2.1. Solids Characterization
X-ray fluorescence (XRF) was used to determine the elemental composition of all solids. The results are presented in
Table 1. Phonolite treatment with acids (HCl and oxalic acid) indicated a reduction in the Al, Na, Fe, and Ca contents. The results for Ni and W contents (impregnated materials) were as expected, with the NiW/Ph-HCl solids containing higher contents of Ni and W compared to the NiW/Ph-Ox catalyst. The acid treatments resulted in solids with a higher porosity, with the Ph-HCl support being the most porous material.
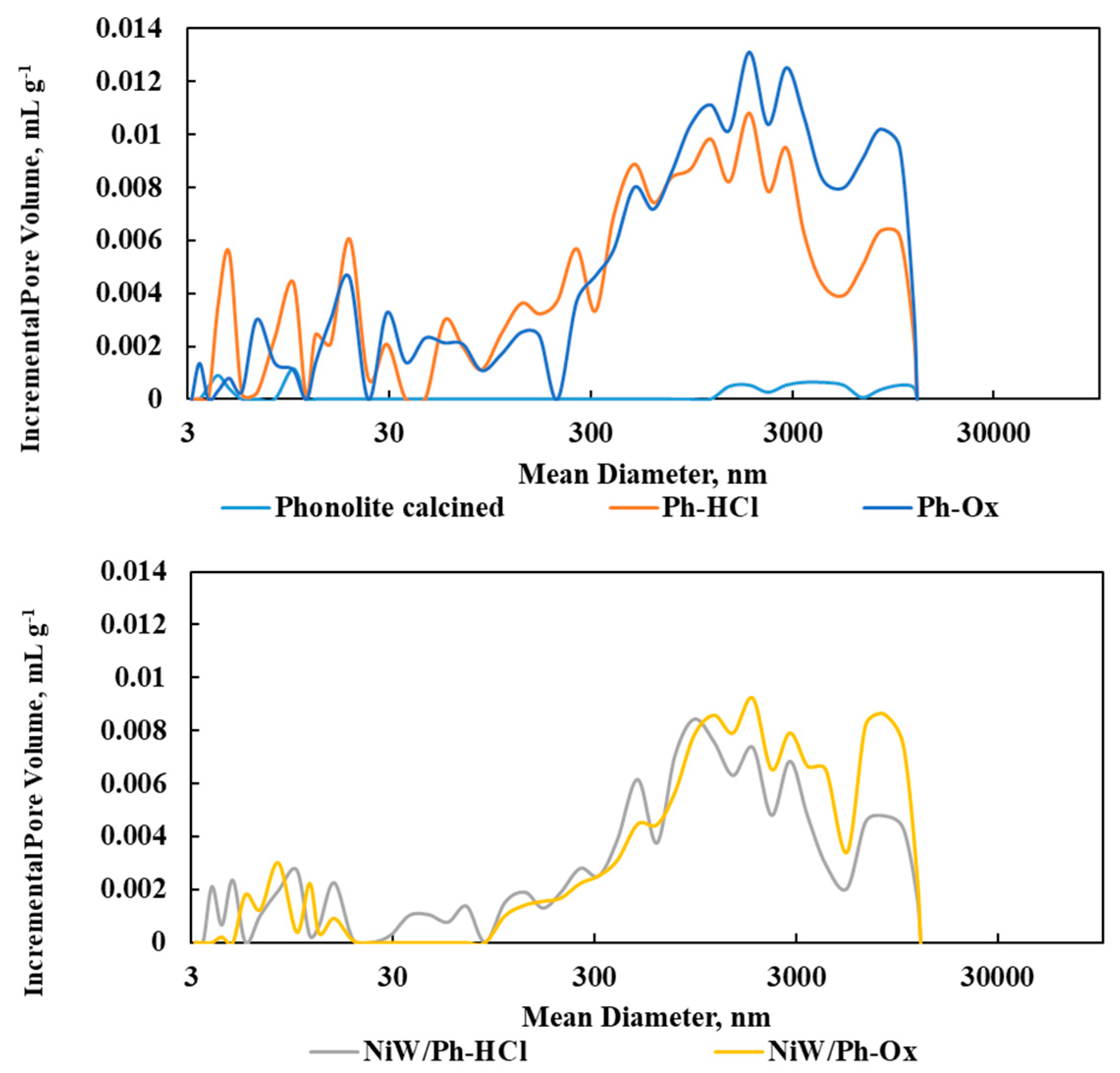
The basic raw material for the preparation of the catalysts (phonolite) has very poor textural properties, and its treatment with acid led to rapid changes in the material’s textural properties (an increase in pore volume and specific surface area). Acid leaching with oxalic acid resulted in a higher total pore volume than the sample leached with HCl. On the other hand, a higher volume of “mesopores” (i.e., pores with dimensions of 3 to 50 nm) can be seen here (
Table 2,
Figure 1). The measured parameters for catalysts prepared on the basis of these modified phonolite supports were all lower in value, which is related to the blocking of the pores by the deposited active metals (Ni and W).
X-ray diffraction (XRD) patterns were acquired to obtain more information about the materials’ structures. The XRD diagrams (
Figure 2) were similar for all solids (i.e., the signals had the same angle). However, only peaks related (XRD patterns) to minerals from the feldspar group were clearly found (sanidine ((PDF 19-1227), analcime (PDF 72-0445), and nepheline (PDF 70-1582) diagrams) [
15].
There was no evidence of NiO crystal phase for the solids containing Ni at 2
θ about 38° (111), 43° (200), and 62.5° (220) [
16]. In addition, the diffraction peaks at 2
θ = 32 and 35° assigned to the reflections associated with tungsten oxide (WO
3, (220)) [
17] were not clear. This shows that the active phase is very well distributed on the support surface.
Ammonia temperature programmed desorption (NH
3-TPD) characterization was carried out to study the acidity of the supports prior to being impregnated (
Figure 3). Ph-HCl presented two main zones of desorption peaks: the first at 100–400 °C and the second at 400–800 °C. The peaks from 100 to 250 °C correspond to weak and intermediate acid sites, those from 250 to 400 °C correspond to intermediate acid sites, and those at >400 °C correspond to strong acid sites. PhOx presented more strong acid sites than Ph-HCl, with the largest number of weak acid sites at 130 °C (100–200 °C temperature zone) and a larger amount of stronger acid sites as represented in the curve from 200 to 800 °C. However, from 450 °C, the observed signals are not only due to the desorption of ammonia, but also to water produced from the dehydroxylation of –OH surface groups.
Ph-Ox presented a larger number of strong acid sites compared to Ph-HCl. However, Ph-HCl presented many more weak–intermediate acid sites than Ph-Ox. Thus, these two supports presented different acidity values, as shown in
Figure 3. The total amount of desorbed ammonia for the temperature up to a maximum of 450 °C is shown in
Table 3.
2.2. Hydrotreating Tests Results
The DO and HDS mass balance results are shown in
Table 4. Large-scale production of gases was found for all the tests due to the decomposition of oxalic acid to gases and water (CO + CO
2 + H
2O). This decomposition has previously been reported [
5,
18,
19,
20,
21,
22]. In our case, oxalic acid decomposition appeared to yield only CO
2 and H
2, as shown in
Table 5. However, the CO that was being produced after each decomposition was rather reacting with water, producing CO
2 and H
2 [
23]. Even some oxygenated compounds were created and retained in the aqueous phases (liquid products) and in the solid products (
Table 2).
For DO products, an organic phase immiscible with a small amount of the aqueous phase was produced. Some solids were also produced when the phonolite-derived catalysts were used. No solids were found for the commercial catalysts compared to the solids produced by the two phonolite-derived catalysts. This solid production could be due to the reaction of glycerol [
24]. Here, glycerol can be transformed into propane and propylene, and into carbonaceous solids when phonolite catalysts are present, in which case the gaseous products include lower amounts of C3 gases (propylene, propane) (
Table 5). The higher Ni and W contents in the SiO
2–Al
2O
3 commercial material could explain the lower production of solid products. The production of aqueous phase was due to water production.
For the HDS tests, the amount of aqueous phase was higher compared to the DO tests, which presented a lower wt% of organic liquid products. Solid products were found for all HDS tests—in this case, presenting a higher quantity of solid products for the commercial catalyst but a lower amount of the product compared to the aqueous phase, indicating the possible production of polar compounds (water and others) that favor the final production of solids. Another reason for solid carbonaceous production is the commercial catalyst’s greater cracking capacity, resulting from its higher tungsten content, which thereby provides higher content of gases in its products, with more methane and less hydrogen. This is indicative of a hydrocracking reaction, with the gas production indicating higher solid production.
The simulated distillation (SimDis) (
Figure 4,
Table 5) of the organic liquids for the DO tests indicated that phonolite-derived materials were able to catalyze the production of only 17–18 wt% of paraffin (C17) and 4 wt% of lighter compounds from the RSO oil. The commercial catalyst was able to catalyze the production of 69 wt% of C17 compounds, with 5 wt% of other lighter compounds. Thus, the commercial solid was more capable of deoxygenating the RSO oil than the phonolite materials, which presented similar activity (NiW/Ph-HCl and NiW/Ph-Ox).
For the HDS tests, an elemental analysis for % C, H, N, and S of the organic liquid phase was performed, in which similar results were obtained for the hydrogen and carbon contents (
Table 6). This is the first time that oxalic acid was used for the HDS reaction. In this case, the atmospheric gas oil was used as feedstock, and the HDS decreased from 1 wt% to 0.2 and 0.5 wt% for tests using commercial and phonolite catalysts, respectively. The sulfur contents differed for each product depending on each test. The highest decrease in sulfur content was found for products using the catalyst NiW/Comm. Phonolite catalysts presented a similar sulfur decrease (0.56 and 0.52 wt% sulfur content for NiW/Ph-HCl and NiW/Ph-Ox, respectively, compared to 1 wt% sulfur content in the feedstock).
The density decreased in all cases after the HDS tests, with the product obtained from the NiW/Comm catalyst having a lower density. This product also presented the lowest refractive index. As shown in the literature, the refractive index and density are closely related properties [
19].
The SimDis results (
Figure 5) were similar for the feedstock and products, with the products having a slightly higher content of lighter compounds. The phonolite catalysts presented similar SimDis results, and NiW/Comm presented the highest increase in light compounds due to cracking during the reaction, producing not only methane (
Table 5), but also other lighter compounds, as shown in
Figure 5 (and having the greatest amount of solid products).
The gaseous products (
Table 7) were mainly CO
2 and hydrogen. Methane, ethane, propane, and propylene were found as secondary products. An unusually high content of propane and propylene was found under the DO tests using NiW/Comm, which could be due to the conversion of glycerol into propane and propylene.
For HDS tests (
Table 5), the main gases were CO
2 and hydrogen, with methane and ethane being the main byproducts. The hydrogen sulfide content was similar for the phonolite catalysts, but low for the test carried out using NiW/Comm, indicating that some sulfur could possibly be transferred to the solid residual product.