Implementation of Bismuth Chalcogenides as an Efficient Anode: A Journey from Conventional Liquid Electrolyte to an All-Solid-State Li-Ion Battery
Abstract
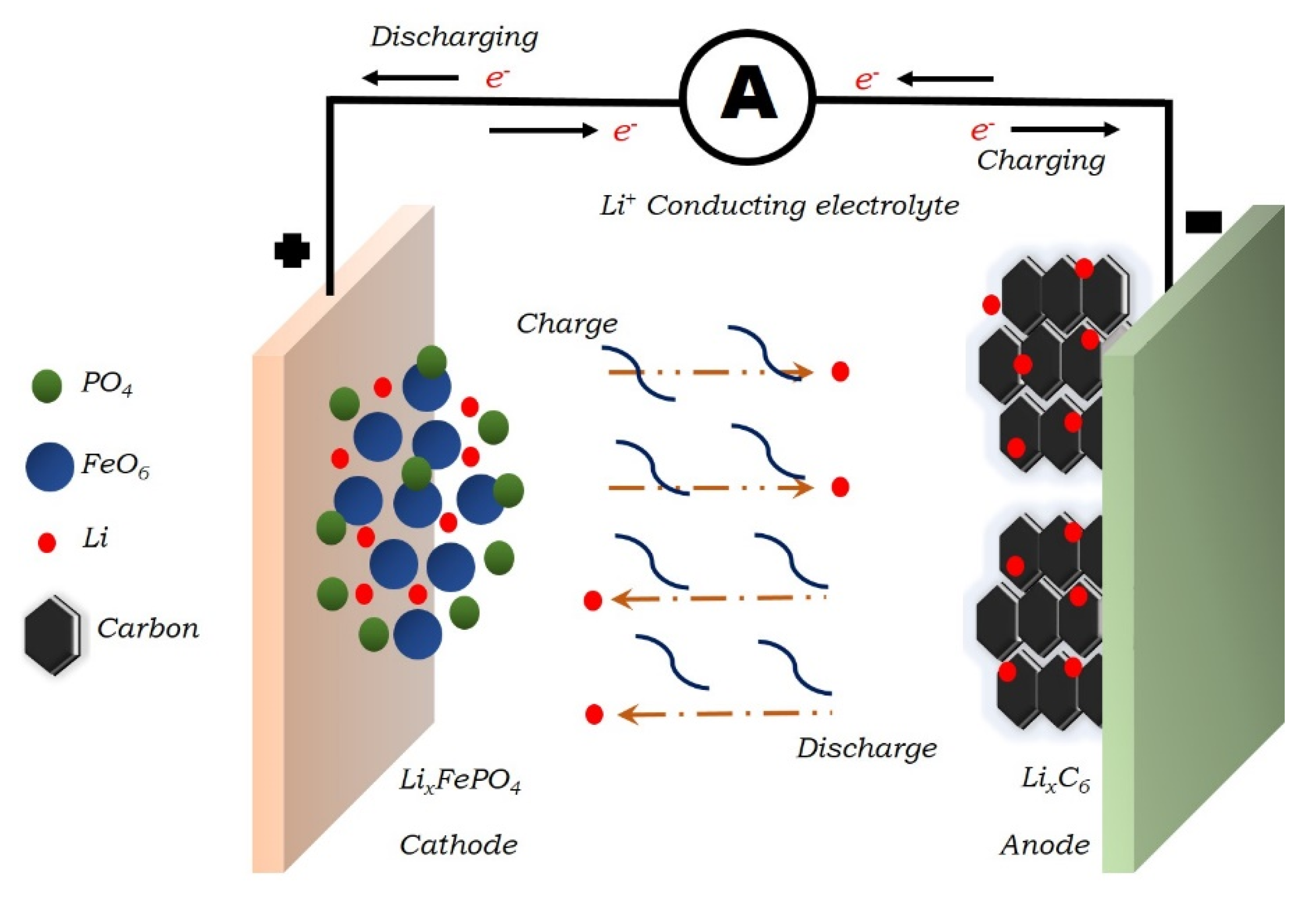
:1. Introduction
2. Development of Bi-Based Anodes for Li-Ion Batteries
- Discharging: Bi (Rhombohedral)→LiBi (Tetragonal)→Li3Bi (Cubic).
- Charging: Li3Bi (Cubic)→LiBi (Tetragonal)→Bi (Rhombohedral).
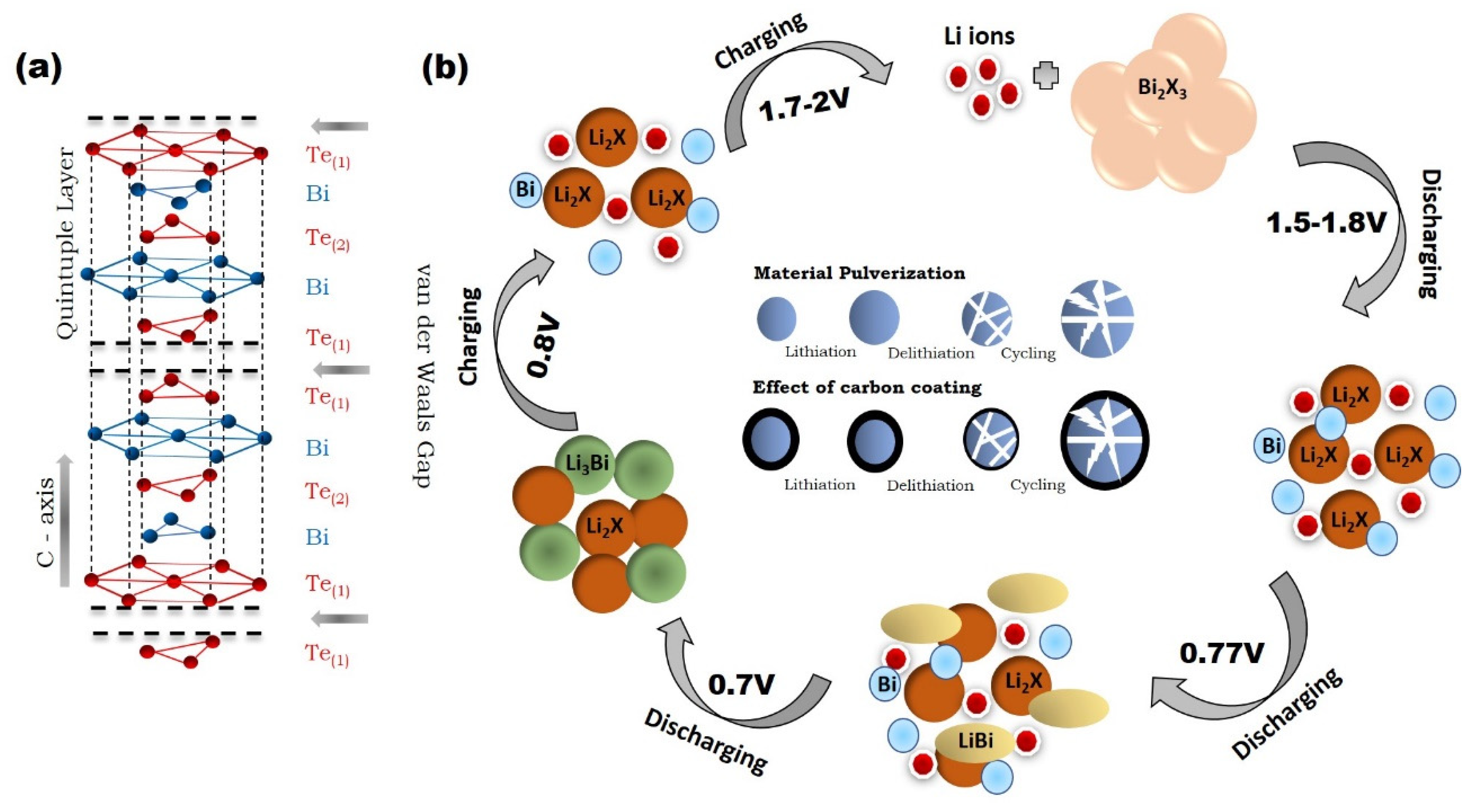
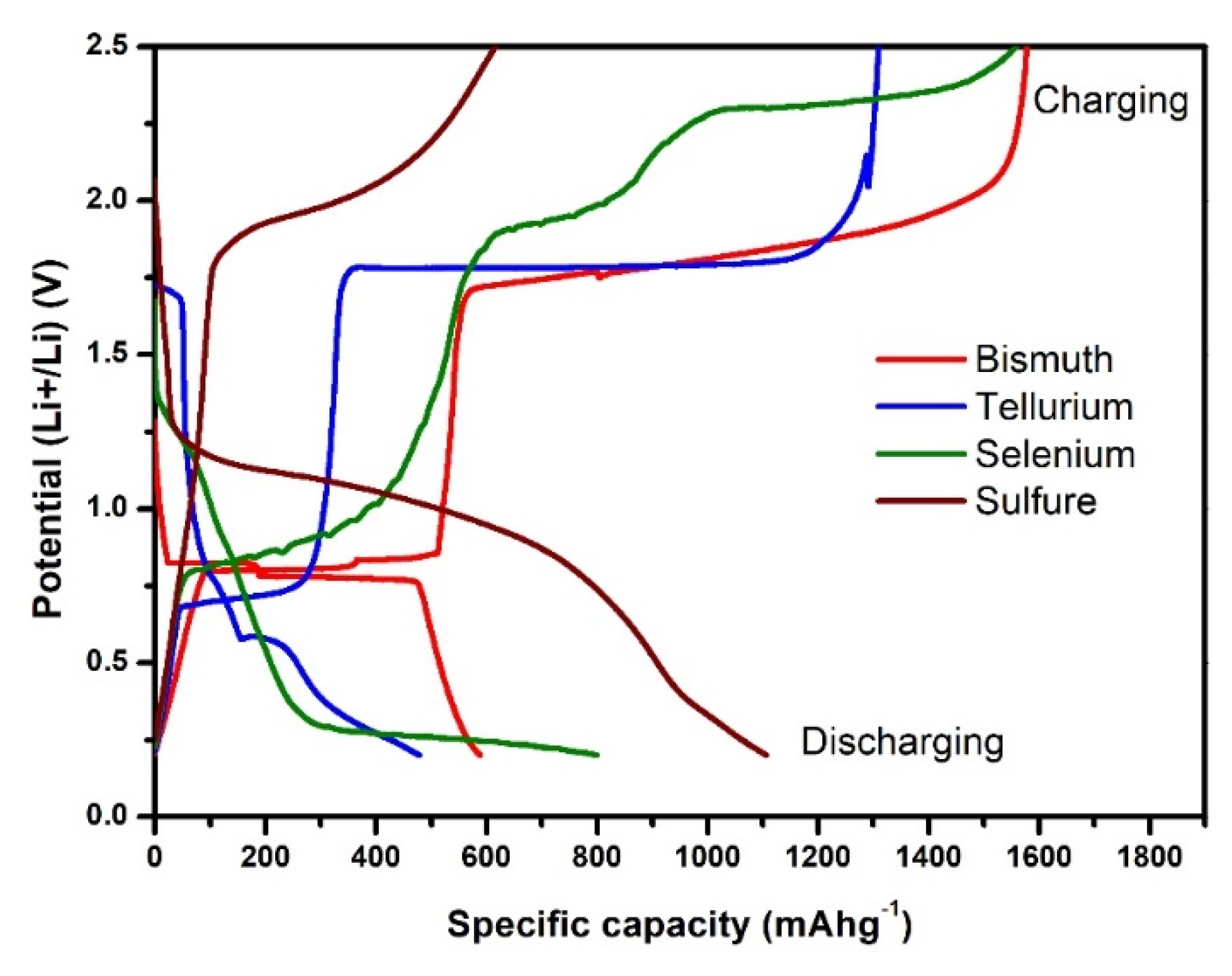
3. Reaction Mechanism of Bismuth Based Chalcogenides Bi2X3 (X = S, Se, and Te)
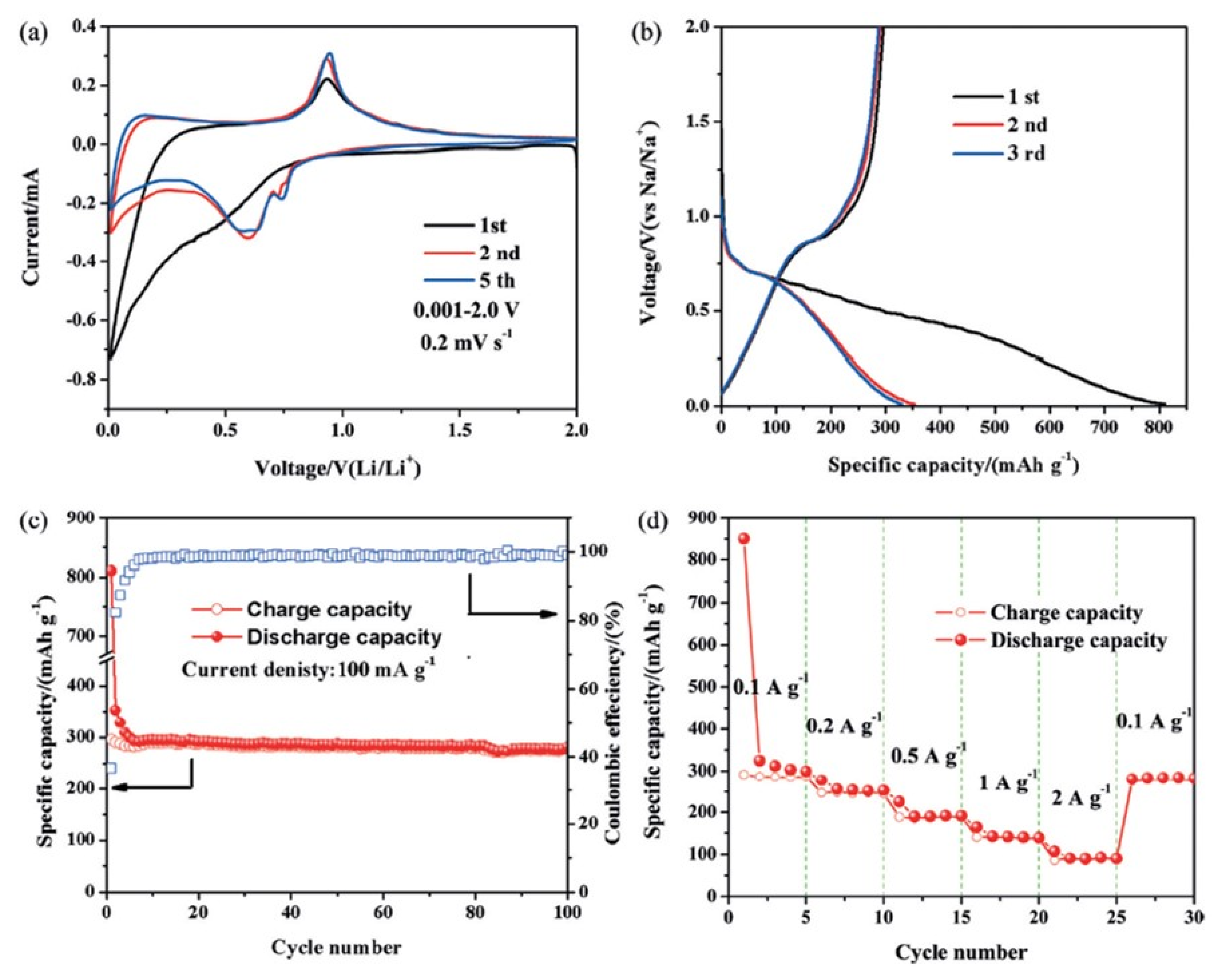
4. Bismuth Chalcogenides Materials as an Efficient Anode for Li-Ion Batteries
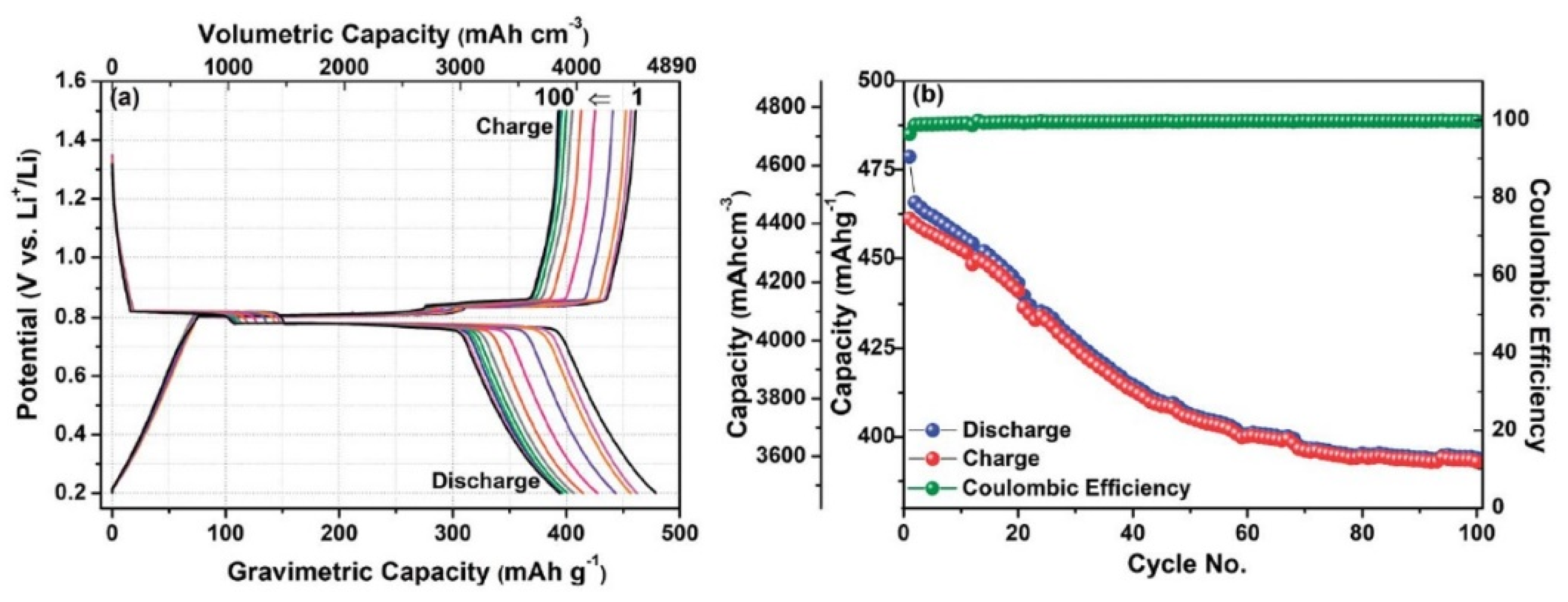
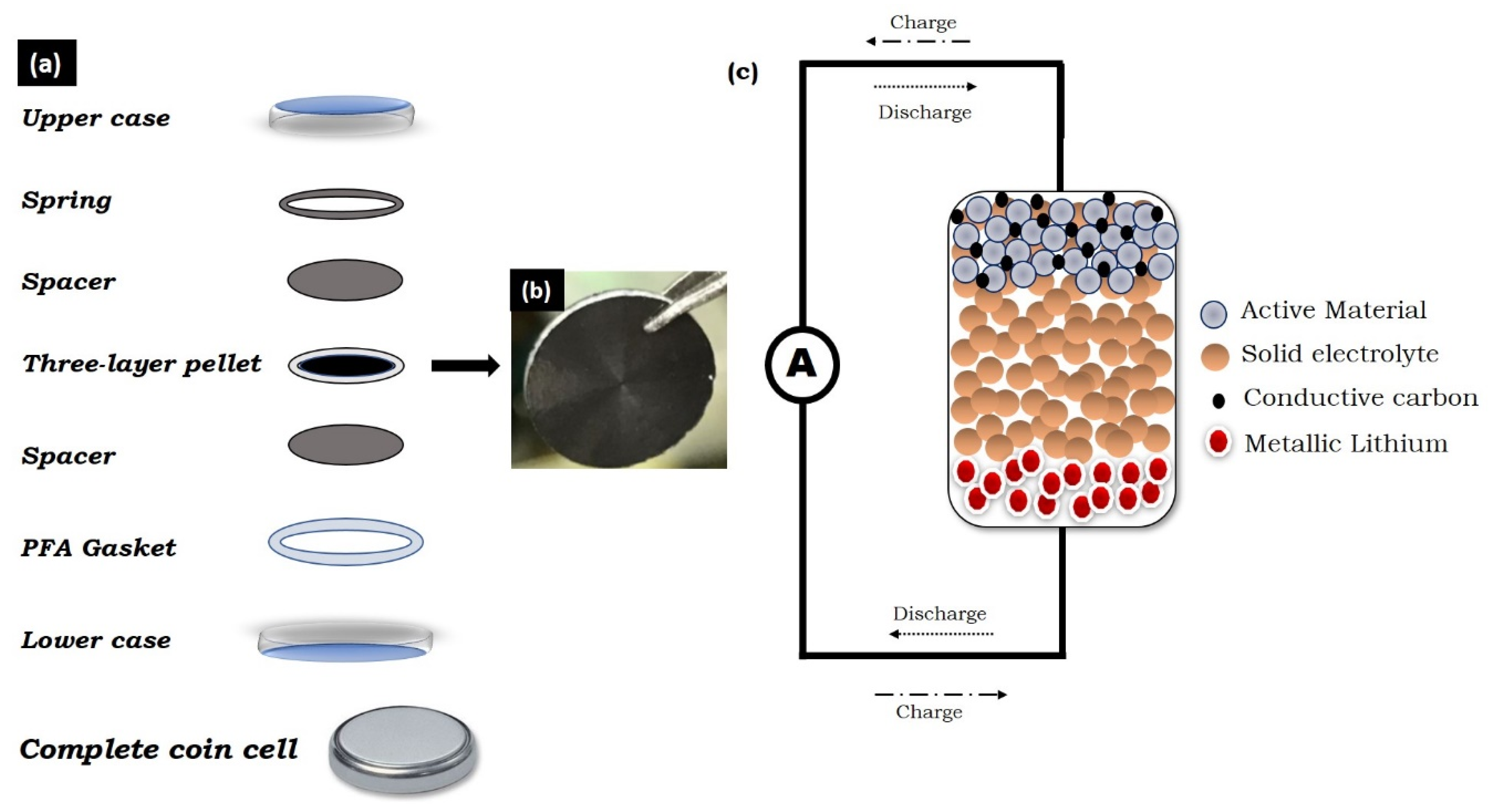
5. Bismuth Chalcogenides as the Anode in All-Solid-State Lithium-Ion Batteries
6. Summary and Future Outlook
Funding
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Abruña, H.D.; Kiya, Y.; Henderson, J.C. Batteries and electrochemical capacitors. Phys. Today 2008, 61, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells: Fundamentals Technology and Applications; Wiley: New York, NY, USA, 2003; Volume 2. [Google Scholar]
- Linden, D. Handbook of Batteries and Fuel Cells; McGraw-Hill Education: New York, NY, USA, 1984. [Google Scholar]
- Hovel, H.J. Solar cells. STIA 1975, 76, 20650. [Google Scholar]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent progress in Zn-based anodes for advanced lithium ion batteries. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Xu, J.-J.; Zhang, X.-B. Li–air batteries: Decouple to stabilize. Nat. Energy 2017, 2, 1–2. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Van Schalkwijk, W.; Scrosati, B. Advances in lithium ion batteries introduction. In Advances in Lithium-Ion Batteries; Springer: London, UK, 2002; pp. 1–5. [Google Scholar]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-solid-state lithium-ion microbatteries: A review of various three-dimensional concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef] [Green Version]
- Quartarone, E.; Mustarelli, P. Review—emerging trends in the design of electrolytes for lithium and post-lithium batteries. J. Electrochem. Soc. 2020, 167, 50508. [Google Scholar] [CrossRef]
- Tuller, H. Ionic conduction and applications BT. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: Basel, Switzerland, 2017; p. 1. ISBN 978-3-319-48933-9. [Google Scholar]
- Wang, Z.; Shao, G. Theoretical design of solid electrolytes with superb ionic conductivity: Alloying effect on Li+ transportation in cubic Li6PA5X chalcogenides. J. Mater. Chem. A 2017, 5, 21846–21857. [Google Scholar] [CrossRef] [Green Version]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent progress in all-solid-state Lithium−Sulfur batteries using high Li-ion conductive solid electrolytes. Electrochem. Energy Rev. 2019, 1–32. [Google Scholar] [CrossRef]
- Uitz, M.; Epp, V.; Bottke, P.; Wilkening, M. Ion dynamics in solid electrolytes for lithium batteries. J. Electroceramics. 2017, 38, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Blomgren, G.E. Liquid electrolytes for lithium and lithium-ion batteries. J. Power Sources 2003, 119–121, 326–329. [Google Scholar]
- Tirado, J.L. Inorganic materials for the negative electrode of lithium-ion batteries: State-of-the-art and future prospects. Mater. Sci. Eng. R Rep. 2003, 40, 103–136. [Google Scholar] [CrossRef]
- Zhang, W.-J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Masood, K.B.; Jain, N.; Singh, J. Electrochemical performance of Bi2Te3/GO composite anode for LIB application. Int. J. Appl. Ceram. Technol. 2020, 17, 1422–1429. [Google Scholar] [CrossRef]
- Wang, R.Y.; Feser, J.P.; Gu, X.; Yu, K.M.; Segalman, R.A.; Majumdar, A.; Milliron, D.J.; Urban, J.J. Universal and solution-processable precursor to bismuth chalcogenide thermoelectrics. Chem. Mater. 2010, 22, 1943–1945. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Omachi, A.; Goto, Y.; Kamihara, Y.; Matoba, M.; Hiroi, T.; Kajitani, J.; Miura, O. Enhancement of thermoelectric properties by Se substitution in layered bismuth-chalcogenide LaOBiS2-xSex. J. Appl. Phys. 2014, 116, 163915. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.Y.; Hogan, T.; Schindler, J.; Iordarridis, L.; Brazis, P.; Kannewurf, C.R.; Chen, B.; Uher, C.; Kanatzidis, M.G. Complex bismuth chalcogenides as thermoelectrics. In Proceedings of the XVI ICT’97. Proceedings ICT’97. 16th International Conference on Thermoelectrics (Cat. No.97TH8291), Dresden, Germany, 26–29 August 1997; IEEE: Piscataway, NJ, USA, 1997; pp. 459–462. [Google Scholar]
- Kim, S.; Lee, Y.-I.; Choi, Y.-M.; Lim, H.-R.; Lim, J.-H.; Myung, N.V.; Choa, Y.-H. Thermochemical hydrogen sensor based on chalcogenide nanowire arrays. Nanotechnology 2015, 26, 145503. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharyya, B.; Srivastava, A.K.; Senguttuvan, T.D.; Husale, S. High performance broadband photodetector using fabricated nanowires of bismuth selenide. Sci. Rep. 2016, 6, 19138. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.D.; Shao, J.M.; Yang, G.W. Ultra-broadband and high-responsive photodetectors based on bismuth film at room temperature. Sci. Rep. 2015, 5, 12320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, C.; Huang, W.; Xie, Z.; Zhao, J.; Ma, D.; Fan, T.; Liang, W.; Ge, Y.; Dong, B.; Li, J.; et al. Ultrasmall Bismuth quantum dots: Facile liquid-phase exfoliation, characterization, and application in high-performance UV–vis photodetector. ACS Photonics 2018, 5, 621–629. [Google Scholar] [CrossRef]
- Savariraj, A.D.; Vinoth, V.; Mangalaraja, R.V.; Arun, T.; Contreras, D.; Akbari-Fakhrabadi, A.; Valdés, H.; Banat, F. Microwave-assisted synthesis of localized surface plasmon resonance enhanced bismuth selenide (Bi2Se3) layers for non-enzymatic glucose sensing. J. Electroanal. Chem. 2020, 856, 113629. [Google Scholar] [CrossRef]
- Li, X.; Zhong, L.; Liu, R.; Wei, X.; Li, J. A molecularly imprinted photoelectrochemical sensor based on the use of Bi2S3 for sensitive determination of dioctyl phthalate. Microchim. Acta 2019, 186, 688. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, Z.-Y. Electronic structure and optical properties of bismuth chalcogenides Bi2Q3 (Q = O, S, Se, Te) by first-principles calculations. Comput. Mater. Sci. 2018, 142, 312–319. [Google Scholar] [CrossRef]
- Mishra, S.K.; Satpathy, S.; Jepsen, O. Electronic structure and thermoelectric properties of bismuth telluride and bismuth selenide. J. Phys. Condens. Matter 1997, 9, 461. [Google Scholar] [CrossRef]
- Ding, Z.; Bux, S.K.; King, D.J.; Chang, F.L.; Chen, T.-H.; Huang, S.-C.; Kaner, R.B. Lithium intercalation and exfoliation of layered bismuth selenide and bismuth telluride. J. Mater. Chem. 2009, 19, 2588–2592. [Google Scholar] [CrossRef]
- Julien, C.; Sararas, I.; Chevy, A. Studies of lithium insertion in bismuth chalcogenide compounds. Solid State Ion. 1989, 36, 113–120. [Google Scholar] [CrossRef]
- Bludská, J.; Jakubec, I.; Karamazov, S.; Horák, J.; Uher, C. Lithium ions in the van der Waals gap of Bi2Se3 single crystals. J. Solid State Chem. 2010, 183, 2813–2817. [Google Scholar] [CrossRef]
- Bludská, J.; Jakubec, I.; Lošťák, P.; Horák, J. Lithium intercalation into the tetradymite-type selenides Bi2-xInxSe3. Philos. Mag. B 1997, 76, 867–873. [Google Scholar] [CrossRef]
- Sarakonsri, T.; Johnson, C.S.; Hackney, S.A.; Thackeray, M.M. Solution route synthesis of InSb, Cu6Sn5 and Cu2Sb electrodes for lithium batteries. J. Power Sources 2006, 153, 319–327. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.B.; Cao, G.S.; Zhong, Y.D.; Zhao, M.J.; Tu, J.P. Solvothermal synthesis of nanosized CoSb2 alloy anode for Li-ion batteries. Electrochim. Acta 2005, 50, 1903–1907. [Google Scholar] [CrossRef]
- Chen, W.X.; Lee, J.Y.; Liu, Z. The nanocomposites of carbon nanotube with Sb and SnSb0.5 as Li-ion battery anodes. Carbon 2003, 41, 959–966. [Google Scholar]
- Hassoun, J.; Derrien, G.; Panero, S.; Scrosati, B. The role of the morphology in the response of Sb-C nanocomposite electrodes in lithium cells. J. Power Sources 2008, 183, 339–343. [Google Scholar] [CrossRef]
- Applestone, D.; Yoon, S.; Manthiram, A. Mo3Sb7–C composite anodes for lithium-ion batteries. J. Phys. Chem. C 2011, 115, 18909–18915. [Google Scholar] [CrossRef]
- Xianming, W.; Nishina, T.; Uchida, I. Lithium alloy formation at bismuth thin layer and its kinetics in propylene carbonate electrolyte. J. Power Sources 2002, 104, 90–96. [Google Scholar] [CrossRef]
- Park, C.-M.; Yoon, S.; Lee, S.-I.; Sohn, H.-J. Enhanced electrochemical properties of nanostructured bismuth-based composites for rechargeable lithium batteries. J. Power Sources 2009, 186, 206–210. [Google Scholar] [CrossRef]
- Yang, F.; Yu, F.; Zhang, Z.; Zhang, K.; Lai, Y.; Li, J. Bismuth nanoparticles embedded in carbon spheres as anode materials for sodium/lithium-ion batteries. Chem. Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Y.; Da, P.; Wu, H.; Xu, M.; Zheng, G. Indirect growth of mesoporous Bi@C core–shell nanowires for enhanced lithium-ion storage. Nanoscale 2014, 6, 13236–13241. [Google Scholar] [CrossRef]
- Crosnier, O.; Brousse, T.; Devaux, X.; Fragnaud, P.; Schleich, D.M. New anode systems for lithium ion cells. J. Power Sources 2001, 94, 169–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Manthiram, A. High-capacity, high-rate Bi–Sb alloy anodes for lithium-ion and sodium-ion batteries. Chem. Mater. 2015, 27, 3096–3101. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, B.; Li, S.; Xu, S.; Pan, Z.; Huang, Q.; Xing, L.; Wang, C.; Li, W. Bi nanoparticles anchored in N-doped porous carbon as anode of high energy density lithium ion battery. Nano-Micro Lett. 2018, 10, 56. [Google Scholar]
- Kumari, P.; Sharma, K.; Pal, P.; Kumar, M.; Ichikawa, T.; Jain, A. Highly efficient & stable Bi & Sb anodes using lithium borohydride as solid electrolyte in Li-ion batteries. RSC Adv. 2019, 9, 13077–13081. [Google Scholar]
- Jung, H.; Park, C.-M.; Sohn, H.-J. Bismuth sulfide and its carbon nanocomposite for rechargeable lithium-ion batteries. Electrochim. Acta 2011, 56, 2135–2139. [Google Scholar] [CrossRef]
- Kumari, P.; Awasthi, K.; Agarwal, S.; Ichikawa, T.; Kumar, M.; Jain, A. Flower-like Bi2S3 nanostructures as highly efficient anodes for all-solid-state lithium-ion batteries. RSC Adv. 2019, 9, 29549–29555. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Singh, R.; Awasthi, K.; Ichikawa, T.; Kumar, M.; Jain, A. Highly stable nanostructured Bi2Se3 anode material for all solid-state lithium-ion batteries. J. Alloys Compd. 2020, 838, 155403. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, D.; Ni, J.; Gao, L.; Yang, J.; Li, Y. One-pot facile fabrication of carbon-coated Bi2S3 nanomeshes with efficient Li-storage capability. Nano Res. 2014, 7, 765–773. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Huang, L.; Wang, X.; Qu, Y.; Lai, Y.; Li, J. Synthesis of bismuth sulfide/reduced graphene oxide composites and their electrochemical properties for lithium ion batteries. Electrochim. Acta 2013, 114, 88–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Lu, H.; Jia, M.; Lai, Y.; Li, J. Facile synthesis of dandelion-like Bi2S3 microspheres and their electrochemical properties for lithium-ion batteries. Mater. Lett. 2013, 91, 100–102. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Xia, H.; Zhang, L.; Jiang, J.; Shen, M.; Ni, J.; Gao, L. Branch-structured Bi2S3–CNT hybrids with improved lithium storage capability. J. Mater. Chem. A 2014, 2, 13854–13858. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Liu, T.; Zheng, H.; Gao, L.; Yan, C.; Li, L. Strongly coupled Bi2S3@ CNT hybrids for robust lithium storage. Adv. Energy Mater. 2014, 4, 1400798. [Google Scholar] [CrossRef]
- Tang, C.; Li, N.; Sheng, J.; Zhou, L.; He, L.; Zhu, J.; Li, F.; Liu, Y.; Mai, L. Facile synthesis of Bi2S3@ SiO2 core-shell microwires as high-performance anode materials for lithium-ion batteries. J. Electrochem. Soc. 2016, 164, A6110. [Google Scholar] [CrossRef]
- Hu, P.; Cao, Y.; Lu, B. Flowerlike assemblies of Bi2S3 nanorods by solvothermal route and their electrochemical hydrogen storage performance. Mater. Lett. 2013, 106, 297–300. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Lian, J.; Duan, X.; Kim, T.; Peng, P.; Liu, X.; Chen, Q.; Yao, G.; Zheng, W. Ionic liquids-assisted synthesis and electrochemical properties of Bi2S3 nanostructures. CrystEngComm 2011, 13, 3072–3079. [Google Scholar] [CrossRef]
- Zhou, H.; Xiong, S.; Wei, L.; Xi, B.; Zhu, Y.; Qian, Y. Acetylacetone-directed controllable synthesis of Bi2S3 nanostructures with tunable morphology. Cryst. Growth Des. 2009, 9, 3862–3867. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Jiao, L.; Wang, T.; Lian, J.; Duan, X.; Zheng, W. Bi2S3 nanomaterials: Morphology manipulation and related properties. Dalt. Trans. 2011, 40, 10100–10108. [Google Scholar] [CrossRef] [PubMed]
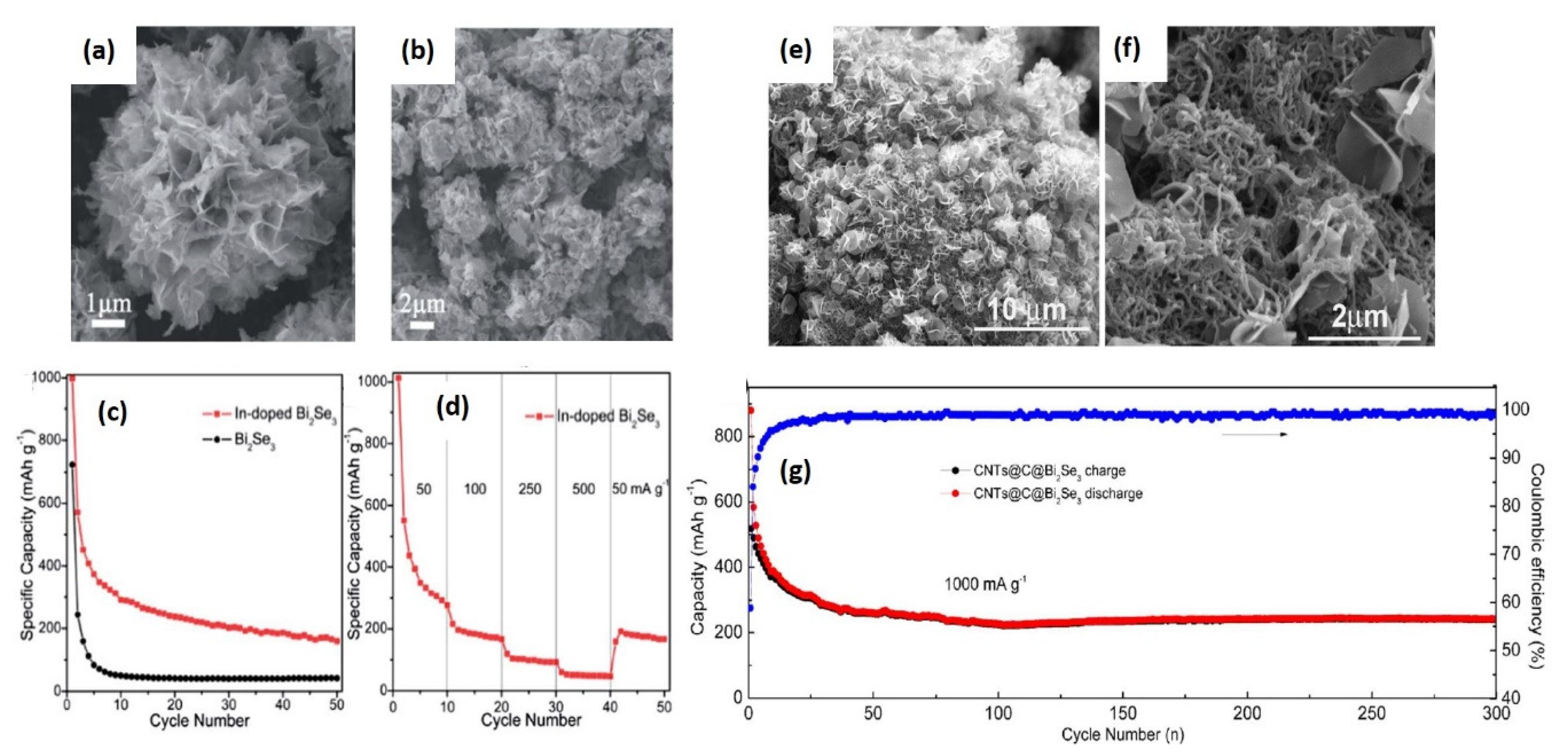
- Han, G.; Chen, Z.-G.; Ye, D.; Yang, L.; Wang, L.; Drennan, J.; Zou, J. In-doped Bi2Se3 hierarchical nanostructures as anode materials for Li-ion batteries. J. Mater. Chem. A 2014, 2, 7109–7116. [Google Scholar] [CrossRef]
- Ali, Z.; Cao, C.; Li, J.; Wang, Y.; Cao, T.; Tanveer, M.; Tahir, M.; Idrees, F.; Butt, F.K. Effect of synthesis technique on electrochemical performance of bismuth selenide. J. Power Sources 2013, 229, 216–222. [Google Scholar] [CrossRef]
- Jin, R.; Liu, J.; Xu, Y.; Li, G.; Chen, G.; Yang, L. Hierarchical Bi2Se3-xSx microarchitectures assembled from ultrathin polycrystalline nanosheets: Solvothermal synthesis and good electrochemical performance. J. Mater. Chem. A 2013, 1, 10942–10950. [Google Scholar] [CrossRef]
- Xu, H.; Chen, G.; Jin, R.; Pei, J.; Wang, Y.; Chen, D. Hierarchical Bi2Se3 microrods: Microwave-assisted synthesis, growth mechanism and their related properties. CrystEngComm 2013, 15, 1618–1625. [Google Scholar] [CrossRef]
- Jin, R.; Sun, M.; Li, G. CNTs@C@Bi2Se3 composite as an improved-performance anode for lithium ion batteries. Ceram. Int. 2017, 43, 17093–17099. [Google Scholar] [CrossRef]
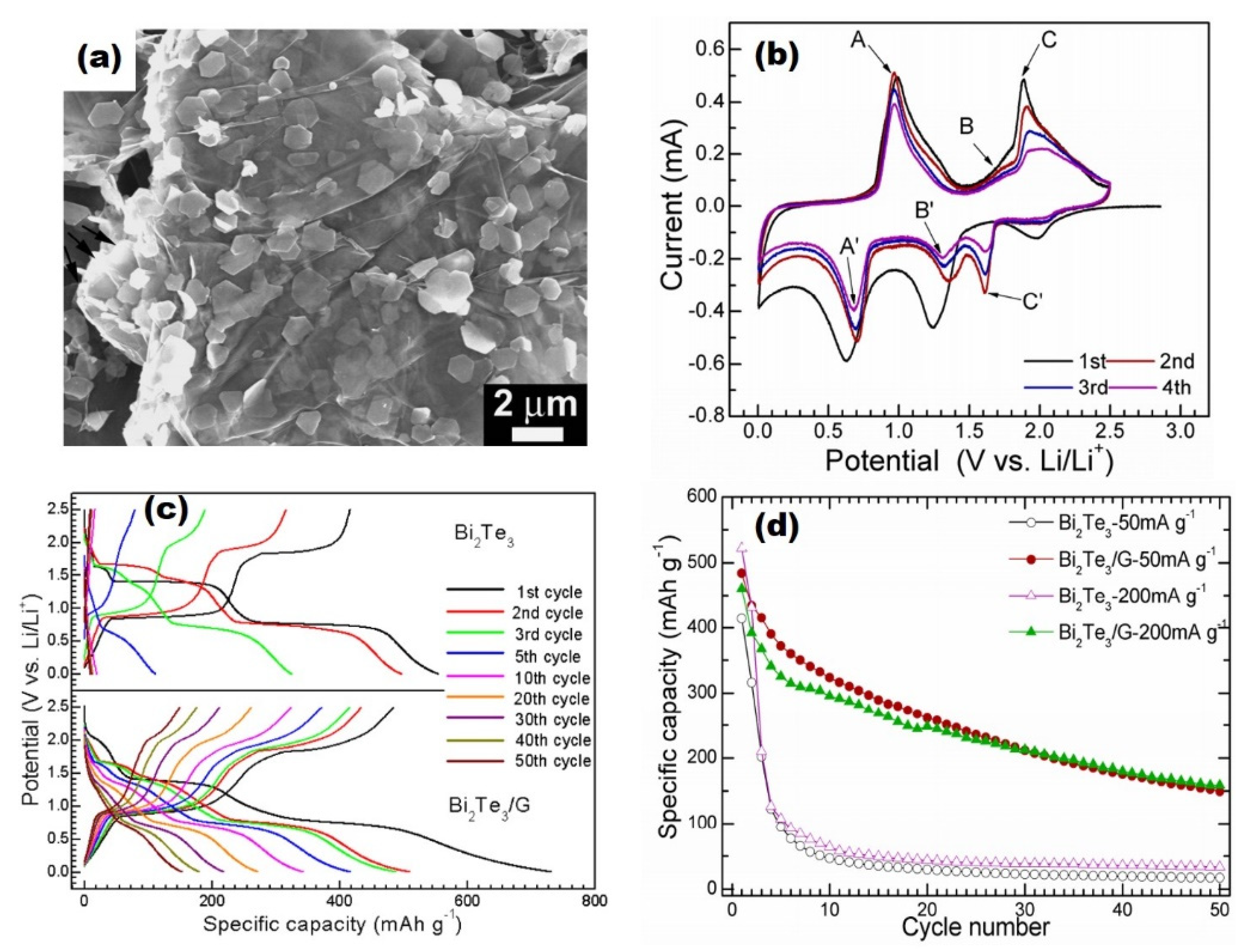
- Tu, F.; Xie, J.; Cao, G.; Zhao, X. Self-assembly of Bi2Te3-nanoplate/graphene-nanosheet hybrid by one-pot route and its improved Li-storage properties. Materials 2012, 5, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.T.; Johnson, S.A.; Cairns, E.J. Phase Equilibria in Lithium-Chalcogen Systems: II. Lithium-Sulfur. J. Electrochem. Soc. 1972, 119, 1448. [Google Scholar]
- Arya, A.; Sharma, A.L. A glimpse on all-solid-state Li-ion battery (ASSLIB) performance based on novel solid polymer electrolytes: A topical review. J. Mater. Sci. 2020, 1–63. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A long cycle life, all-solid-state lithium battery with a ceramic–polymer composite electrolyte. ACS Appl. Energy Mater. 2020, 3, 2916–2924. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Li, J.; An, C. Interfaces: Key issue to be solved for all solid-state lithium battery technologies. J. Electrochem. Soc. 2020, 167, 70541. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 1–11. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef]
- Zeng, L.; Kawahito, K.; Ikeda, S.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Metal hydride-based materials towards high performance negative electrodes for all-solid-state lithium-ion batteries. Chem. Commun. 2015, 51, 9773–9776. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Unemoto, A.; Yasaku, S.; Nogami, G.; Tazawa, M.; Taniguchi, M.; Matsuo, M.; Ikeshoji, T.; Orimo, S. Development of bulk-type all-solid-state lithium-sulfur battery using LiBH4 electrolyte. Appl. Phys. Lett. 2014, 105, 83901. [Google Scholar] [CrossRef]
- Takahashi, K.; Hattori, K.; Yamazaki, T.; Takada, K.; Matsuo, M.; Orimo, S.; Maekawa, H.; Takamura, H. All-solid-state lithium battery with LiBH4 solid electrolyte. J. Power Sources 2013, 226, 61–64. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, P.; Rathore, R.K.; Shinzato, K.; Ichikawa, T.; Verma, A.S.; Saraswat, V.K.; Awasthi, K.; Jain, A.; Kumar, M. LiBH4 as solid electrolyte for Li-ion batteries with Bi2Te3 nanostructured anode. Int. J. Hydrogen Energy 2018, 43, 21709–21714. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, R.; Awasthi, K.; Ichikawa, T.; Jain, A.; Kumar, M. Electrochemical reaction mechanism for Bi2Te3 based anode material in highly durable all solid state lithium ion batteries. J. Mater. Sci. Mater. Electron. 2020. [Google Scholar] [CrossRef]
- Kumari, P.; Pal, P.; Shinzato, K.; Awasthi, K.; Ichikawa, T.; Jain, A.; Kumar, M. Nanostructured Bi2Te3 as anode material as well as a destabilizing agent for LiBH4. Int. J. Hydrogen Energy 2020, 45, 16992–16999. [Google Scholar] [CrossRef]
- Chen, X.; Tang, H.; Huang, Z.; Zhou, J.; Ren, X.; Huang, K.; Qi, X.; Zhong, J. Flexible Bismuth selenide/graphene composite paper for lithium-ion batteries. Ceram. Int. 2017, 43, 1437–1442. [Google Scholar] [CrossRef]
- Li, Z.; Pan, H.; Wei, W.; Dong, A.; Zhang, K.; Lv, H.; He, X. Bismuth metal–organic frameworks derived bismuth selenide nanosheets/nitrogen–doped carbon hybrids as anodes for Li–ion batteries with improved cyclic performance. Ceram. Int. 2019, 45, 11861–11867. [Google Scholar] [CrossRef]


| Samples | Method of Synthesis | Electrolyte | 1st Discharge Capacity (mAhg−1) | Cyclic Stability | Potential (V vs. Li+/Li) | Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| Dandelion like Bi2S3 | Facile reflux method | Liquid | 826 | 74.7 mAhg−1 after 50 cycles | 0.001–3 V | 100 mAg−1 | [65] |
| Bi2S3/CNT composite | Facile reflux method | Liquid | 899 | 247.9 mAh/g after 50 cycles | 0.001–3 V | 100 mAg−1 | [65] |
| Bi2S3/C composite | Mechanical milling | Liquid | 747 | 500 mAhg−1 after 100 cycles | 0–2.5 V | 100 mAg−1 | [60] |
| Bi2S3 | Mechanical milling | Liquid | 746 | ~100 mAhg−1 after 25 cycles | 0–2.5 V | 100 mAg−1 | [60] |
| Bulk Bi2S3 | As purchased | Solid | 662 | 311 mAhg−1 after 50 cycles | 0.2–1.5 V | 0.1 C | [61] |
| Flower-like Bi2S3 nanostructures | Hydrothermal | Solid | 685 | 375 mAhg−1 after 50 cycle | 0.2–1.5 V | 0.1 C | [61] |
| Bi2Se3/graphene | Hydrothermal/Hummers method | Liquid | 695 | 232 mAhg−1 after 50 cycle | 0.001–3.0 | 50 mAg−1 | [97] |
| Bi2Se3 microrods | Microwave assisted route | Liquid | 870 | 55 mAhg−1 after 50 cycle | 0.01–3.0 | 50 mAg−1 | [76] |
| Bi2Se3 nanosheets/N doped carbon | Solvothermal synthesis | Liquid | 942.6 | 410.6 mAhg−1 after 50 cycles | 0–3 V | 100 mAg−1 | [98] |
| Bulk Bi2Se3 | As purchased | Solid | 621 | 306 mAhg−1 after 50 cycles | 0.1–1.5 V | 0.1 C | [62] |
| Bi2Se3 nanostructures | Hydrothermal | Solid | 594 | 343 mAhg−1 after 50 cycles | 0.2–1.5 V | 0.1 C | [62] |
| Bi2Te3/GO composite | Polyol method | Liquid | 752 | ~110 mAhg−1 after 500 cycles | 0.01–3 V | 0.1 Ag−1 | [31] |
| Bi2Te3 nanoplate/graphene | Solvothermal/Hummers method | Liquid | 731 | 200 mAhg−1 after 50 cycles | 0.1–2.5 V | 50 mAg−1 | [78] |
| Bare Bi2Te3 | Solvothermal | Liquid | ~560 | 50 mAhg−1 after 50 cycles | 0.1–2.5 V | 50 mAg−1 | [78] |
| Bi2Te3 nanorods | Hydrothermal | Solid | 533 | 219 mAhg−1 after 50 cycles | 0.1–1.5 V | 0.1 C | [95] |
| Bulk Bi2Te3 | As purchased | Solid | 489 | 235 mAhg−1 after 50 cycles | 0.1–2.5 V | 0.1 C | [95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Kumari, P.; Kumar, M.; Ichikawa, T.; Jain, A. Implementation of Bismuth Chalcogenides as an Efficient Anode: A Journey from Conventional Liquid Electrolyte to an All-Solid-State Li-Ion Battery. Molecules 2020, 25, 3733. https://doi.org/10.3390/molecules25163733
Singh R, Kumari P, Kumar M, Ichikawa T, Jain A. Implementation of Bismuth Chalcogenides as an Efficient Anode: A Journey from Conventional Liquid Electrolyte to an All-Solid-State Li-Ion Battery. Molecules. 2020; 25(16):3733. https://doi.org/10.3390/molecules25163733
Chicago/Turabian StyleSingh, Rini, Pooja Kumari, Manoj Kumar, Takayuki Ichikawa, and Ankur Jain. 2020. "Implementation of Bismuth Chalcogenides as an Efficient Anode: A Journey from Conventional Liquid Electrolyte to an All-Solid-State Li-Ion Battery" Molecules 25, no. 16: 3733. https://doi.org/10.3390/molecules25163733
APA StyleSingh, R., Kumari, P., Kumar, M., Ichikawa, T., & Jain, A. (2020). Implementation of Bismuth Chalcogenides as an Efficient Anode: A Journey from Conventional Liquid Electrolyte to an All-Solid-State Li-Ion Battery. Molecules, 25(16), 3733. https://doi.org/10.3390/molecules25163733









