressed high: testis, bone marrow, and heart moderate/low: other tissues | EEEGEFEEEAEEEVA | [43,63] | |
| βV | TUBB6 | ubiquitous at low levels | related to skeletal muscle regeneration | NDGEEAFEDEEEEIDG | [43,64,65] |
| βVI | TUBB1 | very low level in all tissues, highest in bone marrow and spleen | platelet cytoskeleton | VLEEDEEVTEEAEMEPEDKGH | [43,66] |
| βVIII | TUBB8 | very low in all tissues, highest in testis | oocyte maturation, early development | EEEEDEEYAEEEEVA | [67] |
3.2. Post-Translational Modifications of Microtubules
3.3. Microtubule-Associated Proteins and Microtubule Dynamics
4. Extrinsic Factors Affecting Microtubule Dynamics
4.1. Microtubule-Targeting Agents
4.2. Tubulin Pockets
4.2.1. Taxane Site
4.2.2. Laulimalide/Peloruside Site
4.2.3. Vinca Site
4.2.4. Maytansine Site
4.2.5. Colchicine Site
4.2.6. Pironetin Site
5. Factors Affecting Microtubule Dynamics in Cancer Cells
5.1. Tubulin Isotypes in Cancer and Anticancer Drug Resistance
| High Level of Tubulin Expression | Cancer | Prognosis | Resistance to | Reference |
|---|---|---|---|---|
| αIa | renal | poor | n/a | [38] |
| αIb | hepatocellular carcinoma | poor | paclitaxel | [199] |
| renal, breast | n/a | [38] | ||
| αIc | liver, renal, pancreatic, colon, breast, lung | poor | n/a | [38] |
| αIVa | liver | poor | n/a | [38] |
| βI | ovarian | not determined | paclitaxel | [191] |
| breast | not determined | docetaxel | [200] | |
| NSCLC adenocarcinomas | poor | paclitaxel and eribulin | [193] | |
| βII | breast | not determined | docetaxel | [196,201] |
| lung adenocarcinoma cell line | not determined | paclitaxel | [191] | |
| βIIa | urothelial | poor | n/a | [38] |
| renal | good | n/a | [38] | |
| βIIb | endometrial | poor | n/a | [38] |
| βIII | prostate | poor | docetaxel | [202,203] |
| colon | poor | paclitaxel | [204] | |
| bladder, cisplatin resistant | poor after paclitaxel chemotherapy | n/a | [205] | |
| gastric | poor | n/a | [206] | |
| gastric metastatic | poor after taxane chemotherapy | n/a | [207] | |
| uterine serous carcinoma | poor | paclitaxel, sensitivity to epothilone | [208] | |
| lung carcinoma cell line | n/a | epothilone | [209] | |
| NSCLC | poor | vinorelbine | [210] | |
| NSCLC stage III/IV | poor | vinorelbine | [211] | |
| NSCLC stage I/II | good after cisplatin/vinorelbine adjuvant chemotherapy | n/a | [212] | |
| ovarian | poor | n/a | [213] | |
| ovarian clear cell carcinoma | good after taxane based chemotherapy | [214] | ||
| breast | poor | n/a | [215] | |
| breast | not determined | sensitivity to taxanes | [216] * | |
| metastatic breast | invariant | sensitivity to docetaxel treatment | [217] | |
| melanoma | difference not statistically significant | paclitaxel | [218] | |
| βIVa | endometrial | poor | n/a | [38] |
| βIVb | liver | poor | n/a | [38] |
| thyroid, endometrial | good | n/a | [38] | |
| βV | renal, urothelial | poor | n/a | [38] |
| NSCLC | good | good response to paclitaxel and vinorelbine | [219] | |
| breast | not determined | sensitivity to taxanes | [216] * |
5.2. Microtubule PTMs and Cancer
| PMT | Changes | Cancer | Outcome | Reference |
|---|---|---|---|---|
| α-, βIII-, βIV-tubulin tyrosination | elevated level | breast cancer cell lines | paclitaxel resistance | [261] |
| α-tubulin detyrosination | TTL down regulation | breast cancer lines, | increasing metastasis and tumor aggressiveness | [245] |
| non-epithelial tumor of different origin | tumor growth correlates with loss of TTL activity | [262] | ||
| primary neuroblastomas | impaired neuronal differentiation and poor prognosis | [248] | ||
| ∆2 α-tubulin | elevated level | prostate cancer cell lines | n/a | [246] |
| non-small-cell lung cancer | poor outcome, vinorelbine resistance | [211] | ||
| breast cancers | high aggressiveness and poor prognosis | [247] | ||
| ∆2 β IVb-tubulin | hepatic carcinoma (rat) | increased in cancer with respect to healthy liver | [263] | |
| α-tubulin acetylation | HDAC6 knockdown | ovarian, breast epidermoid carcinoma cell lines | mitotic arrest, and cell death | [259] |
| HDAC6 inhibition | nsclc cell lines | marker of better prognosis | [264] | |
| HDAC6 overexpression | breast cancer | good prognosis | [265] | |
| oral squamous cell carcinoma | correlates with tumor stage | [258] | ||
| MEC-17 overexpression | lung cancer animal model | cancer cells migration and facilitated invasiveness | [256] | |
| MEC-17 downregulation | higher tumor grade | [256] | ||
| elevated level of tubulin acetylation | head and neck cancer | correlates with tumor grade | [253] | |
| primary breast tumors | correlates with metastatic phenotype | [255] | ||
| breast cancer cell lines | colchicine-resistance | [254] | ||
| ovarian and breast cancer cell lines | paclitaxel sensitivity | [257] | ||
| Glutamylation | elevated levels | prostate cancer cells | n/a | [246] |
| breast cancer cell lines | colchicine-resistance | [254] | ||
| Glycylation | TTLL3 downregulation | colorectal cancer | risk factor for carcinoma development | [266] |
| Phosphorylation of α-tubulin (Ser 165) | dephosphorylated (S165D) α-tubulin | breast cancer cell lines | hyperproliferation and increased metastatic potential | [267] |
5.3. Microtubule-Associated Proteins and Cancer
6. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roostalu, J.; Surrey, T. Microtubule Nucleation: Beyond the Template. Nat. Rev. Mol. Cell Biol. 2017, 18, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Control of Microtubule Organization and Dynamics: Two Ends in the Limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Muroyama, A.; Lechler, T. Microtubule Organization, Dynamics and Functions in Differentiated Cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho-Santos, Z.; Azimzadeh, J.; Pereira-Leal, J.B.; Bettencourt-Dias, M. Tracing the Origins of Centrioles, Cilia, and Flagella. J. Cell Biol. 2011, 194, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Sun, X. Regulation of Paclitaxel Activity by Microtubule-Associated Proteins in Cancer Chemotherapy. Cancer Chemother. Pharmacol. 2017, 80, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.F. Structure and Utilization of Tubulin Isotypes. Annu. Rev. Cell Biol. 1988, 4, 687–716. [Google Scholar] [CrossRef]
- Ludueña, R.F. Multiple Forms of Tubulin: Different Gene Products and Covalent Modifications. Int. Rev. Cytol. 1997, 178, 207–275. [Google Scholar] [CrossRef]
- Lu, Q.; Luduena, R.F. In Vitro Analysis of Microtubule Assembly of Isotypically Pure Tubulin Dimers. Intrinsic Differences in the Assembly Properties of Alpha Beta II, Alpha Beta III, and Alpha Beta IV Tubulin Dimers in the Absence of Microtubule-Associated Proteins. J. Biol. Chem. 1994, 269, 2041–2047. [Google Scholar]
- Panda, D.; Miller, H.P.; Banerjee, A.; Ludueña, R.F.; Wilson, L. Microtubule Dynamics in Vitro Are Regulated by the Tubulin Isotype Composition. Proc. Natl. Acad. Sci. USA 1994, 91, 11358–11362. [Google Scholar] [CrossRef] [Green Version]
- Newton, C.N.; Deluca, J.G.; Himes, R.H.; Miller, H.P.; Jordan, M.A.; Wilson, L. Intrinsically Slow Dynamic Instability of HeLa Cell Microtubules in Vitro. J. Biol. Chem. 2002, 277, 42456–42462. [Google Scholar] [CrossRef] [Green Version]
- Verhey, K.J.; Gaertig, J. The Tubulin Code. Cell Cycle 2007, 6, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Wloga, D.; Joachimiak, E.; Fabczak, H. Tubulin Post-Translational Modifications and Microtubule Dynamics. Int. J. Mol. Sci. 2017, 18, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavallaris, M.; Don, S.; Verrills, N.M. Microtubule-Associated Proteins. In The Role of Microtubules in Cell Biology, Neurobiology, and Oncology; Humana Press: New York, NY, USA, 2009; pp. 83–104. [Google Scholar] [CrossRef]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau Stabilizes Microtubules by Binding at the Interface between Tubulin Heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-Binding Agents: A Dynamic Field of Cancer Therapeutics. Nat. Rev. Drug Disc. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.N.; Zheng, L.L.; Wang, D.; Liang, X.X.; Gao, F.; Zhou, X.L. Recent Advances in Microtubule-Stabilizing Agents. Eur. J. Med. Chem. 2018, 143, 806–828. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Structure of the Aβ Tubulin Dimer by Electron Crystallography. Nature 1998, 391, 199–203. [Google Scholar] [CrossRef]
- Löwe, J.; Li, H.; Downing, K.H.; Nogales, E. Refined Structure of Aβ-Tubulin at 3.5 Å Resolution. J. Mol. Biol. 2001, 313, 1045–1057. [Google Scholar] [CrossRef]
- Inclán, Y.F.; Nogales, E. Structural Models for the Self-Assembly and Microtubule Interactions of γ-, δ- and ε-Tubulin. J. Cell Sci. 2001, 114, 413–422. [Google Scholar]
- Keays, D.A.; Tian, G.; Poirier, K.; Huang, G.J.; Siebold, C.; Cleak, J.; Oliver, P.L.; Fray, M.; Harvey, R.J.; Molnár, Z.; et al. Mutations in α-Tubulin Cause Abnormal Neuronal Migration in Mice and Lissencephaly in Humans. Cell 2007, 128, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. Microtubule Dynamics: An Interplay of Biochemistry and Mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Dorléans, A.; Gigant, B.; Ravelli, R.B.G.; Mailliet, P.; Mikol, V.; Knossow, M. Variations in the Colchicine-Binding Domain Provide Insight into the Structural Switch of Tubulin. Proc. Natl. Acad. Sci. USA 2009, 106, 13775–13779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alushin, G.M.; Lander, G.C.; Kellogg, E.H.; Zhang, R.; Baker, D.; Nogales, E. High-Resolution Microtubule Structures Reveal the Structural Transitions in Aβ-Tubulin upon GTP Hydrolysis. Cell 2014, 157, 1117–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, E.A.; Burns, A.; Lalonde, B.A.; Ye, X.; Piedra, F.A.; Huffaker, T.C.; Rice, L.M. A Mutation Uncouples the Tubulin Conformational and GTPase Cycles, Revealing Allosteric Control of Microtubule Dynamics. Elife 2015, 4, e10113. [Google Scholar] [CrossRef] [PubMed]
- Ti, S.C.; Pamula, M.C.; Howes, S.C.; Duellberg, C.; Cade, N.I.; Kleiner, R.E.; Forth, S.; Surrey, T.; Nogales, E.; Kapoor, T.M. Mutations in Human Tubulin Proximal to the Kinesin-Binding Site Alter Dynamic Instability at Microtubule Plus- and Minus-Ends. Dev. Cell 2016, 37, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.L.; Teo, W.S.; Pandzic, E.; Vicente, J.J.; McCarroll, J.A.; Wordeman, L.; Kavallaris, M. β-Tubulin Carboxy-Terminal Tails Exhibit Isotype-Specific Effects on Microtubule Dynamics in Human Gene-Edited Cells. Life Sci. Alliance 2018, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Prassanawar, S.S.; Panda, D. Tubulin Heterogeneity Regulates Functions and Dynamics of Microtubules and Plays a Role in the Development of Drug Resistance in Cancer. Biochem. J. 2019, 476, 1359–1376. [Google Scholar] [CrossRef]
- Ludueña, R.F. Are Tubulin Isotypes Functionally Significant. Mol. Biol. Cell 1993, 4, 445–457. [Google Scholar] [CrossRef]
- Verdier-Pinard, P.; Pasquier, E.; Xiao, H.; Burd, B.; Villard, C.; Lafitte, D.; Miller, L.M.; Angeletti, R.H.; Horwitz, S.B.; Braguer, D. Tubulin Proteomics: Towards Breaking the Code. Anal. Biochem. 2009, 384, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Tubulins | HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org/data/genegroup/#!/group/778 (accessed on 30 July 2020).
- Gadadhar, S.; Bodakuntla, S.; Natarajan, K.; Janke, C. The Tubulin Code at a Glance. J. Cell Sci. 2017, 130, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrukhin, K.E.; Speer, M.C.; Cayanis, E.; Bonaldo, M.F.; Tantravahi, U.; Soares, M.B.; Fischer, S.G.; Warburton, D.; Gilliam, T.C.; Ott, J. A Microsatellite Genetic Linkage Map of Human Chromosome 13. Genomics 1993, 15, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Stanchi, F.; Corso, V.; Scannapieco, P.; Ievolella, C.; Negrisolo, E.; Tiso, N.; Lanfranchi, G.; Valle, G. TUBA8: A New Tissue-Specific Isoform of Alpha-Tubulin That Is Highly Conserved in Human and Mouse. Biochem. Biophys. Res. Commun. 2000, 270, 1111–1118. [Google Scholar] [CrossRef]
- Braun, A.; Breuss, M.; Salzer, M.C.; Flint, J.; Cowan, N.J.; Keays, D.A. Tuba8 Is Expressed at Low Levels in the Developing Mouse and Human Brain. Am. J. Hum. Genet. 2010, 86, 819–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, K.; Keays, D.A.; Francis, F.; Saillour, Y.; Bahi, N.; Manouvrier, S.; Fallet-Bianco, C.; Pasquier, L.; Toutain, A.; Tuy, F.P.D.; et al. Large Spectrum of Lissencephaly and Pachygyria Phenotypes Resulting from de Novo Missense Mutations in Tubulin Alpha 1A (TUBA1A). Hum. Mutat. 2007, 28, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A Subcellular Map of the Human Proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Belvindrah, R.; Natarajan, K.; Shabajee, P.; Bruel-Jungerman, E.; Bernard, J.; Goutierre, M.; Moutkine, I.; Jaglin, X.H.; Savariradjane, M.; Irinopoulou, T.; et al. Mutation of the α-Tubulin Tuba1a Leads to Straighter Microtubules and Perturbs Neuronal Migration. J. Cell Biol. 2017, 216, 2443–2461. [Google Scholar] [CrossRef]
- Strassel, C.; Magiera, M.M.; Dupuis, A.; Batzenschlager, M.; Hovasse, A.; Pleines, I.; Guéguen, P.; Eckly, A.; Moog, S.; Mallo, L.; et al. An Essential Role for A4A-Tubulin in Platelet Biogenesis. Life Sci. Alliance 2019, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Diggle, C.P.; Martinez-Garay, I.; Molnar, Z.; Brinkworth, M.H.; White, E.; Fowler, E.; Hughes, R.; Hayward, B.E.; Carr, I.M.; Watson, C.M.; et al. A tubulin Alpha 8 Mouse Knockout Model Indicates a Likely Role in Spermatogenesis but Not in Brain Development. PLoS ONE 2017, 12, e0174264. [Google Scholar] [CrossRef] [Green Version]
- Leandro-García, L.J.; Leskelä, S.; Landa, I.; Montero-Conde, C.; López-Jiménez, E.; Letón, R.; Cascón, A.; Robledo, M.; Rodríguez-Antona, C. Tumoral and Tissue-Specific Expression of the Major Human β-Tubulin Isotypes. Cytoskeleton 2010, 67, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Walss-Bass, C.; Ludueña, R.F. The Beta Isotypes of Tubulin in Neuronal Differentiation. Cytoskeleton 2010, 67, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.; Yan, Z.; Li, B.; Yu, M.; Sang, Q.; Tian, G.; Xu, Y.; Chen, B.; Qu, R.; Sun, Z.; et al. Mutations in TUBB8 Cause a Multiplicity of Phenotypes in Human Oocytes and Early Embryos. J. Med. Genet. 2016, 53, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, K.; Shibutani, M.; Matsuda, H. Distribution of the Class II β-Tubulin in Developmental and Adult Rat Tissues. Cell Motil. Cytoskelet. 2002, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.H.; Hiser, L.; Davis, J.A.; Thomas, N.S.; Tucci, M.A.; Benghuzzi, H.A.; Frankfurter, A.; Correia, J.J.; Lobert, S. Beta Class II Tubulin Predominates in Normal and Tumor Breast Tissues. Breast Cancer Res. 2003, 5, R157–R169. [Google Scholar] [CrossRef] [Green Version]
- Latremoliere, A.; Cheng, L.; DeLisle, M.; Wu, C.; Chew, S.; Hutchinson, E.B.; Sheridan, A.; Alexandre, C.; Latremoliere, F.; Sheu, S.H.; et al. Neuronal-Specific TUBB3 Is Not Required for Normal Neuronal Function but Is Essential for Timely Axon Regeneration. Cell Rep. 2018, 24, 1865–1879. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.; Bey, G.R.; Takanohashi, A.; Bugiani, M.; Fu, X.; Wolf, N.I.; Nmezi, B.; Schiffmann, R.; Bugaighis, M.; Pierson, T.; et al. TUBB4A Mutations Result in Specific Neuronal and Oligodendrocytic Defects That Closely Match Clinically Distinct Phenotypes. Hum. Mol. Genet. 2017, 26, 4506–4518. [Google Scholar] [CrossRef] [Green Version]
- Burley, K.; Westbury, S.K.; Mumford, A.D. TUBB1 Variants and Human Platelet Traits. Platelets 2018, 29, 209–211. [Google Scholar] [CrossRef] [Green Version]
- TUBA1A tubulin alpha 1a [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/7846 (accessed on 2 August 2020).
- TUBA1B tubulin alpha 1b [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/10376 (accessed on 30 July 2020).
- TUBA1C tubulin alpha 1c [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/84790 (accessed on 2 August 2020).
- TUBA3C tubulin alpha 3c [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/7278 (accessed on 2 August 2020).
- TUBA3E tubulin alpha 3e [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/112714 (accessed on 2 August 2020).
- TUBA4A tubulin alpha 4a [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/7277 (accessed on 2 August 2020).
- TUBA8 tubulin alpha 8 [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/51807 (accessed on 2 August 2020).
- TUBB tubulin beta class I [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/203068 (accessed on 2 August 2020).
- TUBB2A tubulin beta 2A class IIa [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/7280 (accessed on 2 August 2020).
- TUBB2B tubulin beta 2B class IIb [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/347733 (accessed on 2 August 2020).
- TUBB3 tubulin beta 3 class III [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/10381 (accessed on 2 August 2020).
- TUBB4A tubulin beta 4A class IVa [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/10382 (accessed on 2 August 2020).
- TUBB4B tubulin beta 4B class IVb [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/10383 (accessed on 2 August 2020).
- Randazzo, D.; Khalique, U.; Belanto, J.J.; Kenea, A.; Talsness, D.M.; Olthoff, J.T.; Tran, M.D.; Zaal, K.J.; Pak, K.; Pinal-Fernandez, I.; et al. Persistent Upregulation of the β-Tubulin Tubb6, Linked to Muscle Regeneration, Is a Source of Microtubule Disorganization in Dystrophic Muscle. Hum. Mol. Genet. 2018, 28, 1117–1135. [Google Scholar] [CrossRef] [Green Version]
- TUBB6 tubulin beta 6 class V [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/84617 (accessed on 2 August 2020).
- TUBB1 tubulin beta 1 class VI [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/81027 (accessed on 2 August 2020).
- TUBB8 tubulin beta 8 class VIII [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/347688 (accessed on 2 August 2020).
- Ti, S.C.; Alushin, G.M.; Kapoor, T.M. Human β-Tubulin Isotypes Can Regulate Microtubule Protofilament Number and Stability. Dev. Cell 2018, 47, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Ludueña, R.F.; Zimmermann, H.P.; Little, M. Identification of the Phosphorylated β-Tubulin Isotype in Differentiated Neuroblastoma Cells. FEBS Lett. 1988, 230, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Nido, J.; Serrano, L.; López-Otín, C.; Vandekerckhove, J.; Avila, J. Phosphorylation of a Neuronal-Specific Beta-Tubulin Isotype. J. Biol. Chem. 1990, 265, 13949–13954. [Google Scholar] [PubMed]
- Pamula, M.C.; Ti, S.C.; Kapoor, T.M. The Structured Core of Human β Tubulin Confers Isotype-Specific Polymerization Properties. J. Cell Biol. 2016, 213, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Vemu, A.; Atherton, J.; Spector, J.O.; Moores, C.A.; Roll-Mecak, A. Tubulin Isoform Composition Tunes Microtubule Dynamics. Mol. Biol. Cell 2017, 28, 3564–3572. [Google Scholar] [CrossRef] [Green Version]
- Janke, C.; Magiera, M.M. The Tubulin Code and Its Role in Controlling Microtubule Properties and Functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Roll-Mecak, A. How Cells Exploit Tubulin Diversity to Build Functional Cellular Microtubule Mosaics. Curr. Opin. Cell Biol. 2019, 56, 102–108. [Google Scholar] [CrossRef]
- Chu, C.-W.; Hou, F.; Zhang, J.; Phu, L.; Loktev, A.V.; Kirkpatrick, D.S.; Jackson, P.K.; Zhao, Y.; Zou, H. A Novel Acetylation of β-Tubulin by San Modulates Microtubule Polymerization via down-Regulating Tubulin Incorporation. Mol. Biol. Cell 2011, 22, 448–456. [Google Scholar] [CrossRef]
- Ori-McKenney, K.M.; McKenney, R.J.; Huang, H.H.; Li, T.; Meltzer, S.; Jan, L.Y.; Vale, R.D.; Wiita, A.P.; Jan, Y.N. Phosphorylation of β-Tubulin by the Down Syndrome Kinase, Minibrain/DYRK1a, Regulates Microtubule Dynamics and Dendrite Morphogenesis. Neuron 2016, 90, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Fourest-Lieuvin, A.; Peris, L.; Gache, V.; Garcia-Saez, I.; Juillan-Binard, C.; Lantez, V.; Job, D. Microtubule Regulation in Mitosis: Tubulin Phosphorylation by the Cyclin-Dependent Kinase Cdk1. Mol. Biol. Cell 2006, 17, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Abeyweera, T.P.; Chen, X.; Rotenberg, S.A. Phosphorylation of A6-Tubulin by Protein Kinase Cα Activates Motility of Human Breast Cells. J. Biol. Chem. 2009, 284, 17648–17656. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Tsimounis, A.; Chen, X.; Rotenberg, S.A. Phosphorylation of α-Tubulin by Protein Kinase C Stimulates Microtubule Dynamics in Human Breast Cells. Cytoskeleton 2014, 71, 257–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Xiong, Y.; Ren, Y.; Zhang, L.; He, X.; Wang, X.; Liu, M.; Li, D.; Shui, W.; Zhou, J. Proteomic Profiling and Functional Characterization of Multiple Post-Translational Modifications of Tubulin. J. Proteome Res. 2015, 14, 3292–3304. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Kang, J.G.; Park, S.Y.; Lee, J.; Oh, Y.J.; Cho, J.W. O-GlcNAcylation of Tubulin Inhibits Its Polymerization. Amino Acids 2011, 40, 809–818. [Google Scholar] [CrossRef]
- Janke, C.; Bulinski, J.C. Post-Translational Regulation of the Microtubule Cytoskeleton: Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef]
- Van der Laan, S.; Lévêque, M.F.; Marcellin, G.; Vezenkov, L.; Lannay, Y.; Dubra, G.; Bompard, G.; Ovejero, S.; Urbach, S.; Burgess, A.; et al. Evolutionary Divergence of Enzymatic Mechanisms for Tubulin Detyrosination. Cell Rep. 2019, 29, 4159–4171. [Google Scholar] [CrossRef] [Green Version]
- Paturle-Lafanechère, L.; Job, D.; Eddé, B.; Denoulet, P.; Van Dorsselaer, A.; Mazarguil, H.; Le Caer, J.P.; Wehland, J. Characterization of a Major Brain Tubulin Variant Which Cannot Be Tyrosinated. Biochemistry 1991, 30, 10523–10528. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, K.; van Dijk, J.; Magiera, M.M.; Bosc, C.; Deloulme, J.C.; Bosson, A.; Peris, L.; Gold, N.D.; Lacroix, B.; Grau, M.B.; et al. A Family of Protein-Deglutamylating Enzymes Associated with Neurodegeneration. Cell 2010, 143, 564–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezniuk, I.; Vu, H.T.; Lyons, P.J.; Sironi, J.J.; Xiao, H.; Burd, B.; Setou, M.; Angeletti, R.H.; Ikegami, K.; Fricker, L.D. Cytosolic Carboxypeptidase 1 Is Involved in Processing α- and β-Tubulin. J. Biol. Chem. 2012, 287, 6503–6517. [Google Scholar] [CrossRef] [Green Version]
- Berezniuk, I.; Lyons, P.J.; Sironi, J.J.; Xiao, H.; Setou, M.; Angeletti, R.H.; Ikegami, K.; Fricker, L.D. Cytosolic Carboxypeptidase 5 Removes α- And γ-Linked Glutamates from Tubulin. J. Biol. Chem. 2013, 288, 30445–30453. [Google Scholar] [CrossRef] [Green Version]
- Ersfeld, K.; Wehland, J.; Plessmann, U.; Dodemont, H.; Gerke, V.; Weber, K. Characterization of the Tubulin-Tyrosine Ligase. J. Cell Biol. 1993, 120, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Prota, A.E.; Magiera, M.M.; Kuijpers, M.; Bargsten, K.; Frey, D.; Wieser, M.; Jaussi, R.; Hoogenraad, C.C.; Kammerer, R.A.; Janke, C.; et al. Structural Basis of Tubulin Tyrosination by Tubulin Tyrosine Ligase. J. Cell Biol. 2013, 200, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeDizet, M.; Piperno, G. Cytoplasmic Microtubules Containing Acetylated α-Tubulin in Chlamydomonas Reinhardtii: Spatial Arrangement and Properties. J. Cell Biol. 1986, 103, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Akella, J.S.; Wloga, D.; Kim, J.; Starostina, N.G.; Lyons-Abbott, S.; Morrissette, N.S.; Dougan, S.T.; Kipreos, E.T.; Gaertig, J. MEC-17 Is an α-Tubulin Acetyltransferase. Nature 2010, 467, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Wloga, D.; Joachimiak, E.; Louka, P.; Gaertig, J. Posttranslational Modifications of Tubulin and Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The Major Alpha-Tubulin K40 Acetyltransferase AlphaTAT1 Promotes Rapid Ciliogenesis and Efficient Mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.F.; Yao, T.P. HDAC6 Is a Microtubule-Associated Deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The Human Sir2 Ortholog, SIRT2, Is an NAD+-Dependent Tubulin Deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-Tubulin Acetylation on Microtubule Structure and Stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [Green Version]
- Portran, D.; Schaedel, L.; Xu, Z.; Théry, M.; Nachury, M.V. Tubulin Acetylation Protects Long-Lived Microtubules against Mechanical Ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Schaedel, L.; Portran, D.; Aguilar, A.; Gaillard, J.; Peter Marinkovich, M.; Théry, M.; Nachury, M.V. Microtubules Acquire Resistance from Mechanical Breakage through Intralumenal Acetylation. Science 2017, 356, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Kirkpatrick, L.L.; Schilling, A.B.; Helseth, D.L.; Chabot, N.; Keillor, J.W.; Johnson, G.V.W.; Brady, S.T. Transglutaminase and Polyamination of Tubulin: Posttranslational Modification for Stabilizing Axonal Microtubules. Neuron 2013, 78, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Manka, S.W.; Moores, C.A. Microtubule Structure by Cryo-EM: Snapshots of Dynamic Instability. Essays Biochem. 2018, 62, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodakuntla, S.; Jijumon, A.S.; Villablanca, C.; Gonzalez-Billault, C.; Janke, C. Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Biol. 2019, 29, 804–819. [Google Scholar] [CrossRef] [PubMed]
- McNally, F.J.; Roll-Mecak, A. Microtubule-Severing Enzymes: From Cellular Functions to Molecular Mechanism. J. Cell Biol. 2018, 217, 4057–4069. [Google Scholar] [CrossRef]
- Moore, A.; Wordeman, L. The Mechanism, Function and Regulation of Depolymerizing Kinesins during Mitosis. Trends Cell Biol. 2004, 14, 537–546. [Google Scholar] [CrossRef]
- Moore, A.T.; Rankin, K.E.; Von Dassow, G.; Peris, L.; Wagenbach, M.; Ovechkina, Y.; Andrieux, A.; Job, D.; Wordeman, L. MCAK Associates with the Tips of Polymerizing Microtubules. J. Cell Biol. 2005, 169, 391–397. [Google Scholar] [CrossRef]
- Reid, T.A.; Schuster, B.M.; Mann, B.J.; Keshavan Balchand, S.; Plooster, M.; Mcclellan, M.; Coombes, C.E.; Wadsworth, P.; Gardner, M.K. Suppression of Microtubule Assembly Kinetics by the Mitotic Protein TPX2. J. Cell Sci. 2016, 129, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Steinmetz, M.O. Microtubule +TIPs at a Glance. J. Cell Sci. 2010, 123, 3415–3419. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Steinmetz, M.O. Microtubule Minus-End Regulation at a Glance. J. Cell Sci. 2019, 132, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cassimeris, L. The Oncoprotein 18/Stathmin Family of Microtubule Destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef]
- Steinmetz, M.O. Structure and Thermodynamics of the Tubulin-Stathmin Interaction. J. Struct. Biol. 2007, 158, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Li, C.; Duan, A.; Alberico, E.O.; Kim, O.V.; Alber, M.S.; Goodson, H.V. Mechanism for the Catastrophe-Promoting Activity of the Microtubule Destabilizer Op18/Stathmin. Proc. Natl. Acad. Sci. USA 2013, 110, 20449–20454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendershott, M.C.; Vale, R.D. Regulation of Microtubule Minus-End Dynamics by CAMSAPs and Patronin. Proc. Natl. Acad. Sci. USA 2014, 111, 5860–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuveillier, C.; Delaroche, J.; Seggio, M.; Gory-Fauré, S.; Bosc, C.; Denarier, E.; Bacia, M.; Schoehn, G.; Mohrbach, H.; Kulić, I.; et al. MAP6 Is an Intraluminal Protein That Induces Neuronal Microtubules to Coil. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Cassimeris, L.; Spittle, C. Regulation of Microtubule-Associated Proteins. Int. Rev. Cytol. 2001, 210, 163–226. [Google Scholar] [CrossRef]
- Bieling, P.; Kandels-Lewis, S.; Telley, I.A.; Van Dijk, J.; Janke, C.; Surrey, T. CLIP-170 Tracks Growing Microtubule Ends by Dynamically Recognizing Composite EB1/Tubulinbinding Sites. J. Cell Biol. 2008, 183, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Peris, L.; Thery, M.; Fauré, J.; Saoudi, Y.; Lafanechère, L.; Chilton, J.K.; Gordon-Weeks, P.; Galjart, N.; Bornens, M.; Wordeman, L.; et al. Tubulin Tyrosination Is a Major Factor Affecting the Recruitment of CAP-Gly Proteins at Microtubule plus Ends. J. Cell Biol. 2006, 174, 839–849. [Google Scholar] [CrossRef]
- Peris, L.; Wagenbach, M.; Lafanechère, L.; Brocard, J.; Moore, A.T.; Kozielski, F.; Job, D.; Wordeman, L.; Andrieux, A. Motor-Dependent Microtubule Disassembly Driven by Tubulin Tyrosination. J. Cell Biol. 2009, 185, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, C.; Boucher, D.; Lazereg, S.; Pedrotti, B.; Islam, K.; Denoulet, P.; Larcher, J.C. Differential Binding Regulation of Microtubule-Associated Proteins MAP1A, MAP1B, and MAP2 by Tubulin Polyglutamylation. J. Biol. Chem. 2001, 276, 12839–12848. [Google Scholar] [CrossRef] [Green Version]
- Boucher, D.; Larcher, J.C.; Gros, F.; Denoulet, P. Polyglutamylation of Tubulin as a Progressive Regulator of in Vitro Interactions between the Microtubule-Associated Protein Tau and Tubulin. Biochemistry 1994, 33, 12471–12477. [Google Scholar] [CrossRef] [PubMed]
- Valenstein, M.L.; Roll-Mecak, A. Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell 2016, 164, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Haider, M.R.; Shafi, S.; Yar, M.S. Anti-Tubulin Agents of Natural Origin: Targeting Taxol, Vinca, and Colchicine Binding Domains. Eur. J. Med. Chem. 2019, 171, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; Mcphail, A.T. Plant Antitumor Agents.VI.The Isolation and Structure of Taxol, a Novel Antileukemic and Antitumor Agent from Taxus Brevifolia2. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A Novel Investigational Antimicrotubule Agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs That Target Dynamic Microtubules: A New Molecular Perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Bollag, D.M.; McQueney, P.A.; Zhu, J.; Hensens, O.; Koupal, L.; Liesch, J.; Goetz, M.; Lazarides, E.; Woods, C.M. Epothilones, a New Class of Microtubule-Stabilizing Agents with a Taxol-like Mechanism of Action. Cancer Res. 1995, 55, 2325–2333. [Google Scholar]
- Rugo, H.S.; Barry, W.T.; Moreno-Aspitia, A.; Lyss, A.P.; Cirrincione, C.; Leung, E.; Mayer, E.L.; Naughton, M.; Toppmeyer, D.; Carey, L.A.; et al. Randomized Phase III Trial of Paclitaxel Once per Week Compared with Nanoparticle Albumin-Bound Nab-Paclitaxel Once per Week or Ixabepilone with Bevacizumab as First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol. 2015, 33, 2361–2369. [Google Scholar] [CrossRef]
- McMeekin, S.; Dizon, D.; Barter, J.; Scambia, G.; Manzyuk, L.; Lisyanskaya, A.; Oaknin, A.; Ringuette, S.; Mukhopadhyay, P.; Rosenberg, J.; et al. Phase III Randomized Trial of Second-Line Ixabepilone versus Paclitaxel or Doxorubicin in Women with Advanced Endometrial Cancer. Gynecol. Oncol. 2015, 138, 18–23. [Google Scholar] [CrossRef]
- Field, J.J.; Northcote, P.T.; Paterson, I.; Altmann, K.-H.; Díaz, J.F.; Miller, J.H. Zampanolide, a Microtubule-Stabilizing Agent, Is Active in Resistant Cancer Cells and Inhibits Cell Migration. Int. J. Mol. Sci. 2017, 18, 971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Li, G.-B.; Li, S.-A.; Wu, C.; Gigant, B.; Qin, W.; Chen, H.; Wu, Y.; Chen, Q.; et al. Mechanism of Microtubule Stabilization by Taccalonolide AJ. Nat. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Choy, H. Taxanes in Combined Modality Therapy for Solid Tumors. Crit. Rev. Oncol. Hematol. 2001, 37, 237–247. [Google Scholar] [CrossRef]
- Engels, F.K.; Sparreboom, A.; Mathot, R.A.A.; Verweij, J. Potential for Improvement of Docetaxel-Based Chemotherapy: A Pharmacological Review. Br. J. Cancer 2005, 93, 173–177. [Google Scholar] [CrossRef]
- Larkin, J.M.G.; Kaye, S.B. Potential Clinical Applications of Epothilones: A Review of Phase II Studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A Novel Second-Line Treatment for Metastatic Castration-Resistant Prostate Cancer. Drug Des. Devel. Ther. 2011, 5, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diéras, V.; Limentani, S.; Romieu, G.; Tubiana-Hulin, M.; Lortholary, A.; Kaufman, P.; Girre, V.; Besenval, M.; Valero, V. Phase II Multicenter Study of Larotaxel (XRP9881), a Novel Taxoid, in Patients with Metastatic Breast Cancer Who Previously Received Taxane-Based Therapy. Ann. Oncol. 2008, 19, 1255–1260. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Skoneczna, I.A.; Castellano, D.; Theodore, C.; Blais, N.; Voog, E.; Bellmunt, J.; Peters, F.; Le-Guennec, S.; Cerbone, L.; et al. Larotaxel with Cisplatin in the First-Line Treatment of Locally Advanced/Metastatic Urothelial Tract or Bladder Cancer: A Randomized, Active-Controlled, Phase III Trial (CILAB). Oncology 2013, 85, 208–215. [Google Scholar] [CrossRef]
- Kellogg, E.H.; Hejab, N.M.A.; Howes, S.; Northcote, P.; Miller, J.H.; Díaz, J.F.; Downing, K.H.; Nogales, E. Insights into the Distinct Mechanisms of Action of Taxane and Non-Taxane Microtubule Stabilizers from Cryo-EM Structures. J. Mol. Biol. 2017, 429, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooberry, S.L.; Tien, G.; Hernandez, A.H.; Plubrukarn, A.; Davidson, B.S. Laulimalide and Isolaulimalide, New Paclitaxel-like Microtubule- Stabilizing Agents. Cancer Res. 1999, 59, 653–660. [Google Scholar] [PubMed]
- West, L.M.; Northcote, P.T.; Battershill, C.N. Peloruside A: A Potent Cytotoxic Macrolide Isolated from the New Zealand Marine Sponge Mycale Sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.A.; West, L.M.; Rouwé, B.; Northcote, P.T.; Berridge, M.V.; Wakefield, S.J.; Miller, J.H. Peloruside A, a Novel Antimitotic Agent with Paclitaxel-like Microtubule-Stabilizing Activity. Cancer Res. 2002, 62, 3356–3360. [Google Scholar]
- Prota, A.E.; Bargsten, K.; Northcote, P.T.; Marsh, M.; Altmann, K.-H.; Miller, J.H.; Díaz, J.F.; Steinmetz, M.O. Structural Basis of Microtubule Stabilization by Laulimalide and Peloruside A. Angew. Chem. Int. Ed. Engl. 2014, 53, 1621–1625. [Google Scholar] [CrossRef]
- Ganguly, A.; Cabral, F.; Yang, H.; Patel, K.D. Peloruside A Is a Microtubule-Stabilizing Agent with Exceptional Anti-Migratory Properties in Human Endothelial Cells. Oncoscience 2015, 2, 585–595. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Rowe, M.R.; Field, J.J.; Northcote, P.T.; Teesdale-Spittle, P.H.; Miller, J.H. ΒI-Tubulin Mutations in the Laulimalide/Peloruside Binding Site Mediate Drug Sensitivity by Altering Drug-Tubulin Interactions and Microtubule Stability. Cancer Lett. 2015, 365, 251–260. [Google Scholar] [CrossRef]
- Castro-Alvarez, A.; Pineda, O.; Vilarrasa, J. Further Insight into the Interactions of the Cytotoxic Macrolides Laulimalide and Peloruside A with Their Common Binding Site. ACS Omega 2018, 3, 1770–1782. [Google Scholar] [CrossRef]
- Johnson, I.S.; Wright, H.F.; Svoboda, G.H. Experimental Basis for Clinical Evaluation of Anti Tumor Principles Derived from Vinca-Rosea Linn. In Journal of Laboratory and Clinical Medicine; Mosby-Year Book Inc.: St Louis, MO, USA, 1959; Volume 54, p. 830. [Google Scholar]
- Noble, R.L.; Beer, C.T.; Cutts, J.H. Further Biological Activities of Vincaleukoblastine-an Alkaloid Isolated from Vinca Rosea (L.). Biochem. Pharmacol. 1959, 1, 347–348. [Google Scholar] [CrossRef]
- Liaw, T.; Chang, M.; Kavallaris, M. The Cytoskeleton as a Therapeutic Target in Childhood Acute Leukemia:Obstacles and Opportunities. Curr. Drug Targets 2007, 8, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca Alkaloids and Analogues as Anti-Cancer Agents: Looking Back, Peering Ahead. Bioorg. Med Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, C.; Bourien, H.; Augereau, P.; Patsouris, A.; Frenel, J.-S.; Campone, M. Vinflunine for the Treatment of Breast Cancer. Expert Opin. Pharmacother. 2016, 17, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Gidding, C.E.M.; Kellie, S.J.; Kamps, W.A.; De Graaf, S.S.N. Vincristine Revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef]
- Zhou, H.; Li, L.; Zhou, Y.; Han, Y. Syndrome of Inappropriate Antidiuretic Hormone Secretion from Concomitant Use of Itraconazole and Vindesine. J. Clin. Pharm. Ther. 2018, 43, 137–140. [Google Scholar] [CrossRef]
- Kitagaki, J.; Shi, G.; Miyauchi, S.; Murakami, S.; Yang, Y. Cyclic Depsipeptides as Potential Cancer Therapeutics. Anti-Cancer Drugs 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinoz, M.A.; Ozpinar, A.; Alturfan, E.E.; Elmaci, I. Vinorelbine’s Anti-Tumor Actions May Depend on the Mitotic Apoptosis, Autophagy and Inflammation: Hypotheses with Implications for Chemo-Immunotherapy of Advanced Cancers and Pediatric Gliomas. J. Chemother. 2018, 30, 203–212. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III Trial of Vinflunine plus Best Supportive Care Compared with Best Supportive Care Alone after a Platinum-Containing Regimen in Patients with Advanced Transitional Cell Carcinoma of the Urothelial Tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef]
- Retz, M.; de Geeter, P.; Goebell, P.J.; Matz, U.; de Schultz, W.; Hegele, A. Vinflunine in Routine Clinical Practice for the Treatment of Advanced or Metastatic Urothelial Cell Carcinoma—Data from a Prospective, Multicenter Experience. BMC Cancer 2015, 15, 455. [Google Scholar] [CrossRef] [Green Version]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The Folate Receptor as a Rational Therapeutic Target for Personalized Cancer Treatment. Drug Resist. Updates 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Twelves, C.; Cortes, J.; Vahdat, L.; Wanders, J.; Akerele, C.; Kaufman, P. Phase III Trials of Eribulin Mesylate (E7389) in Extensively Pretreated Patients with Locally Recurrent or Metastatic Breast Cancer. Clin. Breast Cancer 2010, 10, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, R.; Skvortsova, I.; Kumar, V. Mechanisms of Tubulin Binding Ligands to Target Cancer Cells: Updates on Their Therapeutic Potential and Clinical Trials. Curr. Cancer Drug Targets 2016, 17, 357–375. [Google Scholar] [CrossRef]
- Akaiwa, M.; Martin, T.; Mendelsohn, B.A. Synthesis and Evaluation of Linear and Macrocyclic Dolastatin 10 Analogues Containing Pyrrolidine Ring Modifications. ACS Omega 2018, 3, 5212–5221. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, C.; Ravelli, R.B.G.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural Basis for the Regulation of Tubulin by Vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Doodhi, H.; Prota, A.E.; Rodríguez-García, R.; Xiao, H.; Custar, D.W.; Bargsten, K.; Katrukha, E.A.; Hilbert, M.; Hua, S.; Jiang, K.; et al. Termination of Protofilament Elongation by Eribulin Induces Lattice Defects That Promote Microtubule Catastrophes. Curr. Biol. 2016, 26, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alday, P.H.; Correia, J.J. Macromolecular Interaction of Halichondrin B Analogues Eribulin (E7389) and ER-076349 with Tubulin by Analytical Ultracentrifugation. Biochemistry 2009, 48, 7927–7938. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Komoda, Y.; Court, W.A.; Thomas, G.J.; Smith, R.M.; Karim, A.; Gilmore, C.J.; Haltiwanger, R.C.; Bryan, R.F. Maytansine, a Novel Antileukemic Ansa Macrolide from Maytenus Ovatus. J. Am. Chem. Soc. 1972, 94, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Elez, E.; Gomez-Roca, C.; Soto Matos-Pita, A.; Argiles, G.; Valentin, T.; Coronado, C.; Iglesias, J.; Macarulla, T.; Betrian, S.; Fudio, S.; et al. First-in-Human Phase I Study of the Microtubule Inhibitor Plocabulin in Patients with Advanced Solid Tumors. Invest. New Drugs 2019, 37, 674–683. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Ado-Trastuzumab Emtansine For Early Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ado-trastuzumab-emtansine-early-breast-cancer (accessed on 2 August 2020).
- Ozyukseler, D.T.; Basak, M.; Ay, S.; Koseoglu, A.; Arıcı, S.; Oyman, A.; Sürmeli, H.; Turan, M.; Turan, N.; Odabaş, H.; et al. Prognostic Factors of Ado-Trastuzumab Emtansine Treatment in Patients with Metastatic HER-2 Positive Breast Cancer. J. Oncol. Pharm. Pract. 2020. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Diaz, J.F.; Marsh, M.; Cuevas, C.; Liniger, M.; Neuhaus, C.; Andreu, J.M.; Altmann, K.H.; Steinmetz, M.O. A New Tubulin-Binding Site and Pharmacophore for Microtubule-Destabilizing Anticancer Drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 13817–13821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, A.R.; Seetharamalu, P.; Schwarz, P.M.; Hausheer, F.H.; Ludueña, R.F. The Interaction of the B-Ring of Colchicine with Alpha-Tubulin: A Novel Footprinting Approach. J. Mol. Biol. 2000, 303, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Hastie, S.B. Interactions of Colchicine with Tubulin. Pharmacol. Ther. 1991, 51, 377–401. [Google Scholar] [CrossRef]
- Kapoor, S.; Srivastava, S.; Panda, D. Indibulin Dampens Microtubule Dynamics and Produces Synergistic Antiproliferative Effect with Vinblastine in MCF-7 Cells: Implications in Cancer Chemotherapy. Sci. Rep. 2018, 8, 2–13. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Patterson, J.C.; Joughin, B.A.; Prota, A.E.; Mühlethaler, T.; Jonas, O.H.; Whitman, M.A.; Varmeh, S.; Chen, S.; Balk, S.P.; Steinmetz, M.O.; et al. VISAGE Reveals a Targetable Mitotic Spindle Vulnerability in Cancer Cells. Cell Syst. 2019, 9, 74–92. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Kawatani, M.; Muroi, M.; Kondoh, Y.; Futamura, Y.; Aono, H.; Tanaka, M.; Honda, K.; Osada, H. Proteomic Profiling of Small-Molecule Inhibitors Reveals Dispensability of MTH1 for Cancer Cell Survival. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Gul, N.; Karlsson, J.; Tängemo, C.; Linsefors, S.; Tuyizere, S.; Perkins, R.; Ala, C.; Zou, Z.; Larsson, E.; Bergö, M.O.; et al. The MTH1 Inhibitor TH588 Is a Microtubule-Modulating Agent That Eliminates Cancer Cells by Activating the Mitotic Surveillance Pathway. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Gad, H.; Koolmeister, T.; Jemth, A.S.; Eshtad, S.; Jacques, S.A.; Ström, C.E.; Svensson, L.M.; Schultz, N.; Lundbäck, T.; Einarsdottir, B.O.; et al. MTH1 Inhibition Eradicates Cancer by Preventing Sanitation of the DNTP Pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef]
- Samaranayake, G.J.; Huynh, M.; Rai, P. MTH1 as a Chemotherapeutic Target: The Elephant in the Room. Cancers 2017, 9, 47. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into Tubulin Regulation from a Complex with Colchicine and a Stathmin-like Domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Coluccia, A.; Silvestri, R.; Sorba, G.; Brancale, A. The Tubulin Colchicine Domain: A Molecular Modeling Perspective. ChemMedChem 2012, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Tsuchiya, K.; Harada, T.; Nishide, M.; Kurokawa, T.; Nakagawa, T.; Shimada, N.; Kobayashi, K. Pironetin, a Novel Plant Growth Regulator Produced by Streptomyces Sp. NK10958. I. Taxonomy, Production, Isolation and Preliminary Characterization. J. Antibiot. 1994, 47, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Tsuchiya, K.; Kurokawa, T.; Nakagawa, T.; Shimada, N.; Iitaka, Y. Pironetin, a Novel Plant Growth Regulator Produced by Streptomyces Sp. Nk10958. II. Structural Elucidation. J. Antibiot. 1994, 47, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Prota, A.E.; Setter, J.; Waight, A.B.; Bargsten, K.; Murga, J.; Diaz, J.F.; Steinmetz, M.O. Pironetin Binds Covalently to ACys316 and Perturbs a Major Loop and Helix of α-Tubulin to Inhibit Microtubule Formation. J. Mol. Biol. 2016, 428, 2981–2988. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, Y.; Wang, T.; Jiang, J.; Botting, C.H.; Liu, H.; Chen, Q.; Yang, J.; Naismith, J.H.; Zhu, X.; et al. Pironetin Reacts Covalently with Cysteine-316 of α-Tubulin to Destabilize Microtubule. Nat. Commun. 2016. [Google Scholar] [CrossRef]
- Coulup, S.K.; Georg, G.I. Revisiting Microtubule Targeting Agents: α-Tubulin and the Pironetin Binding Site as Unexplored Targets for Cancer Therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865–1873. [Google Scholar] [CrossRef]
- Sun, B.O.; Fang, Y.; Li, Z.; Chen, Z.; Xiang, J. Role of Cellular Cytoskeleton in Epithelial-Mesenchymal Transition Process during Cancer Progression. Biomed. Rep. 2015, 3, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, D.; Duijf, P.H.G.; Khanna, K.K. Mitotic Slippage: An Old Tale with a New Twist. Cell Cycle 2019, 18, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, V.K.; Wang, Q.; Setua, S.; Nagesh, P.K.B.; Chauhan, N.; Kumari, S.; Chowdhury, P.; Miller, D.D.; Yallapu, M.M.; Li, W.; et al. Therapeutic Efficacy of a Novel ΒiII/ΒIV-Tubulin Inhibitor (VERU-111) in Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef] [Green Version]
- Kavallaris, M.; Kuo, D.Y.S.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-Resistant Epithelial Ovarian Tumors Are Associated with Altered Expression of Specific β-Tubulin Isotypes. J. Clin. Invest. 1997, 100, 1282–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, R.E.; Taylor, C.; Stanley, A.; Butler, E.; Joyce, A.; Harnden, P.; Patel, P.M.; Selby, P.J.; Banks, R.E. Resistance to the Tubulin-Binding Agents in Renal Cell Carcinoma: No Mutations in the Class I Beta-Tubulin Gene but Changes in Tubulin Isotype Protein Expression. Clin. Cancer Res. 2005, 11, 3439–3445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhang, Y.; Wu, B.; Kurie, J.M.; Pertsemlidis, A. The MiR-195 Axis Regulates Chemoresistance through TUBB and Lung Cancer Progression through BIRC5. Mol. Ther. Oncolytics 2019, 14, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohishi, Y.; Oda, Y.; Basaki, Y.; Kobayashi, H.; Wake, N.; Kuwano, M.; Tsuneyoshi, M. Expression of Beta-Tubulin Isotypes in Human Primary Ovarian Carcinoma. Gynecol. Oncol. 2007, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.I.; Valoti, G.; Giannakakou, P.; Zhan, Z.; Kim, J.H.; Lucchini, V.; Landoni, F.; Mayo, J.G.; Giavazzi, R.; Fojo, T. Expression of β-Tubulin Isotypes in Human Ovarian Carcinoma Xenografts and in a Sub-Panel of Human Cancer Cell Lines from the NCI-Anticancer Drug Screen: Correlation with Sensitivity to Microtubule Active Agents. Clin. Cancer Res. 2001, 7, 2912–2922. [Google Scholar] [PubMed]
- Shalli, K.; Brown, I.; Heys, S.D.; Schofield, C.A. Alterations of Β-tubulin Isotypes in Breast Cancer Cells Resistant to Docetaxel. FASEB J. 2005, 19, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Mi, R.; Pan, C.; Zhou, Y.; Liu, Y.; Jin, G.; Liu, F. Identification of the Metastasis Potential and Its Associated Genes in Melanoma Multinucleated Giant Cells Using the PHA-ECM830 Fusion Method. Oncol. Rep. 2016, 35, 211–218. [Google Scholar] [CrossRef]
- Cullen, K.J.; Schumaker, L.; Nikitakis, N.; Goloubeva, O.; Tan, M.; Sarlis, N.J.; Haddad, R.I.; Posner, M.R. Beta-Tubulin-II Expression Strongly Predicts Outcome in Patients Receiving Induction Chemotherapy for Locally Advanced Squamous Carcinoma of the Head and Neck: A Companion Analysis of the TAX 324 Trial. J. Clin. Oncol. 2009, 27, 6222–6228. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J.; He, S.; Wan, C.; Shan, A.; Wang, Y.; Yu, L.; Liu, G.; Chen, K.; Shi, J.; et al. Increased α-Tubulin1b Expression Indicates Poor Prognosis and Resistance to Chemotherapy in Hepatocellular Carcinoma. Dig. Dis. Sci. 2013, 58, 2713–2720. [Google Scholar] [CrossRef]
- Hasegawa, S.; Miyoshi, Y.; Egawa, C.; Ishitobi, M.; Taguchi, T.; Tamaki, Y.; Monden, M.; Noguchi, S. Prediction of Response to Docetaxel by Quantitative Analysis of Class I and III β-Tubulin Isotype MRNA Expression in Human Breast Cancers. Clin. Cancer Res. 2003, 9, 2992–2997. [Google Scholar]
- Bernard-Marty, C.; Treilleux, I.; Dumontet, C.; Cardoso, F.; Fellous, A.; Gancberg, D.; Bissery, M.C.; Paesmans, M.; Larsimont, D.; Piccart, M.J.; et al. Microtuble-Associated Parameters as Predictive Markers of Docetaxel Activity in Advanced Breast Cancer Patients: Results of a Pilot Study. Clin. Breast Cancer 2002, 3, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Maahs, L.; Sanchez, B.E.; Gupta, N.; Van Harn, M.; Barrack, E.R.; Reddy, P.V.; Hwang, C. Class III β-Tubulin Expression as a Predictor of Docetaxel-Resistance in Metastatic Castrationresistant Prostate Cancer. PLoS ONE 2019, 14, e0222510. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Terry, S.; Maillé, P.; Allory, Y.; Sirab, N.; Kheuang, L.; Soyeux, P.; Nicolaiew, N.; Coppolani, E.; Paule, B.; et al. Class III β-Tubulin Expression Predicts Prostate Tumor Aggressiveness and Patient Response to Docetaxel-Based Chemotherapy. Cancer Res. 2010, 70, 9253–9264. [Google Scholar] [CrossRef] [Green Version]
- Öztop, S.; Işik, A.; Güner, G.; Gürdal, H.; Karabulut, E.; Yilmaz, E.; Akyol, A. Class III β-Tubulin Expression in Colorectal Neoplasms Is a Potential Predictive Biomarker for Paclitaxel Response. Anticancer Res. 2019, 39, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Nakamura, Y.; Yasuda, T.; Ohba, K.; Takehara, K.; Sakai, H. Expression of Class III Beta-Tubulin Predicts Prognosis in Patients with Cisplatin-Resistant Bladder Cancer Receiving Paclitaxel-Based Second-Line Chemotherapy. Anticancer Res. 2018, 38, 1629–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höflmayer, D.; Öztürk, E.; Schroeder, C.; Hube-Magg, C.; Blessin, N.C.; Simon, R.; Lang, D.S.; Neubauer, E.; Göbel, C.; Heinrich, M.C.; et al. High Expression of Class III β-Tubulin in Upper Gastrointestinal Cancer Types. Oncol. Lett. 2018, 16, 7139–7145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.E.; Hong, J.Y.; Kim, K.; Kim, S.H.; Choi, W.Y.; Kim, M.J.; Jung, S.H.; Shim, H.J.; Bae, W.K.; Hwang, E.C.; et al. Class III β-Tubulin Is a Predictive Marker for Taxane-Based Chemotherapy in Recurrent and Metastatic Gastric Cancer. BMC Cancer 2013, 13, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Roque, D.M.; Bellone, S.; English, D.P.; Buza, N.; Cocco, E.; Gasparrini, S.; Bortolomai, I.; Ratner, E.; Silasi, D.A.; Azodi, M.; et al. Tubulin-β-III Overexpression by Uterine Serous Carcinomas Is a Marker for Poor Overall Survival after Platinum/Taxane Chemotherapy and Sensitivity to Epothilones. Cancer 2013, 119, 2582–2592. [Google Scholar] [CrossRef] [Green Version]
- Narvi, E.; Jaakkola, K.; Winsel, S.; Oetken-Lindholm, C.; Halonen, P.; Kallio, L.; Kallio, M.J. Altered TUBB3 Expression Contributes to the Epothilone Response of Mitotic Cells. Br. J. Cancer 2013, 108, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Yu, X.M.; Zhou, X.M.; Wang, X.H.; Su, D. Correlation between Microtubule-Associated Gene Expression and Chemosensitivity of Patients with Stage II Non-Small Cell Lung Cancer. Exp. Ther. Med. 2013, 5, 1506–1510. [Google Scholar] [CrossRef] [Green Version]
- Sève, P.; Isaac, S.; Trédan, O.; Souquet, P.J.; Pachéco, Y.; Pérol, M.; Lafanéchère, L.; Penet, A.; Peiller, E.L.; Dumontet, C. Expression of Class III β-Tubulin Is Predictive of Patient Outcome in Patients with Non—Small Cell Lung Cancer Receiving Vinorelbine-Based Chemotherapy. Clin. Cancer Res. 2005, 11, 5481–5486. [Google Scholar] [CrossRef] [Green Version]
- Sève, P.; Lai, R.; Ding, K.; Winton, T.; Butts, C.; Mackey, J.; Dumontet, C.; Dabbagh, L.; Aviel-Ronen, S.; Seymour, L.; et al. Class III Beta-Tubulin Expression and Benefit from Adjuvant Cisplatin/Vinorelbine Chemotherapy in Operable Non-Small Cell Lung Cancer: Analysis of NCIC JBR.10. Clin. Cancer Res. 2007, 13, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class III β-Tubulin Overexpression Is a Marker of Poor Clinical Outcome in Advanced Ovarian Cancer Patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, D.; Oda, Y.; Hattori, S.; Taguchi, K.I.; Ohishi, Y.; Basaki, Y.; Oie, S.; Suzuki, N.; Kono, S.; Tsuneyoshi, M.; et al. Overexpression of Class III β-Tubulin Predicts Good Response to Taxane-Based Chemotherapy in Ovarian Clear Cell Adenocarcinoma. Clin. Cancer Res. 2009, 15, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebok, P.; Öztürk, M.; Heilenkötter, U.; Jaenicke, F.; Müller, V.; Paluchowski, P.; Geist, S.; Wilke, C.; Burandt, E.; Lebeau, A.; et al. High Levels of Class III β-Tubulin Expression Are Associated with Aggressive Tumor Features in Breast Cancer. Oncol. Lett. 2016, 11, 1987–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nami, B.; Wang, Z. Genetics and Expression Profile of the Tubulin Gene Superfamily in Breast Cancer Subtypes and Its Relation to Taxane Resistance. Cancers 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galmarini, C.M.; Treilleux, I.; Cardoso, F.; Bernard-Marty, C.; Durbecq, V.; Gancberg, D.; Bissery, M.C.; Paesmans, M.; Larsimont, D.; Piccart, M.J.; et al. Class III β-Tubulin Isotype Predicts Response in Advanced Breast Cancer Patients Randomly Treated Either with Single-Agent Doxorubicin or Docetaxel. Clin. Cancer Res. 2008, 14, 4511–4516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akasaka, K.; Maesawa, C.; Shibazaki, M.; Maeda, F.; Takahashi, K.; Akasaka, T.; Masuda, T. Loss of Class III Β-Tubulin Induced by Histone Deacetylation Is Associated with Chemosensitivity to Paclitaxel in Malignant Melanoma Cells. J. Invest. Dermatol. 2009, 129, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoph, D.C.; Kasper, S.; Gauler, T.C.; Loesch, C.; Engelhard, M.; Theegarten, D.; Poettgen, C.; Hepp, R.; Peglow, A.; Loewendick, H.; et al. Βv-Tubulin Expression Is Associated With Outcome Following Taxane-Based Chemotherapy in Non-Small Cell Lung Cancer. Br. J. Cancer 2012, 107, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Gan, P.P.; Kavallaris, M. Tubulin-Targeted Drug Action: Functional Significance of Class II and Class IVB β-Tubulin in Vinca Alkaloid Sensitivity. Cancer Res. 2008, 68, 9817–9824. [Google Scholar] [CrossRef] [Green Version]
- Yeh, I.-T.; Ludueña, R.F. The BetaII Isotype of Tubulin Is Present in the Cell Nuclei of a Variety of Cancers. Cell Motil. Cytoskelet. 2004, 57, 96–106. [Google Scholar] [CrossRef]
- Ruksha, K.; Mezheyeuski, A.; Nerovnya, A.; Bich, T.; Tur, G.; Gorgun, J.; Luduena, R.; Portyanko, A. Over-Expression of ΒII-Tubulin and Especially Its Localization in Cell Nuclei Correlates with Poorer Outcomes in Colorectal Cancer. Cells 2019, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Person, F.; Wilczak, W.; Hube-Magg, C.; Burdelski, C.; Möller-Koop, C.; Simon, R.; Noriega, M.; Sauter, G.; Steurer, S.; Burdak-Rothkamm, S.; et al. Prevalence of ΒIII-Tubulin (TUBB3) Expression in Human Normal Tissues and Cancers. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, A.; Mangia, A.; Chiriatti, A.; Tommasi, S.; Zito, A.; Latorre, A.; Schittulli, F.; Lorusso, V. Biomarkers Predictive for Clinical Efficacy of Taxol-Based Chemotherapy in Advanced Breast Cancer. Ann. Oncol. 2005, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; McCarroll, J.A.; Byrne, F.L.; Garner, J.; Kavallaris, M. Specific β-Tubulin Isotypes Can Functionally Enhance or Diminish Epothilone B Sensitivity in Non-Small Cell Lung Cancer Cells. PLoS ONE 2011, 6, e21717. [Google Scholar] [CrossRef] [Green Version]
- Tsourlakis, M.C.; Weigand, P.; Grupp, K.; Kluth, M.; Steurer, S.; Schlomm, T.; Graefen, M.; Huland, H.; Salomon, G.; Steuber, T.; et al. ΒIII-Tubulin Overexpression Is an Independent Predictor of Prostate Cancer Progression Tightly Linked to ERG Fusion Status and PTEN Deletion. Am. J. Pathol. 2014, 184, 609–617. [Google Scholar] [CrossRef]
- Hetland, T.E.; Hellesylt, E.; Flørenes, V.A.; Tropé, C.; Davidson, B.; Kærn, J. Class III β-Tubulin Expression in Advanced-Stage Serous Ovarian Carcinoma Effusions Is Associated with Poor Survival and Primary Chemoresistance. Hum. Pathol. 2011, 42, 1019–1026. [Google Scholar] [CrossRef]
- Zhang, H.L.; Ruan, L.; Zheng, L.M.; Whyte, D.; Tzeng, C.M.; Zhou, X.W. Association between Class III β-Tubulin Expression and Response to Paclitaxel/Vinorebine-Based Chemotherapy for Non-Small Cell Lung Cancer: A Meta-Analysis. Lung Cancer 2012, 77, 9–15. [Google Scholar] [CrossRef]
- Koh, Y.; Jang, B.; Han, S.-W.; Kim, T.-M.; Oh, D.-Y.; Lee, S.-H.; Kang, C.H.; Kim, D.-W.; Im, S.-A.; Chung, D.H.; et al. Expression of Class III Beta-Tubulin Correlates with Unfavorable Survival Outcome in Patients with Resected Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 320–325. [Google Scholar] [CrossRef] [Green Version]
- McCarroll, J.A.; Sharbeen, G.; Liu, J.; Youkhana, J.; Goldstein, D.; McCarthy, N.; Limbri, L.F.; Dischl, D.; Ceyhan, G.O.; Erkan, M.; et al. ΒIII-Tubulin: A Novel Mediator of Chemoresistance and Metastases in Pancreatic Cancer. Oncotarget 2015, 6, 2235–2249. [Google Scholar] [CrossRef] [Green Version]
- Levallet, G.; Bergot, E.; Antoine, M.; Creveuil, C.; Santos, A.O.; Beau-Faller, M.; De Fraipont, F.; Brambilla, E.; Levallet, J.; Morin, F.; et al. High TUBB3 Expression, an Independent Prognostic Marker in Patients with Early Non-Small Cell Lung Cancer Treated by Preoperative Chemotherapy, Is Regulated by K-Ras Signaling Pathway. Mol. Cancer Ther. 2012, 11, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Nienstedt, J.C.; Gröbe, A.; Clauditz, T.; Simon, R.; Muenscher, A.; Knecht, R.; Sauter, G.; Moebius, C.; Blessmann, M.; Heiland, M.; et al. High-Level ΒIII-Tubulin Overexpression Occurs in Most Head and Neck Cancers but Is Unrelated to Clinical Outcome. J. Oral Pathol. Med. 2017, 46, 986–990. [Google Scholar] [CrossRef]
- Reader, J.; Harper, A.K.; Legesse, T.; Staats, P.N.; Goloubeva, O.; Rao, G.G.; Fulton, A.; Roque, D.M. EP4 and Class III β-Tubulin Expression in Uterine Smooth Muscle Tumors: Implications for Prognosis and Treatment. Cancers 2019, 11, 1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, K.; Wieczorek, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Wawro, M.E.; Wiktorska, M.; Boncela, J.; Papiewska-Pajak, I.; Kwasniak, P.; Wyroba, E.; et al. β-III Tubulin Modulates the Behavior of Snail Overexpressed during the Epithelial-to-Mesenchymal Transition in Colon Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2221–2233. [Google Scholar] [CrossRef]
- Radakovic, A.; Boger, D.L. High Expression of Class III β-Tubulin Has No Impact on Functional Cancer Cell Growth Inhibition of a Series of Key Vinblastine Analogs. Bioorg. Med. Chem. Lett. 2018, 28, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Sharbeen, G.; McCarroll, J.; Liu, J.; Youkhana, J.; Limbri, L.F.; Biankin, A.V.; Johns, A.; Kavallaris, M.; Goldstein, D.; Phillips, P.A. Delineating the Role of ΒIV-Tubulins in Pancreatic Cancer: ΒIVb-Tubulin Inhibition Sensitizes Pancreatic Cancer Cells to Vinca Alkaloids. Neoplasia 2016, 18, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, K.; Ciszewski, W.M.; Wawro, M.E.; Wieczorek-Szukała, K.; Boncela, J.; Papiewska-Pajak, I.; Niewiarowska, J.; Kowalska, M.A. TUBB4B Downregulation Is Critical for Increasing Migration of Metastatic Colon Cancer Cells. Cells 2019, 8, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, S.K.; Wang, Y.; Verdier-Pinard, P.; Yang, C.-P.H.; Liu, L.; Rodriguez-Gabin, A.; McDaid, H.M.; Horwitz, S.B. Characterization of a Human ΒV-Tubulin Antibody and Expression of This Isotype in Normal and Malignant Human Tissue. Cytoskeleton 2012, 69, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.; Wang, Y.; Van Arsdale, A.; Horwitz, S.B.; Mcdaid, H. Expression of ΒV-Tubulin in Secretory Cells of the Fallopian Tube Epithelium Marks Cellular Atypia. Int. J. Gynecol. Cancer 2018, 28, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Berrieman, H.K.; Lind, M.J.; Cawkwell, L. Do β-Tubulin Mutations Have a Role in Resistance to Chemotherapy? Lancet Oncology. 2004, 5, 158–164. [Google Scholar] [CrossRef]
- Verrills, N.; Kavallaris, M. Improving the Targeting of Tubulin-Binding Agents: Lessons from Drug Resistance Studies. Curr. Pharm. Des. 2005, 11, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Huzil, J.T.; Chen, K.; Kurgan, L.; Tuszynski, J.A. The Roles of β-Tubulin Mutations and Isotype Expression in Acquired Drug Resistance. Cancer Inform. 2007, 3, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, H.; Wang, X.; Patterson, J.; Winter, P.; Graham, K.; Ghosh, S.; Lee, J.C.; Katsetos, C.D.; Mackey, J.R.; et al. Novel Mutations Involving ΒI-, ΒIIA-, or ΒIVB-Tubulin Isotypes with Functional Resemblance to ΒIII-Tubulin in Breast Cancer. Protoplasma 2017, 254, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Magiera, M.M.; Singh, P.; Gadadhar, S.; Janke, C. Tubulin Posttranslational Modifications and Emerging Links to Human Disease. Cell 2018, 173, 1323–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whipple, R.A.; Matrone, M.A.; Cho, E.H.; Balzer, E.M.; Vitolo, M.I.; Yoon, J.R.; Ioffe, O.B.; Tuttle, K.C.; Yang, J.; Martin, S.S. Epithelial-to-Mesenchymal Transition Promotes Tubulin Detyrosination and Microtentacles That Enhance Endothelial Engagement. Cancer Res. 2010, 70, 8127–8137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucek, K.; Kamaid, A.; Phung, A.D.; Kubala, L.; Bulinski, J.C.; Harper, R.W.; Eiserich, J.P. Normal and Prostate Cancer Cells Display Distinct Molecular Profiles of Alpha-Tubulin Posttranslational Modifications. Prostate 2006, 66, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Mialhe, A.; Lafanechère, L.; Job, D.; Treilleux, I.; Peloux, N.; Dumontet, C.; Brémond, A.; Panh, M.H.; Payan, R.; Wehland, J.; et al. Tubulin Detyrosination Is a Frequent Occurrence in Breast Cancers of Poor Prognosis. Cancer Res. 2001, 61, 5024–5027. [Google Scholar]
- Kato, C.; Miyazaki, K.; Nakagawa, A.; Ohira, M.; Nakamura, Y.; Ozaki, T.; Imai, T.; Nakagawara, A. Low Expression of Human Tubulin Tyrosine Ligase and Suppressed Tubulin Tyrosination/Detyrosination Cycle Are Associated with Impaired Neuronal Differentiation in Neuroblastomas with Poor Prognosis. Int. J. Cancer 2004, 112, 365–375. [Google Scholar] [CrossRef]
- Aillaud, C.; Bosc, C.; Peris, L.; Bosson, A.; Heemeryck, P.; Van Dijk, J.; Le Friec, J.; Boulan, B.; Vossier, F.; Sanman, L.E.; et al. Vasohibins/SVBP Are Tubulin Carboxypeptidases (TCPs) That Regulate Neuron Differentiation. Science 2017, 358, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuis, J.; Adamopoulos, A.; Bleijerveld, O.B.; Mazouzi, A.; Stickel, E.; Celie, P.; Altelaar, M.; Knipscheer, P.; Perrakis, A.; Blomen, V.A.; et al. Vasohibins Encode Tubulin Detyrosinating Activity. Science 2017, 358, 1453–1456. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zhao, J.; Hai, L.; Wu, J.; Yi, H.; Shi, Y. The Roles of Vasohibin and Its Family Members: Beyond Angiogenesis Modulators. Cancer Biol. Ther. 2017, 18, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wu, Z.; Xie, W.; Tian, D.; Chen, F.; Qin, C.; Du, Z.; Tang, G.; Gao, Q.; Qiu, X.; et al. The Expression of Vasohibin-1 and Its Prognostic Significance in Bladder Cancer. Exp. Ther. Med. 2017, 14, 3477–3484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saba, N.F.; Magliocca, K.R.; Kim, S.; Muller, S.; Chen, Z.; Owonikoko, T.K.; Sarlis, N.J.; Eggers, C.; Phelan, V.; Grist, W.J.; et al. Acetylated Tubulin (AT) as a Prognostic Marker in Squamous Cell Carcinoma of the Head and Neck. Head Neck Pathol. 2014, 8, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.; Kapoor, S.; Naaz, A.; Kumar Santra, M.; Panda, D. Enhanced Stability of Microtubules Contributes in the Development of Colchicine Resistance in MCF-7 Cells. Biochem. Pharmacol. 2017, 132, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. α-Tubulin Acetylation Elevated in Metastatic and Basal-like Breast Cancer Cells Promotes Microtentacle Formation, Adhesion, and Invasive Migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Cheng, Y.C.; Chang, C.Y.; Lin, C.M.; Chang, J.Y. Alpha-Tubulin Acetyltransferase/MEC-17 Regulates Cancer Cell Migration and Invasion through Epithelial–Mesenchymal Transition Suppression and Cell Polarity Disruption. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lagman, J.; Sayegh, P.; Lee, C.S.; Sulon, S.M.; Jacinto, A.Z.; Sok, V.; Peng, N.; Alp, D.; Benovic, J.L.; So, C.H. G Protein-Coupled Receptor Kinase 5 Modifies Cancer Cell Resistance to Paclitaxel. Mol. Cell. Biochem. 2019, 461, 103–118. [Google Scholar] [CrossRef]
- Sakuma, T.; Uzawa, K.; Onda, T.; Shiiba, M.; Yokoe, H.; Shibahara, T.; Tanzawa, H. Aberrant Expression of Histone Deacetylase 6 in Oral Squamous Cell Carcinoma. Int. J. Oncol. 2006, 29, 117–124. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lim, K.-H.; Guo, X.; Kawaguchi, Y.; Gao, Y.; Barrientos, T.; Ordentlich, P.; Wang, X.-F.; Counter, C.M.; Yao, T.-P. The Cytoplasmic Deacetylase HDAC6 Is Required for Efficient Oncogenic Tumorigenesis. Cancer Res. 2008, 68, 7561–7569. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Liu, Y.; Zhu, B.; Ding, K.; Yao, T.P.; Chen, F.; Zhan, L.; Xu, P.; Ehrlich, M.; Liang, T.; et al. Loss of α-Tubulin Acetylation Is Associated with TGF-β-Induced Epithelial-Mesenchymal Transition. J. Biol. Chem. 2016, 291, 5396–5405. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A. Increased Levels of Tyrosinated α-, ΒIII-, and ΒIV-Tubulin Isotypes in Paclitaxel-Resistant MCF-7 Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2002, 293, 598–601. [Google Scholar] [CrossRef]
- Lafanechere, L.; Courtay-Cahen, C.; Kawakami, T.; Jacrot, M.; Rudiger, M.; Wehland, J.; Job, D.; Margolis, R.L. Suppression of Tubulin Tyrosine Ligase during Tumor Growth. J. Cell Sci. 1998, 111, 171–181. [Google Scholar] [PubMed]
- Miller, L.M.; Menthena, A.; Chatterjee, C.; Verdier-Pinard, P.; Novikoff, P.M.; Horwitz, S.B.; Angeletti, R.H. Increased Levels of a Unique Post-Translationally Modified ΒIVb-Tubulin Isotype in Liver Cancer. Biochemistry 2008, 47, 7572–7582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, A.I.; Zhou, J.; O’Brate, A.; Hamel, E.; Wong, J.; Nivens, M.; El-Naggar, A.; Yao, T.P.; Khuri, F.R.; Giannakakou, P. The Synergistic Combination of the Farnesyl Transferase Inhibitor Lonafarnib and Paclitaxel Enhances Tubulin Acetylation and Requires a Functional Tubulin Deacetylase. Cancer Res. 2005, 65, 3883–3893. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yamashita, H.; Toyama, T.; Sugiura, H.; Omoto, Y.; Ando, Y.; Mita, K.; Hamaguchi, M.; Hayashi, S.I.; Iwase, H. HDAC6 Expression Is Correlated with Better Survival in Breast Cancer. Clin. Cancer Res. 2004, 10, 6962–6968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, C.; Papon, L.; Cacheux, W.; Marques Sousa, P.; Lascano, V.; Tort, O.; Giordano, T.; Vacher, S.; Lemmers, B.; Mariani, P.; et al. Tubulin Glycylases Are Required for Primary Cilia, Control of Cell Proliferation and Tumor Development in Colon. EMBO J. 2014, 33, 2735. [Google Scholar] [CrossRef] [Green Version]
- Markovsky, E.; de Stanchina, E.; Itzkowitz, A.; Haimovitz-Friedman, A.; Rotenberg, S.A. Phosphorylation State of Ser 165 in α-Tubulin Is a Toggle Switch That Controls Proliferating Human Breast Tumors. Cell. Signal. 2018, 52, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.M.R.; Setaluri, V. Microtubule-Associated Proteins as Targets in Cancer Chemotherapy. Clin. Cancer Res. 2007, 13, 2849–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Fu, H.; Zhou, L.; Xu, K.; Pang, Y.; Hu, K.; Wang, J.; Tian, L.; Liu, Y.; Wang, J.; et al. High Expression of MAP7 Predicts Adverse Prognosis in Young Patients with Cytogenetically Normal Acute Myeloid Leukemia. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Chien, T.-M.; Chan, T.-C.; Huang, S.K.-H.; Yeh, B.-W.; Li, W.-M.; Huang, C.-N.; Li, C.-C.; Wu, W.-J.; Li, C.-F. Role of Microtubule-Associated Protein 1b in Urothelial Carcinoma: Overexpression Predicts Poor Prognosis. Cancers 2020, 12, 630. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Ogden, A.; Aneja, R.; Zhou, J. Microtubule-Binding Proteins as Promising Biomarkers of Paclitaxel Sensitivity in Cancer Chemotherapy. Med. Res. Rev. 2016, 36, 300–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melhems, R.F.; Zhu, X.-X.; Hailat, N.; Strahler, J.R.; Hanash, S.M. Characterization of the Gene for a Proliferation-Related Phosphoprotein (Oncoprotein 18) Expressed in High Amounts in Acute Leukemia. J. Biol. Chem. 1991, 266, 17747–17753. [Google Scholar]
- Friedrich, B.; Grönberg, H.; Landström, M.; Gullberg, M.; Bergh, A. Differentiation-Stage Specific Expression of Oncoprotein 18 in Human and Rat Prostatic Adenocarcinoma. Prostate 1995, 27, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Price, D.K.; Ball, J.R.; Bahrani-Mostafavi, Z.; Vachris, J.C.; Kaufman, J.S.; Naumann, R.W.; Higgins, R.V.; Hall, J.B. The Phosphoprotein Op18/Stathmin Is Differentially Expressed in Ovarian Cancer. Cancer Invest. 2000, 18, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Brattsand, G. Correlation of Oncoprotein 18/Stathmin Expression in Human Breast Cancer with Established Prognostic Factors. Br. J. Cancer 2000, 83, 311–318. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Gharib, T.G.; Huang, C.C.; Thomas, D.G.; Shedden, K.A.; Kuick, R.; Taylor, J.M.G.; Kardia, S.L.R.; Misek, D.E.; et al. Overexpression of Oncoprotein 18 Correlates with Poor Differentiation in Lung Adenocarcinomas. Mol. Cell. Proteomics 2003, 2, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Ogino, S.; Nosho, K.; Baba, Y.; Kure, S.; Shima, K.; Irahara, N.; Toyoda, S.; Chen, L.; Kirkner, G.J.; Wolpin, B.M.; et al. A Cohort Study of STMN1 Expression in Colorectal Cancer: Body Mass Index and Prognosis. Am. J. Gastroenterol. 2009, 104, 2047–2056. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Rui, W.; Fang, L.; Ke, D.; Ping, G.; Hui-Zhong, Z. Expression of Stathmin/Op18 as a Signifcant Prognostic Factor for Cervical Carcinoma Patients. J Cancer Res. Clin. Oncol. 2009, 135, 837–846. [Google Scholar] [CrossRef]
- Hsu, H.P.; Li, C.F.; Lee, S.W.; Wu, W.R.; Chen, T.J.; Chang, K.Y.; Liang, S.S.; Tsai, C.J.; Shiue, Y.L. Overexpression of Stathmin 1 Confers an Independent Prognostic Indicator in Nasopharyngeal Carcinoma. Tumor Biol. 2014, 35, 2619–2629. [Google Scholar] [CrossRef]
- Reyes, H.D.; Miecznikowski, J.; Gonzalez-Bosquet, J.; Devor, E.J.; Zhang, Y.; Thiel, K.W.; Samuelson, M.I.; McDonald, M.; Stephan, J.M.; Hanjani, P.; et al. High Stathmin Expression Is a Marker for Poor Clinical Outcome in Endometrial Cancer: An NRG Oncology Group/Gynecologic Oncology Group Study. Gynecol. Oncol. 2017, 146, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Byrne, F.L.; Yang, L.; Phillips, P.A.; Hansford, L.M.; Fletcher, J.I.; Ormandy, C.J.; McCarroll, J.A.; Kavallaris, M. RNAi-Mediated Stathmin Suppression Reduces Lung Metastasis in an Orthotopic Neuroblastoma Mouse Model. Oncogene 2014, 33, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Alli, E.; Bash-Babula, J.; Yang, J.-M.; Hait, W.N. Effect of Stathmin on the Sensitivity to Antimicrotubule Drugs in Human Breast Cancer. Cancer Res. 2002, 62, 6864–6869. [Google Scholar] [PubMed]
- Nishio, K.; Nakamura, T.; Koh, Y.; Kanzawa, F.; Tamura, T.; Saijo, N. Oncoprotein 18 Overexpression Increases the Sensitivity to Vindesine in the Human Lung Carcinoma Cells. Cancer 2001, 91, 1494–1499. [Google Scholar] [CrossRef]
- Lin, X.; Liao, Y.; Xie, J.; Liu, S.; Su, L.; Zou, H. Op18/Stathmin Is Involved in the Resistance of Taxol Among Different Epithelial Carcinoma Cell Lines. Cancer Biother. Radiopharm. 2014, 29, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yu, T.; Zhang, L.; Chen, S.; Chen, X.; Liao, Y.; Long, D.; Shen, F. Silencing Op18/Stathmin by RNA Interference Promotes the Sensitivity of Nasopharyngeal Carcinoma Cells to Taxol and High-Grade Differentiation of Xenografted Tumours in Nude Mice. Basic Clin. Pharmacol. Toxicol. 2016, 119, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Alli, E.; Yang, J.M.; Ford, J.M.; Hait, W.N. Reversal of Stathmin-Mediated Resistance to Paclitaxel and Vinblastine in Human Breast Carcinoma Cells. Mol. Pharmacol. 2007, 71, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Akhtar, J.; Wang, Z. Anti-STMN1 Therapy Improves Sensitivity to Antimicrotubule Drugs in Esophageal Squamous Cell Carcinoma. Tumor Biol. 2015, 36, 7797–7806. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiaoyan, X.; Xuan, Y.; Xiangke, L.; Zichang, Y.; Ran, Z.; Liuxing, W.; Qingxia, F. Silencing Stathmin-Modulating Efficiency of Chemotherapy for Esophageal Squamous Cell Cancer with Paclitaxel. Cancer Gene Ther. 2015, 22, 115–121. [Google Scholar] [CrossRef]
- Shen, F.; Long, D.; Yu, T.; Chen, X.; Liao, Y.; Wu, Y.; Lin, X. Vinblastine Differs from Taxol as It Inhibits the Malignant Phenotypes of NSCLC Cells by Increasingthe Phosphorylation of Op18/Stathmin. Oncol. Rep. 2017, 37, 2481–2489. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Yang, Y.; Ren, Y.; Li, J.; Wang, Z.; Dong, B.; Liu, M.; Zhou, J. Microtubule-Binding Protein CLIP-170 Is a Mediator of Paclitaxel Sensitivity. J. Pathol. 2012, 226, 666–673. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Ran, J.; Yan, B.; Chen, J.; Dong, X.; Liu, Z.; Liu, R.; Zhou, J.; Liu, M. End-Binding Protein 1 Stimulates Paclitaxel Sensitivity in Breast Cancer by Promoting Its Actions toward Microtubule Assembly and Stability. Protein Cell 2014, 5, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoumi, S.; Harisankar, A.; Gracias, A.; Bachinger, F.; Fufa, T.; Chandrasekar, G.; Gaunitz, F.; Walfridsson, J.; Kitambi, S. Drug Design, Development and Therapy Dovepress Understanding Cytoskeleton Regulators in Glioblastoma Multiforme for Therapy Design. Drug Des. Devel. Ther. 2016, 10, 2881–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-Associated Protein Tau: A Marker of Paclitaxel Sensitivity in Breast Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoter, M.; Bodnar, L.; Grala, B.; Stec, R.; Zieniuk, K.; Kozlowski, W.; Szczylik, C. Tau Protein as a Potential Predictive Marker in Epithelial Ovarian Cancer Patients Treated with Paclitaxel/Platinum First-Line Chemotherapy. J. Exp. Clin. Cancer Res. 2013, 32. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, A.; Kobayashi, A.; Oikiri, H.; Yokoyama, Y. Functional Role of the Tau Protein in Epithelial Ovarian Cancer Cells. Reprod. Med. Biol. 2017, 16, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Veitia, R.; Bissery, M.C.; Martinez, C.; Fellous, A. Tau Expression in Model Adenocarcinomas Correlates with Docetaxel Sensitivity in Tumour-Bearing Mice. Br. J. Cancer 1998, 78, 871–877. [Google Scholar] [CrossRef] [Green Version]
- Veitia, R.; David, S.; Barbier, P.; Vantard, M.; Gounon, P.; Bissery, M.C.; Fellous, A. Proteolysis of Microtubule Associated Protein 2 and Sensitivity of Pancreatic Tumours to Docetaxel. Br. J. Cancer 2000, 83, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sturgis, C.D.; Grzybicki, D.M.; Jasnosz, K.M.; Olson, P.R.; Tong, M.; Dabbs, D.D.; Raab, S.S.; Silverman, J.F. Microtubule-Associated Protein-2: A New Sensitive and Specific Marker for Pulmonary Carcinoid Tumor and Small Cell Carcinoma. Mod. Pathol. 2001, 14, 880–885. [Google Scholar] [CrossRef]
- Liu, Y.; Mangini, J.; Saad, R.; Silverman, A.R.; Abell, E.; Tung, M.Y.; Graner, S.R.; Silverman, J.F. Diagnostic Value of Microtubule-Associated Protein-2 in Merkel Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 326–329. [Google Scholar] [CrossRef]
- Chen, J.Y.F.; Chang, Y.L.; Yu, Y.C.; Chao, C.C.; Kao, H.W.; Wu, C.T.; Lin, W.C.; Ko, J.Y.; Jou, Y.S. Specific Induction of the High-Molecular-Weight Microtubule-Associated Protein 2 (Hmw-MAP2) by Betel Quid Extract in Cultural Oral Keratinocytes: Clinical Implications in Betel Quid-Associated Oral Squamous Cell Carcinoma (OSCC). Carcinogenesis 2004, 25, 269–276. [Google Scholar] [CrossRef]
- Song, Z.; He, C.D.; Sun, C.; Xu, Y.; Jin, X.; Zhang, Y.; Xiao, T.; Wang, Y.; Lu, P.; Jiang, Y.; et al. Increased Expression of MAP2 Inhibits Melanoma Cell Proliferation, Invasion and Tumor Growth in Vitro and in Vivo. Exp. Dermatol. 2010, 19, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.H.; Pichardo, R.; Song, Z.; Sangha, N.; Camacho, F.; Satyamoorthy, K.; Sangueza, O.P.; Setaluri, V. Microtubule-Associated Protein 2, a Marker of Neuronal Differentiation, Induces Mitotic Defects, Inhibits Growth of Melanoma Cells, and Predicts Metastatic Potential of Cutaneous Melanoma. Am. J. Pathol. 2005, 166, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Don, S.; Verrills, N.M.; Liaw, T.Y.E.; Liu, M.L.M.; Norris, M.D.; Haber, M.; Kavallaris, M. Neuronal-Associated Microtubule Proteins Class III β-Tubulin Ad MAP2c in Neuroblastoma: Role in Resistance to Microtubule-Targeted Drugs. Mol. Cancer Ther. 2004, 3, 1137–1146. [Google Scholar] [PubMed]
- Kavallaris, M.; Tait, A.S.; Norris, M.D.; Haber, M.; Walsh, B.J.; He, L.; Horwitz, S.B. Multiple Microtubule Alterations Are Associated with Vinca Alkaloid Resistance in Human Leukemia Cells. Cancer Res. 2001, 61, 5803–5809. [Google Scholar]
- Verrills, N.M.; Flemming, C.L.; Liu, M.; Ivery, M.T.; Cobon, G.S.; Norris, M.D.; Haber, M.; Kavallaris, M. Microtubule Alterations and Mutations Induced by Desoxyepothilone B: Implications for Drug-Target Interactions. Chem. Biol. 2003, 10, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Y.; Shang, L.; Shi, Z.Z.; Zhang, T.T.; Ma, S.; Lu, C.C.; Zhang, Y.; Hao, J.J.; Shi, C.; Shi, F.; et al. Microtubule-Associated Protein 4 Is an Important Regulator of Cell Invasion/Migration and a Potential Therapeutic Target in Esophageal Squamous Cell Carcinoma. Oncogene 2016, 35, 4846–4856. [Google Scholar] [CrossRef]
- Xia, X.; He, C.; Wu, A.; Zhou, J.; Wu, J. Microtubule-Associated Protein 4 Is a Prognostic Factor and Promotes Tumor Progression in Lung Adenocarcinoma. Dis. Markers 2018, 2018. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Chen, L.; Suo, Y.; Fan, J.; Zhang, S.; Wang, Y.; Gao, S.; Wang, Y. MAP7 Interacts with RC3H1 and Cooperatively Regulate Cell-Cycle Progression of Cervical Cancer Cells via Activating the NF-ΚB Signaling. Biochem. Biophys. Res. Commun. 2020, 527, 56–63. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Song, L.; Zhai, H.; Chang, C. MAP7 Promotes Migration and Invasion and Progression of Human Cervical Cancer through Modulating the Autophagy. Cancer Cell Int. 2020, 20. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [Green Version]


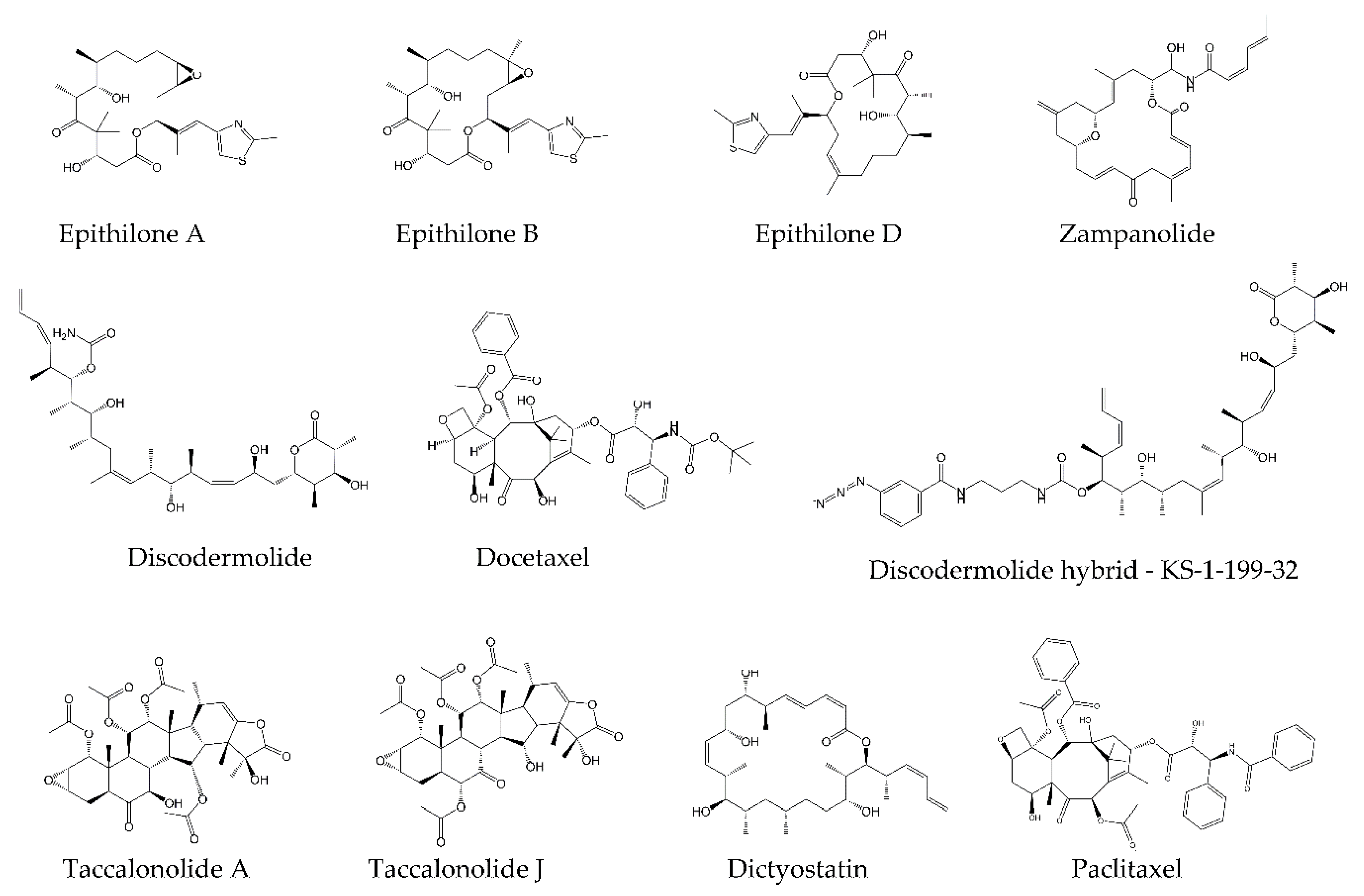
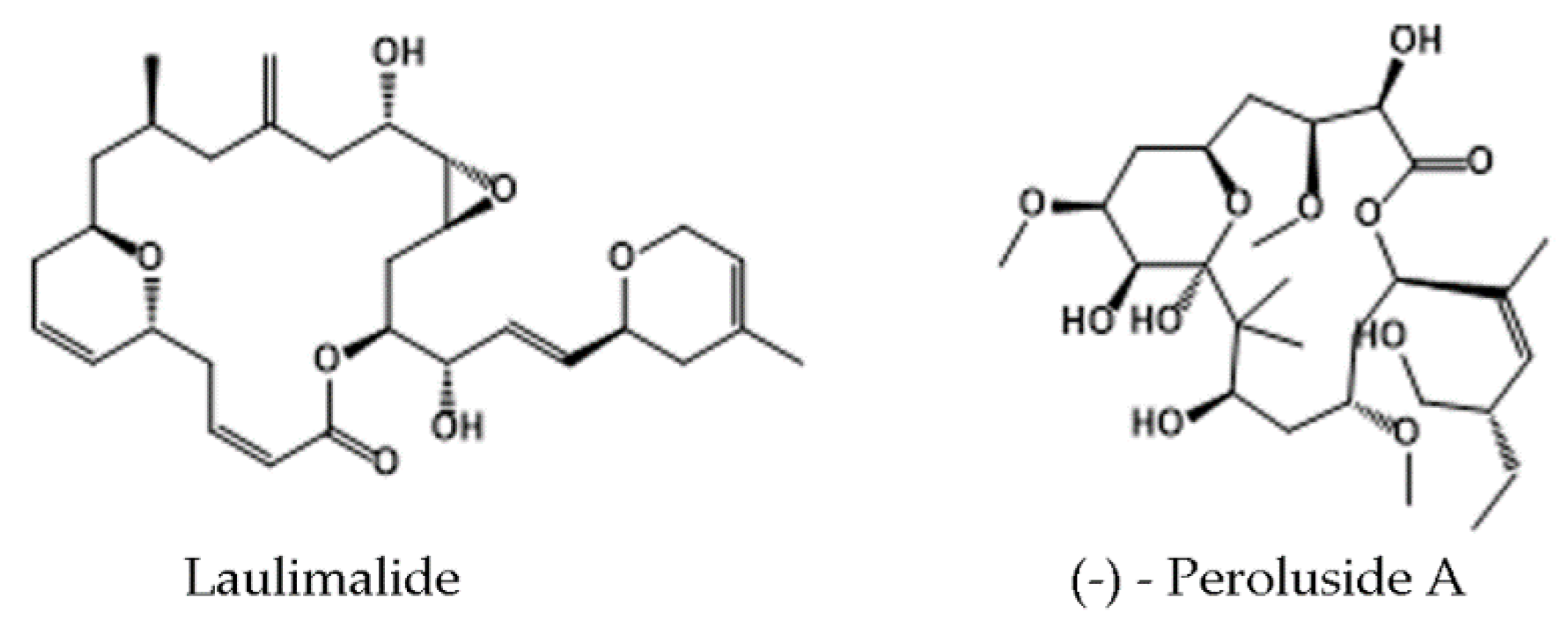
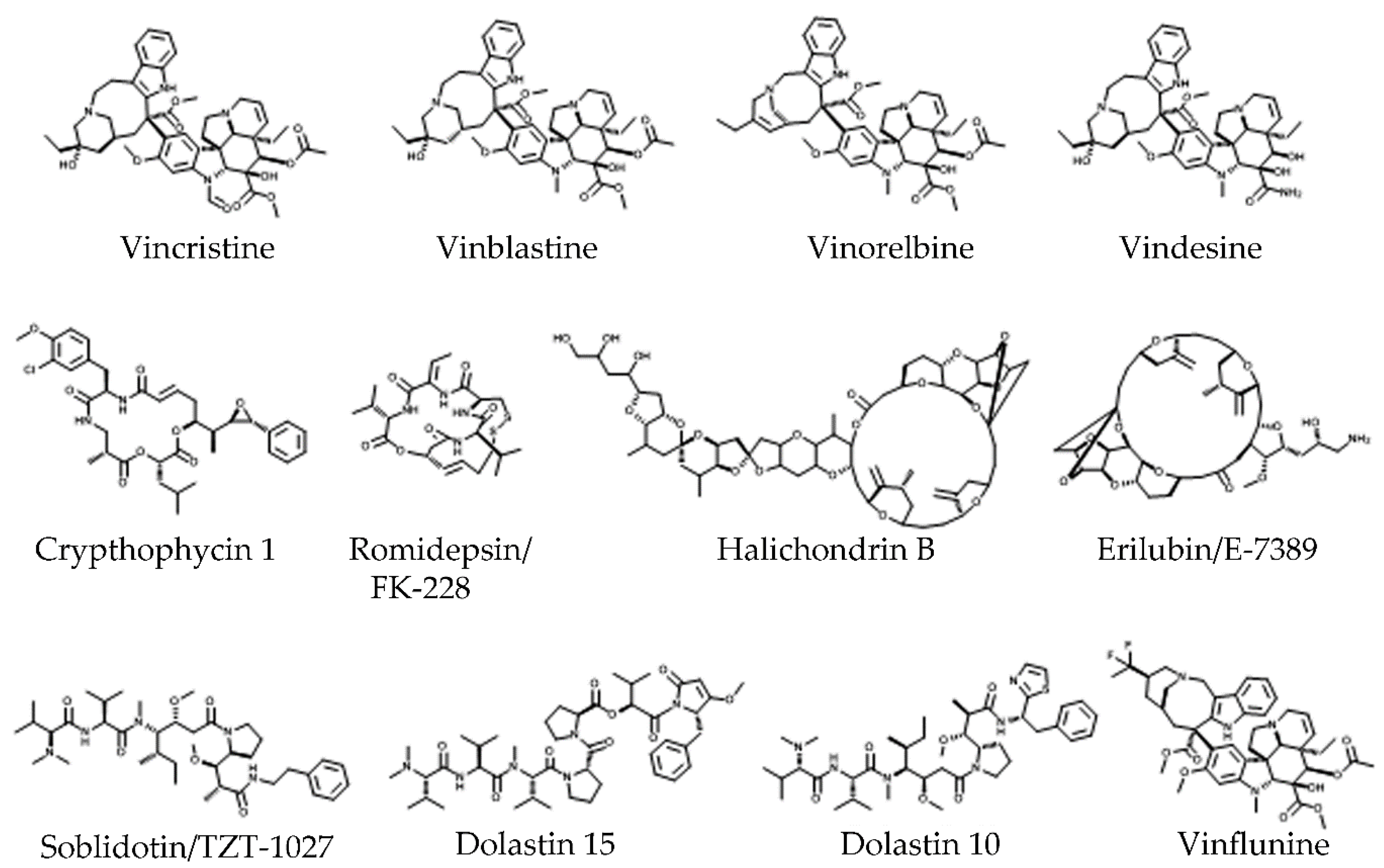
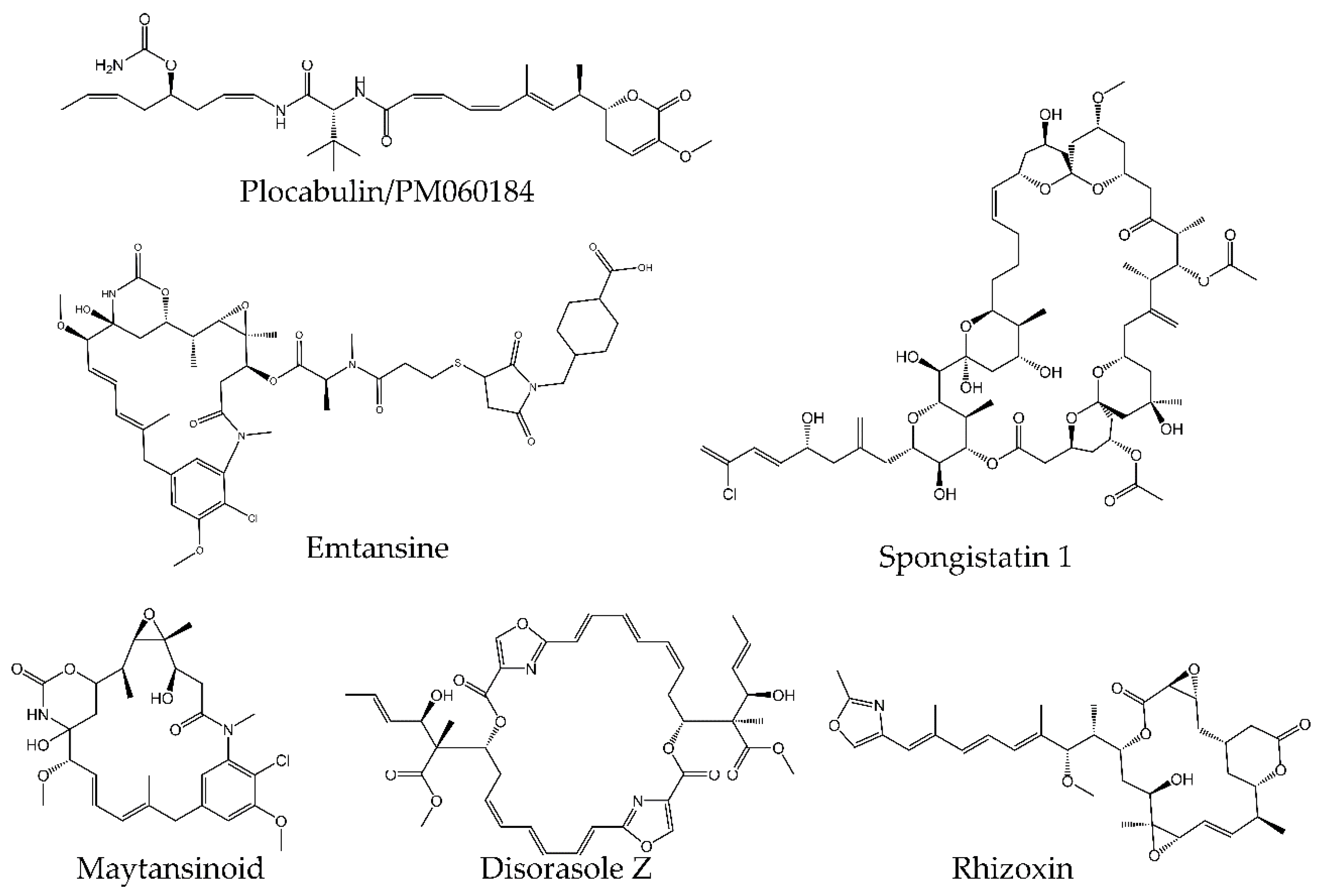
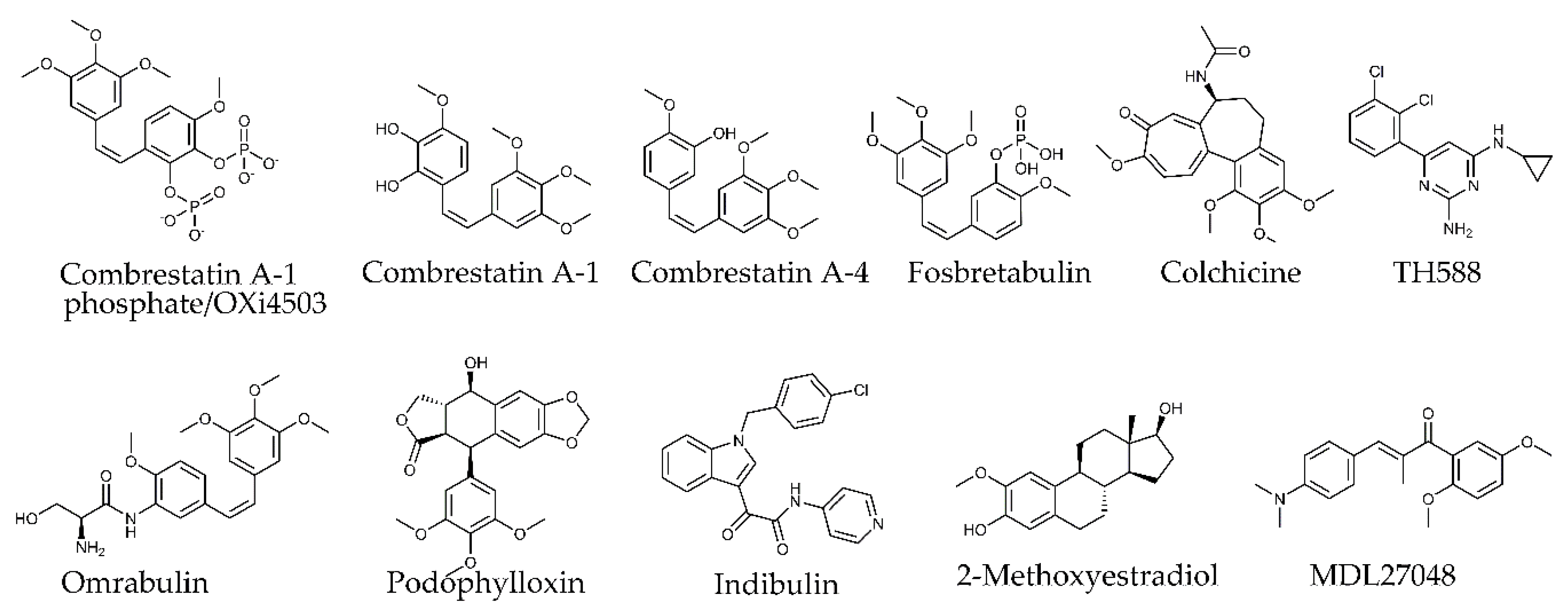

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules 2020, 25, 3705. https://doi.org/10.3390/molecules25163705
Borys F, Joachimiak E, Krawczyk H, Fabczak H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules. 2020; 25(16):3705. https://doi.org/10.3390/molecules25163705
Chicago/Turabian StyleBorys, Filip, Ewa Joachimiak, Hanna Krawczyk, and Hanna Fabczak. 2020. "Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells" Molecules 25, no. 16: 3705. https://doi.org/10.3390/molecules25163705
APA StyleBorys, F., Joachimiak, E., Krawczyk, H., & Fabczak, H. (2020). Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules, 25(16), 3705. https://doi.org/10.3390/molecules25163705






