Nanomechanical Properties of Articular Cartilage Due to the PRP Injection in Experimental Osteoarthritis in Rabbits
Abstract
1. Introduction
2. Results
2.1. Histologic Findings according to the Non-Treated and PRP-Injected Knees
2.2. Submicron Surface Morphology of Rabbits Cartilage and the Mechanical Properties of Rabbits
3. Discussion
4. Methods
4.1. Induced Rabbits Model of Osteoarthritis
4.2. Platelet-Rich Plasma (PRP) Preparation
4.3. Histologic Parameters
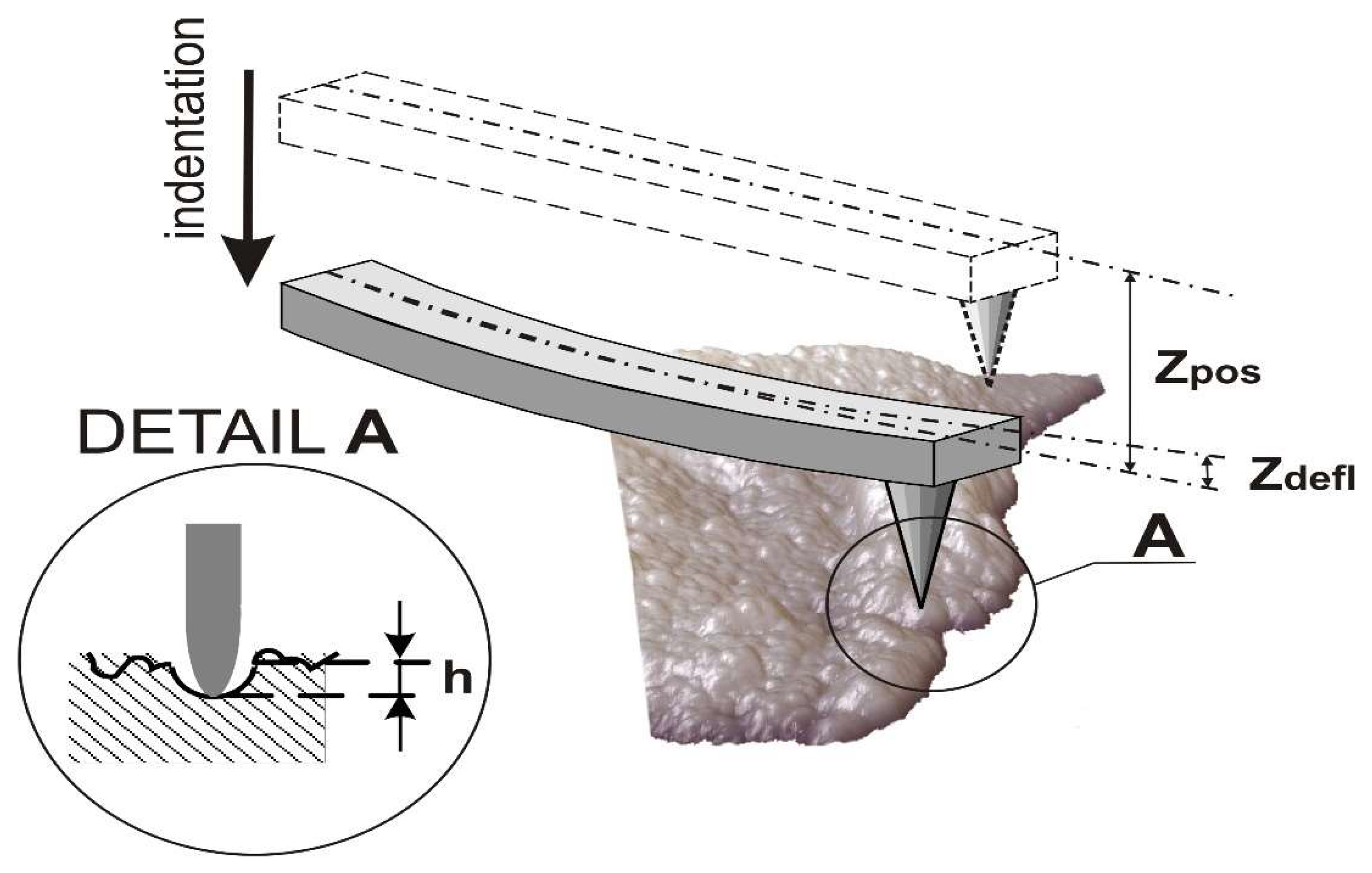
4.4. Measurement Protocol for AFM Mechanical Properties of Rabbits Cartilage
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murphy, L.B.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008, 59, 1207–1213. [Google Scholar] [CrossRef]
- Leitch, K.M.; Birmingham, T.B.; Jones, I.C.; Giffin, J.R.; Jenkyn, T.R. In-shoe plantar pressure measurements for patients with knee osteoarthritis: Reliability and effects of lateral heel wedges. Gait Posture 2011, 34, 391–396. [Google Scholar] [CrossRef]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e1. [Google Scholar] [CrossRef]
- Vos, T. Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef]
- Duncan, R.; Peat, G.; Thomas, E.; Hay, E.; McCall, I.; Croft, P. Symptoms and radiographic osteoarthritis: Not as discordant as they are made out to be? Ann. Rheum. Dis. 2006, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Zhang, Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. N. Am. 2012, 39, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.H. Osteoarthrosis. Can. Fam. Physician Med. Fam. Can. 1984, 30, 1503–1507. [Google Scholar]
- Danalache, M.; Tiwari, A.; Sigwart, V.; Hofmann, U.K. Application of Atomic Force Microscopy to Detect Early Osteoarthritis. J. Vis. Exp. 2020, 159, e61041. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; Choi, H.K. Epidemiology of gout. Rheum. Dis. Clin. N. Am. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Van Zwieten, K.J.; Lambrichts, I.; Baker, B.; Kosten, L.; De Munter, S.; Gervois, P.; Schmidt, K.; Lippens, P. Some morphological substrates in the pathogenesis of arthritis, Conference: FlandersBio’s Annual Life Sciences Convention Knowledge For Growth 2015. In Proceedings of the ICC, 11th Edition of Europe’s Leading Regional Life Sciences Convention, Ghent, Belgium, 21 May 2015. [Google Scholar] [CrossRef]
- Kuyinu, E.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Cook, J.L.; Gerwin, N.; Glasson, S.; Laverty, S.; Little, C.; McIlwraith, C.; Kraus, V.B. Histopathology atlas of animal model systems–Overview of guiding principles. Osteoarthr. Cartil. 2010, 18, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Hoso, M. Long-term histopathological developments in knee-joint components in a rat model of osteoarthritis induced by monosodium iodoacetate. J. Phys. Ther. Sci. 2017, 29, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.K. The effect of intra-articular allogenic platelet rich plasma in Dunkin-Hartley guinea pig model of knee osteoarthritis. Muscle Ligaments Tendons J. 2017, 7, 426–434. [Google Scholar] [CrossRef]
- Tsezou, A. Osteoarthritis Year in Review 2014: Genetics and genomics. Osteoarthr. Cartil. 2014, 22, 2017–2024. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2018, 15, 77–90. [Google Scholar] [CrossRef]
- Wojtowicz, A. Comparison of efficiency of platelet rich plasma, hematopoietic stem cells and bone marrow in augmentation of mandibular bone defects. N.Y. State Dent J. 2007, 73, 41–45. [Google Scholar]
- Gato-Calvo, L.; Magalhaes, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-rich plasma in osteoarthritis treatment: Review of current evidence. Ther. Adv. Chronic Dis. 2019, 10, 2040622319825567. [Google Scholar] [CrossRef]
- Mehrabani, D.; Seghatchian, J.; Acker, J.P. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus. Apher. Sci. 2019, 58, 102675. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Xu, X.; Liu, J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis. Der Orthopäde 2019, 48, 239–247. [Google Scholar] [CrossRef]
- Sengul, A.T.; Buyukkkarabacak, Y.B.; Altunkaynak, B.Z.; Yetim, T.D.; Altun, G.Y.; Sengul, B.; Basoglu, A. Effects of platelet-rich plasma on cartilage regeneration after costal cartilage resection: A stereological and histopathological study. Acta Chir. Belg. 2016, 117, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.S.; Patel, S.; John, R. PRP in OA knee—Update, current confusions and future options. SICOT-J. 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, C.; Frustaci, I.; Armellin, E.; Condò, R.; Arcuri, C.; Cerroni, L. Autologous blood preparations rich in platelets, fibrin and growth factors. Oral Implant. 2016, 8, 96–113. [Google Scholar]
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014, 4, CD010071. [Google Scholar] [CrossRef] [PubMed]
- Sadabad, H.N.; Behzadifar, M.; Arasteh, F.; Behzadifar, M.; Dehghan, H. Efficacy of Platelet-Rich Plasma versus Hyaluronic Acid for treatment of Knee Osteoarthritis: A systematic review and meta-analysis. Electron. Physician 2016, 8, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review With Quantitative Synthesis. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2037–2048. [Google Scholar] [CrossRef]
- Amiel, D.; Toyoguchi, T.; Kobayashi, K.; Bowden, K.; Amiel, M.E.; Healey, R.M. Long-term effect of sodium hyaluronate (Hyalgan) on osteoarthritis progression in a rabbit model. Osteoarthr. Cartil. 2003, 11, 636–643. [Google Scholar] [CrossRef]
- Schmidt, M.; Chen, E.; Lynch, S. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef]
- Song, S.U.; Hong, Y.-J.; Oh, I.-S.; Yi, Y.; Choi, K.B.; Lee, J.W.; Park, K.-W.; Han, J.-U.; Suh, J.-K.; Lee, K.H. Regeneration of Hyaline Articular Cartilage with Irradiated Transforming Growth Factor β1-Producing Fibroblasts. Tissue Eng. 2004, 10, 665–672. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.Y.; Niu, H.J.; Zhang, W.J.; Feng, Q.J.; Che, W.F. Access An ultrasound study of altered hydration behaviour of proteoglycan-degraded articular cartilage. BMC Musculoskelet. Disord. 2013, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Pauk, J.; Wasilewska, A.; Ihnatouski, M. Infrared Thermography Sensor for Disease Activity Detection in Rheumatoid Arthritis Patients. Sensors 2019, 19, 3444. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.T.; Casula, V.; Nevalainen, M.T.; Saarakkala, S. Osteoarthritis year in review 2018: Imaging. Osteoarthr. Cartil. 2019, 27, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Gahunia, H.K.; Lemaire, C.; Cross, A.R.; Babyn, P.; Kessler, M.J.; Pritzker, K.P.H. Osteoarthritis in Rhesus Macaques: Assessment of Cartilage Matrix Quality by Quantitative Magnetic Resonance Imaging. Jt. Destr. Arthritis Osteoarthr. 1993, 39, 255–259. [Google Scholar] [CrossRef]
- Chizhik, S.A.; Arushko, A.V.; Wierzcholski, K. Structure and elastic properties of a cartilage at micro- and nanolevel. Russ. J. Biomech. 2008, 12, 13–22. [Google Scholar]
- Wilusz, R.E.; DeFrate, L.E.; Guilak, F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J. R. Soc. Interface 2012, 9, 2997–3007. [Google Scholar] [CrossRef]
- Krenev, L.; Volkov, S.S.; Sadyrin, E.V.; Zubar’, T.I.; Chizhik, S.A. Mechanical Material Tests by the Nanoindentation Method at Various Indenter and Specimen Temperatures. J. Eng. Phys. Thermophys. 2018, 91, 594–600. [Google Scholar] [CrossRef]
- Zubar, T.I.; Chizhik, S.A. Studying Nanotribological Properties of Functional Materials via Atomic Force Microscopy. J. Frict. Wear 2019, 40, 201–206. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Starodubtseva, M.; Yegorenkov, N.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef]
- Abetkovskaia, S.O.; Chizhik, S.A.; Rudnitsky, V.A.; Kren, A.P. Evaluation of viscoelastic properties of materials by nanoindentation. J. Frict. Wear 2010, 31, 180–183. [Google Scholar] [CrossRef]
- Youssef, D.; El-Ghandoor, H.; Kandel, H.; El-Azab, J.; Elnaby, S.H. Estimation of Articular Cartilage Surface Roughness Using Gray-Level Co-Occurrence Matrix of Laser Speckle Image. Materials 2017, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dang, D.; Li, W.J.; Xi, N.; Wang, Y. Imaging and Force Recognition of Single Molecular Behaviors Using Atomic Force Microscopy. Sensors 2017, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips. J. Bone Jt. Surg.-Am. Vol. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A Review of Translational Animal Models for Knee Osteoarthritis. Arthritis 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Ochi, M.; Cucchiarini, M.; Pape, D.; Seil, R. Large animal models in experimental knee sports surgery: Focus on clinical translation. J. Exp. Orthop. 2015, 2, 9. [Google Scholar] [CrossRef]
- Hunziker, E.B. Biologic repair of articular cartilage: Defect models in experimental animals and matrix requirements. Clin. Orthop. Relat. Res. 1999, 367, 135–146. [Google Scholar] [CrossRef]
- Chevrier, A.; Kouao, A.S.M.; Picard, G.; Hurtig, M.B.; Buschmann, M.D. Interspecies comparison of subchondral bone properties important for cartilage repair. J. Orthop. Res. 2014, 33, 63–70. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Thompson, E.; Matsiko, A.; Schepens, A.; Gleeson, J.P.; O’Brien, F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016, 32, 149–160. [Google Scholar] [CrossRef]
- Yoshimi, T.; Kikuchi, T.; Obara, T.; Yamaguchi, T.; Sakakibara, Y.; Itoh, H.; Iwata, H.; Miura, T. Effects of High-Molecular-Weight Sodium Hyaluronate on Experimental Osteoarthrosis Induced by the Resection of Rabbit Anterior Cruciate Ligament. Clin. Orthop. Relat. Res. 1994, 298, 296–304. [Google Scholar] [CrossRef]
- Funayama, A.; Niki, Y.; Matsumoto, H.; Maeno, S.; Yatabe, T.; Morioka, H.; Yanagimoto, S.; Taguchi, T.; Tanaka, J.; Toyama, Y. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J. Orthop. Sci. 2008, 13, 225–232. [Google Scholar] [CrossRef]
- Kwon, D.R.; Park, G.Y.; Lee, S.-U. The Effects of Intra-Articular Platelet-Rich Plasma Injection According to the Severity of Collagenase-Induced Knee Osteoarthritis in a Rabbit Model. Ann. Rehabil. Med. 2012, 36, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Takahashi, K.A.; Arai, E.; Inoue, A.; Sakao, K.; Tonomura, H.; Honjo, K.; Nakagawa, S.; Inoue, H.; Tabata, Y.; et al. Intraarticular administration of platelet-rich plasma prevents osteoarthritis progression in the rabbit knee. Clin. Exp. Rheumatol. 2009, 27, 201–207. [Google Scholar] [PubMed]
- Serra, C.I.; Soler, C.; Carrillo, J.M.; Sopena, J.J.; Redondo, J.I.; Cugat, R. Erratum to: Effect of autologous platelet-rich plasma on the repair of full-thickness articular defects in rabbits. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 1710. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, Y.; Zhang, C.Q.; Chen, S.B.; Cheng, X.G. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int. Orthop. 2009, 34, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.S.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Kiio, T.M.; Park, S. Nano-scientific Application of Atomic Force Microscopy in Pathology: From Molecules to Tissues. Int. J. Med. Sci. 2020, 17, 844–858. [Google Scholar] [CrossRef]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent progressive use of atomic force microscopy in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef]
- Jin, H.; Liang, Q.; Chen, T.; Wang, X. Resveratrol Protects Chondrocytes from Apoptosis via Altering the Ultrastructural and Biomechanical Properties: An AFM Study. PLoS ONE 2014, 9, e91611. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I.Y. If Cell Mechanics Can Be Described by Elastic Modulus: Study of Different Models and Probes Used in Indentation Experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.B.; Youn, I.; Cao, L.; Leddy, H.A.; Gilchrist, C.L.; Setton, L.A.; Guilak, F. Zonal changes in the three-dimensional morphology of the chondron under compression: The relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J. Biomech. 2007, 40, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, L.G.; Williams, G.M.; Upton, M.L.; Setton, L.A.; Guilak, F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J. Biomech. 2005, 38, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.L.; Dimitriadis, E.K.; Mertz, E.L.; Horkay, F. Microscale mapping of extracellular matrix elasticity of mouse joint cartilage: An approach to extracting bulk elasticity of soft matter with surface roughness. Soft Matter 2018, 14, 2879–2892. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, P.R.; Pouran, B.; Korthagen, N.; Zadpoor, A.A.; Weinans, H. Guidelines for an optimized indentation protocol for measurement of cartilage stiffness: The effects of spatial variation and indentation parameters. J. Biomech. 2016, 49, 3602–3607. [Google Scholar] [CrossRef]
- Ihnatouski, M.; Pauk, J.; Karev, D.; Karev, B. AFM-Based Method for Measurement of Normal and Osteoarthritic Human Articular Cartilage Surface Roughness. Materials 2020, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
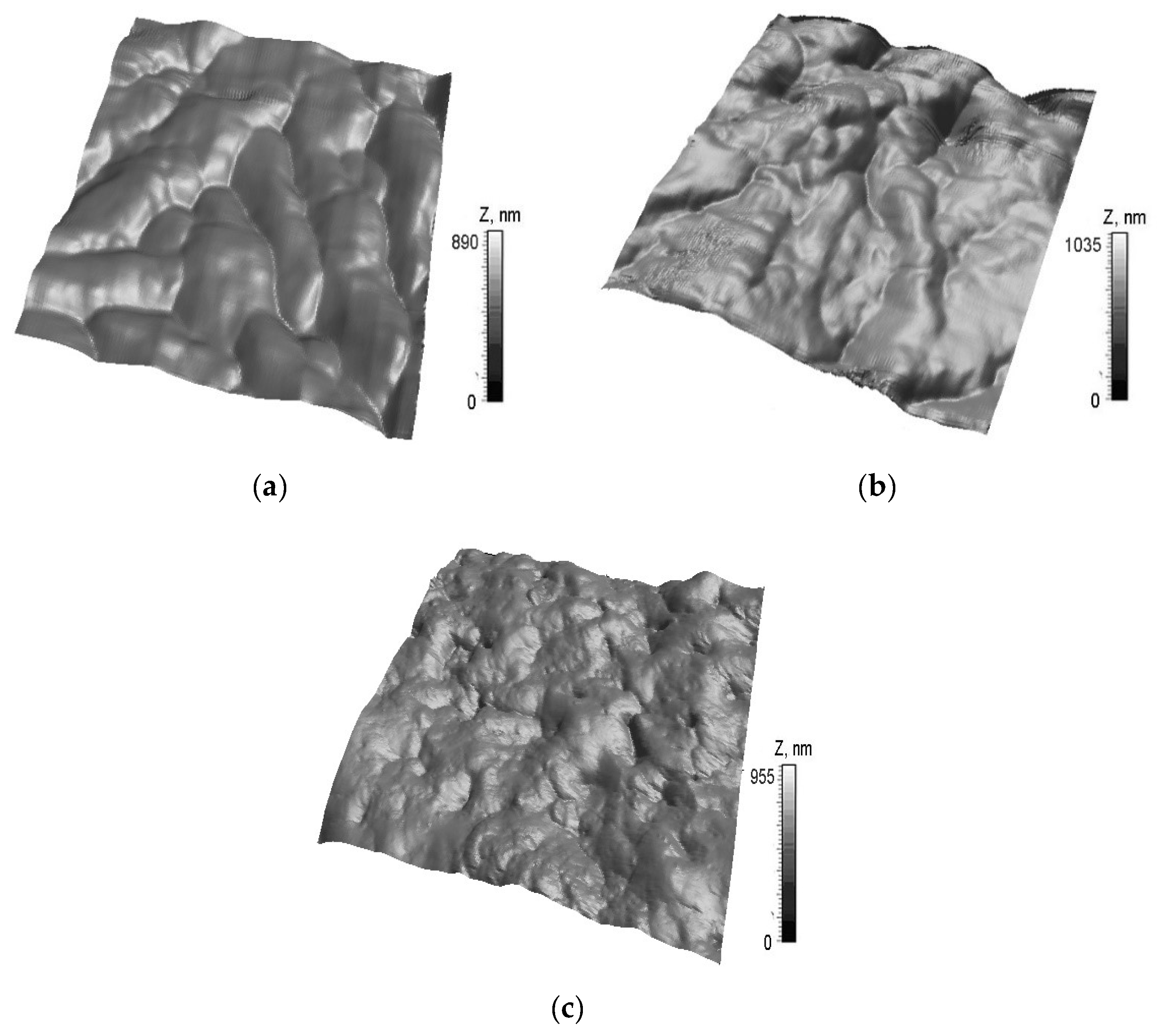
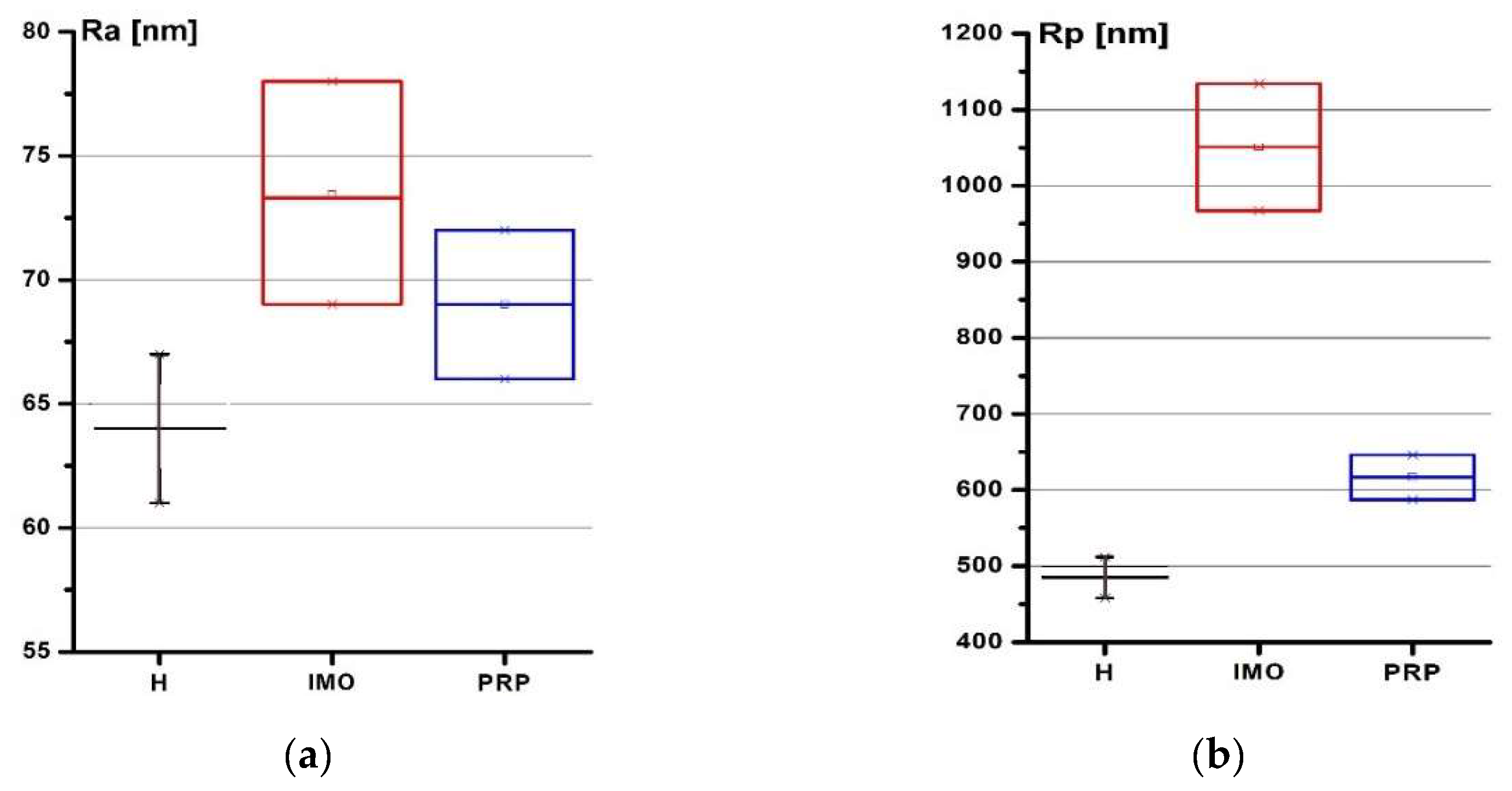

| h, nm | Group 1, MPa (std) | Group 2, MPa (std) | Group 3, MPa (std) |
|---|---|---|---|
| 25 | 1.95 (0.035) | 1.60 (0.027) * | 1.71 (0.022) * |
| 50 | 1.88 (0.046) | 1.53 (0.047) * | 1.62 (0.038) * |
| 75 | 1.37 (0.046) | 1.40 (0.050) * | 1.50 (0.070) * |
| 100 | 0.94 (0.020) | 1.04 (0.183) ** | 1.22 (0.075) ** |
| 125 | 0.75 (0.035) | 0.79 (0.131) ** | 0.87 (0.029) ** |
| 150 | 0.64 (0.029) | 0.70 (0.082) ** | 0.71 (0.024) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihnatouski, M.; Pauk, J.; Karev, B.; Karev, D. Nanomechanical Properties of Articular Cartilage Due to the PRP Injection in Experimental Osteoarthritis in Rabbits. Molecules 2020, 25, 3734. https://doi.org/10.3390/molecules25163734
Ihnatouski M, Pauk J, Karev B, Karev D. Nanomechanical Properties of Articular Cartilage Due to the PRP Injection in Experimental Osteoarthritis in Rabbits. Molecules. 2020; 25(16):3734. https://doi.org/10.3390/molecules25163734
Chicago/Turabian StyleIhnatouski, Mikhail, Jolanta Pauk, Boris Karev, and Dmitrij Karev. 2020. "Nanomechanical Properties of Articular Cartilage Due to the PRP Injection in Experimental Osteoarthritis in Rabbits" Molecules 25, no. 16: 3734. https://doi.org/10.3390/molecules25163734
APA StyleIhnatouski, M., Pauk, J., Karev, B., & Karev, D. (2020). Nanomechanical Properties of Articular Cartilage Due to the PRP Injection in Experimental Osteoarthritis in Rabbits. Molecules, 25(16), 3734. https://doi.org/10.3390/molecules25163734







