Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
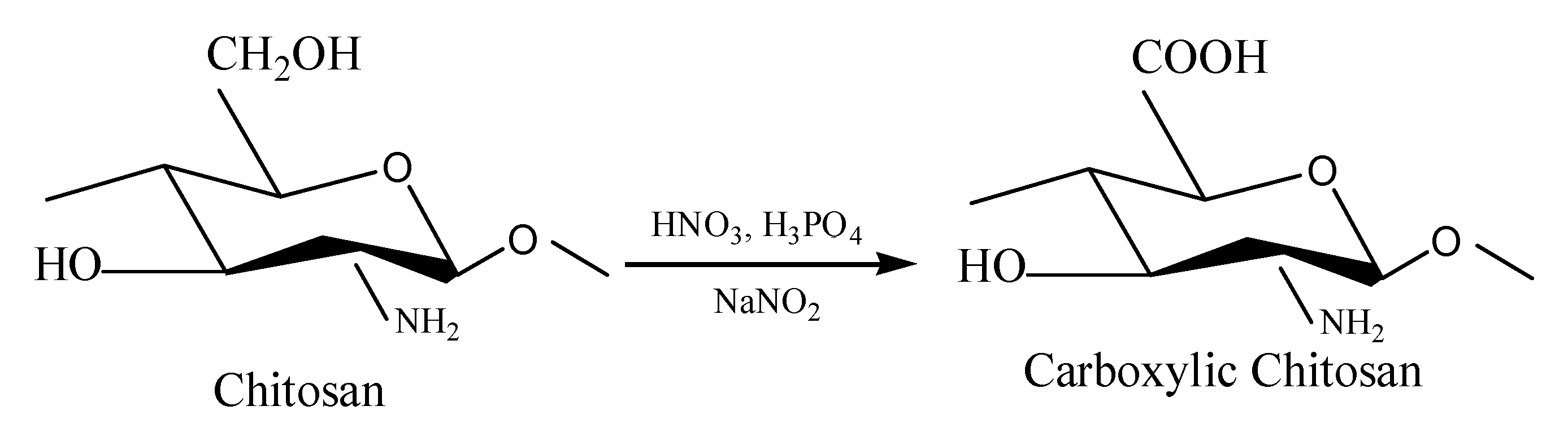
2.2. Oxidation of Chitosan by HNO3/H3PO4–NaNO2-Mediated Oxidization System
2.3. Preparation of Oxidized Chitosan Grafted Cashmere Fiber (OCGCF)
2.4. Oxidation Degree of Chitosan
2.5. Scanning Electron Microscopy Analysis
2.6. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.7. CP/MAS 13C-NMR Analysis of Oxidized Chitosan
2.8. Measurement of Textile Physical Properties of OCGCF
2.9. Antibacterial Testing of OCGCF
3. Results and Discussion
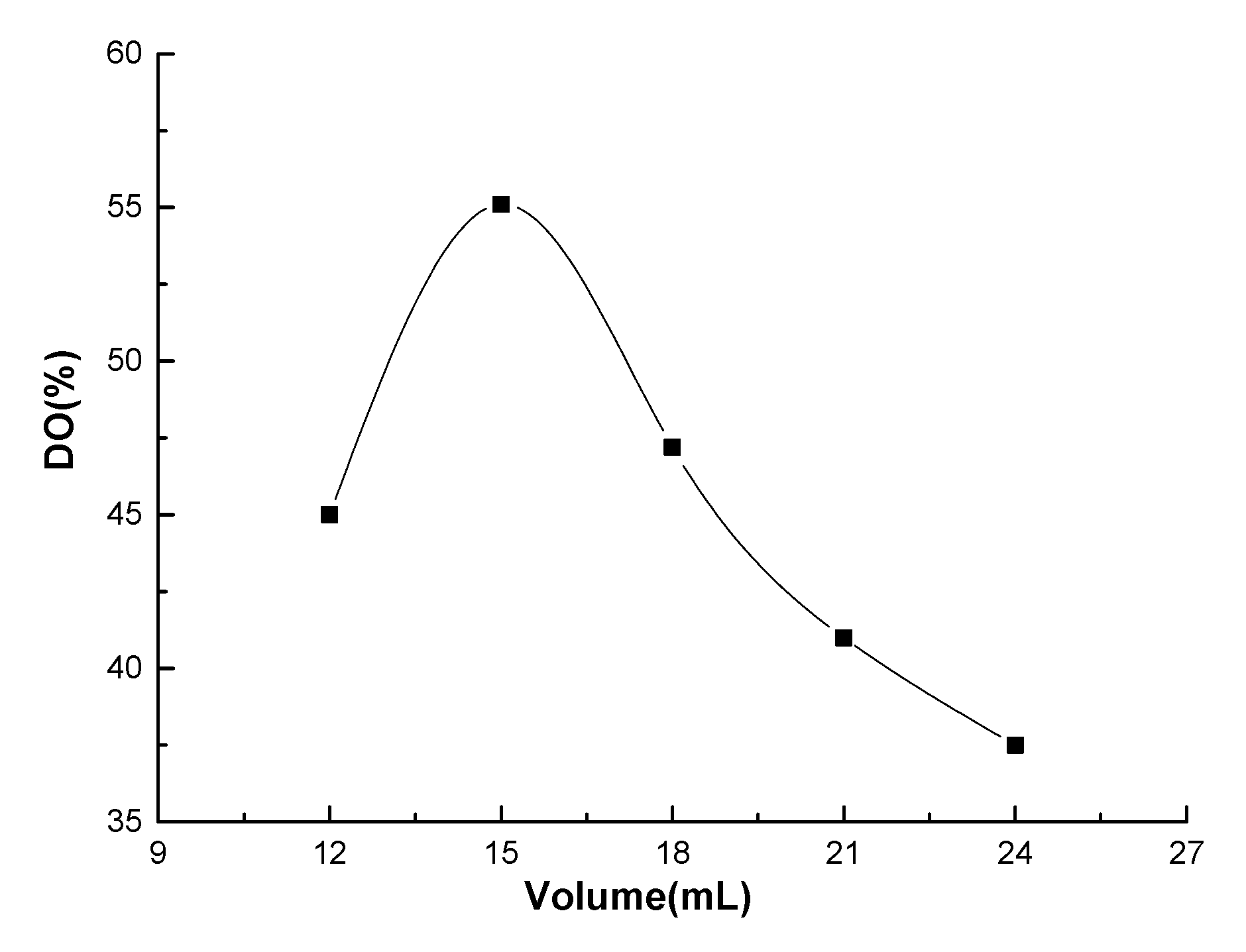
3.1. Effect of HNO3/H3PO4–NaNO2-Mediated Oxidation System on Oxidation Degree
3.2. Morphology Analysis of Oxidized Chitosan
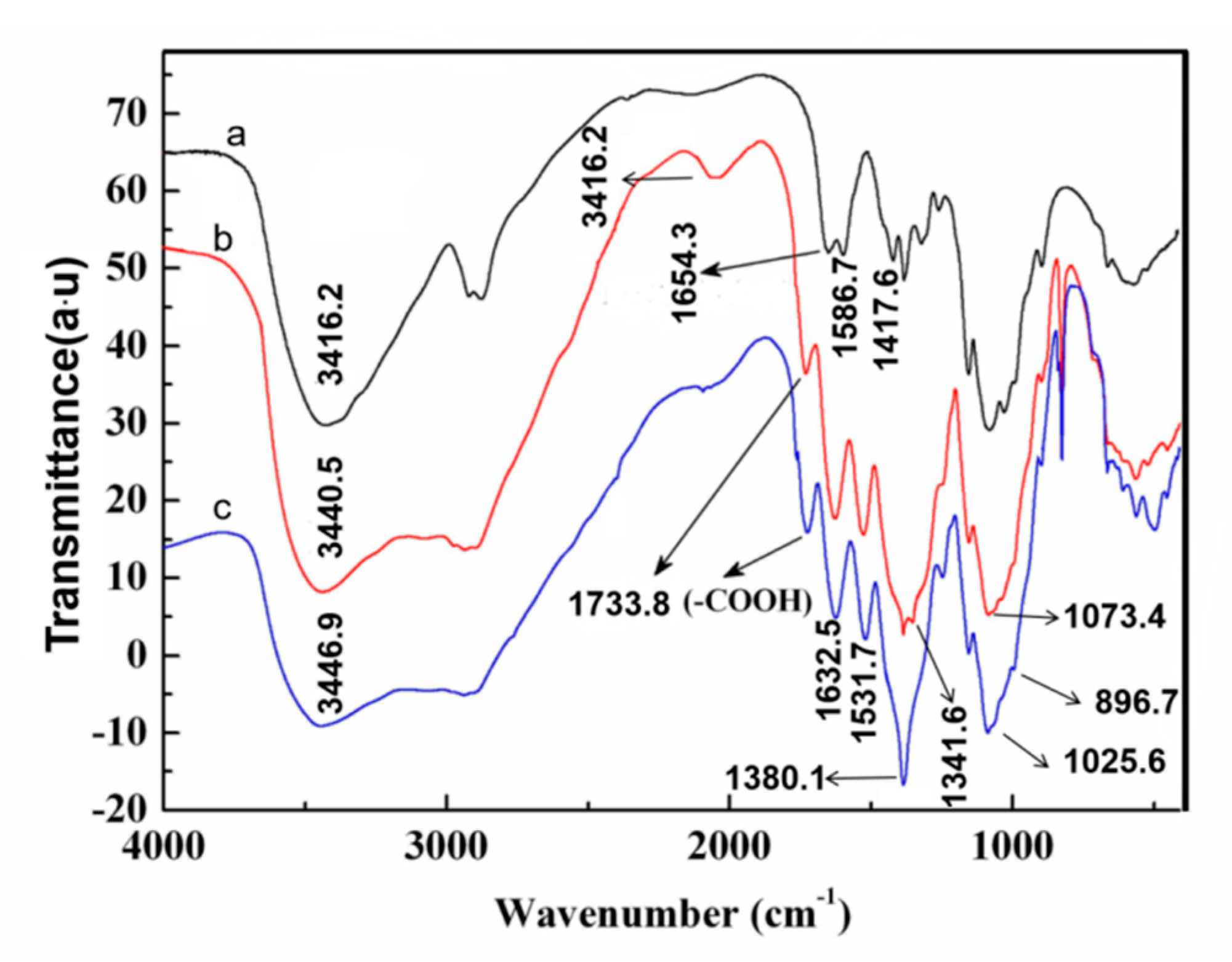
3.3. Characterization of the Oxidized Chitosan
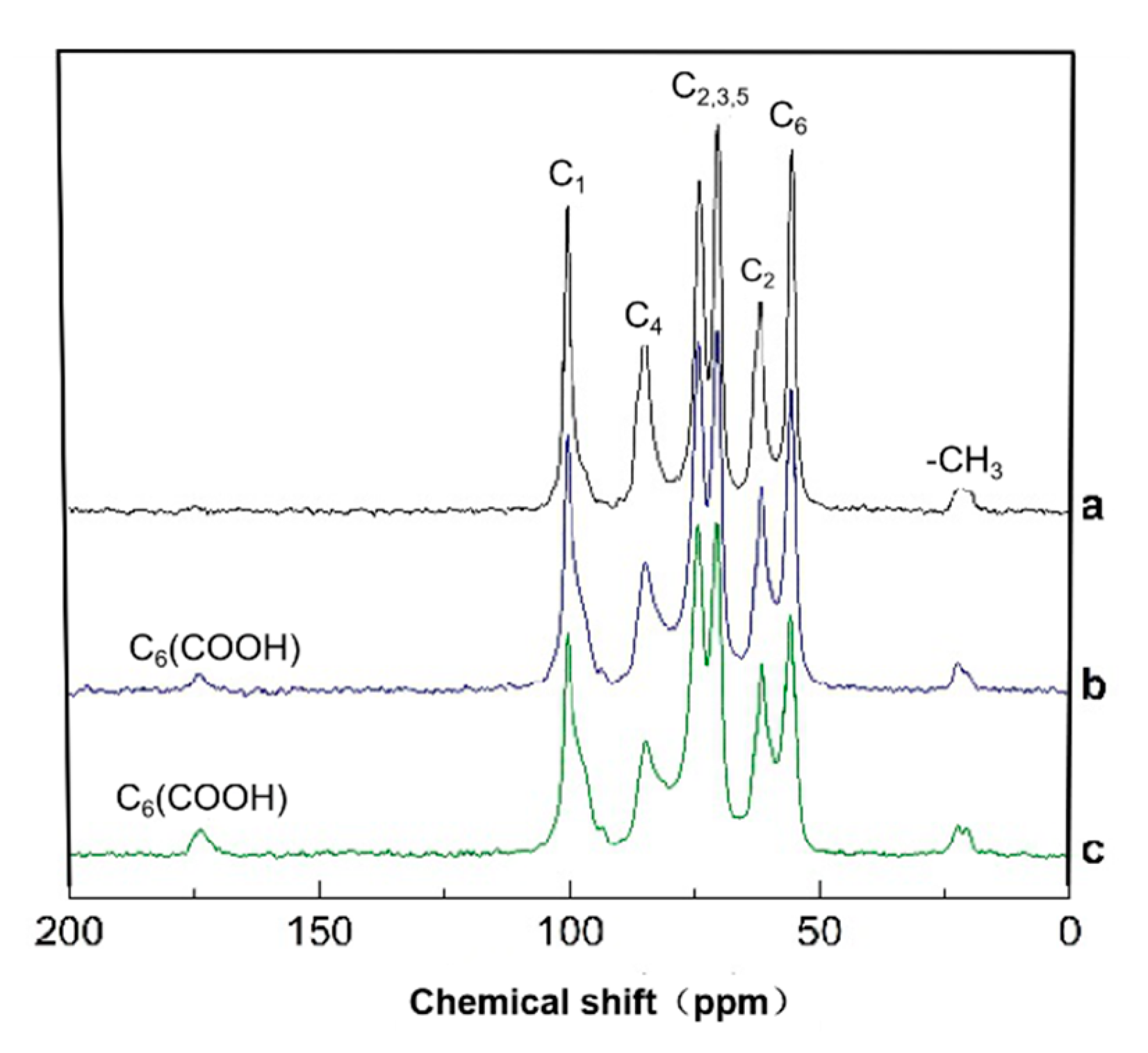
3.4. Solid-State CP/MAS 13C-NMR Analysis of HNO3/H3PO4–NaNO2 Oxidized Chitosan
3.5. Preparation and Characterization of Oxidized Chitosan Grafted Cashmere Fiber
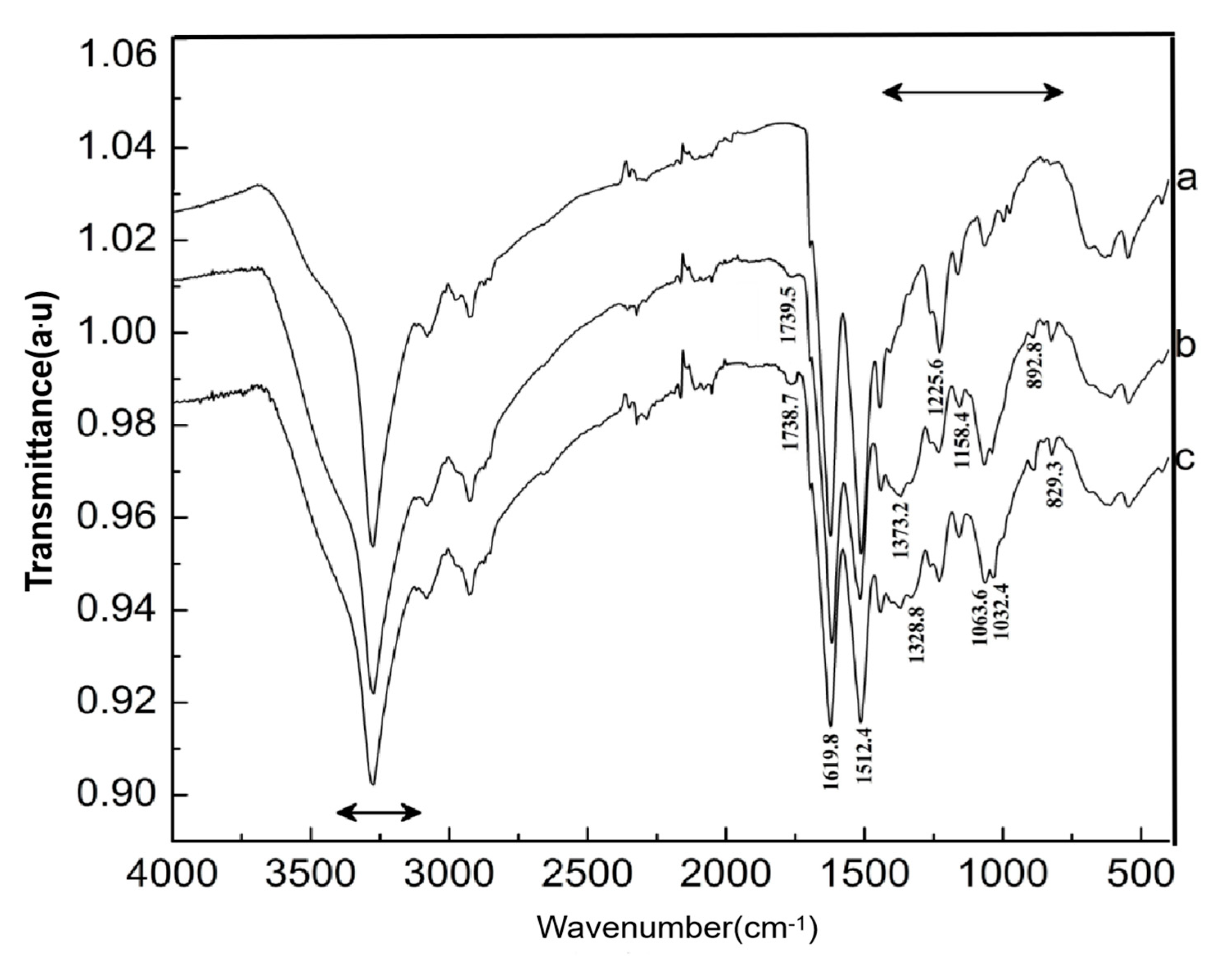
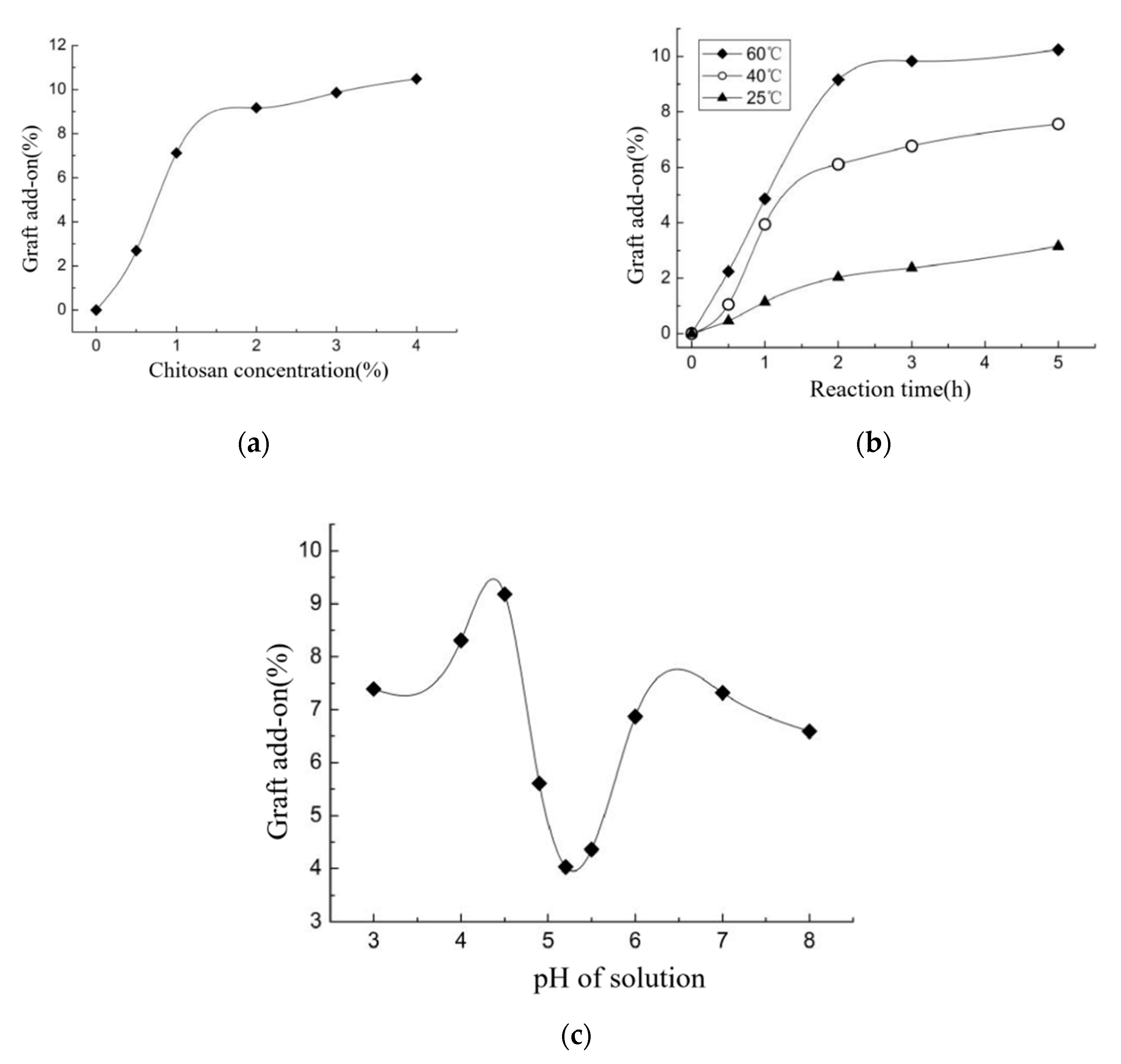
3.6. Grafted Reaction between Cashmere Fiber and Carboxylic Chitosan
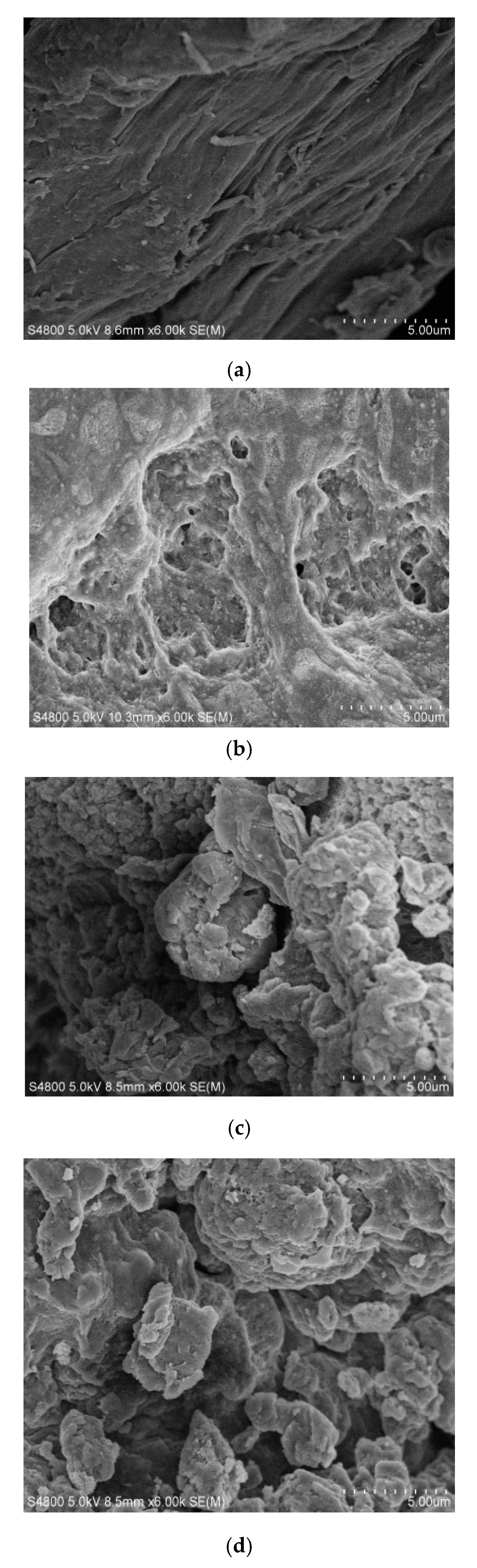
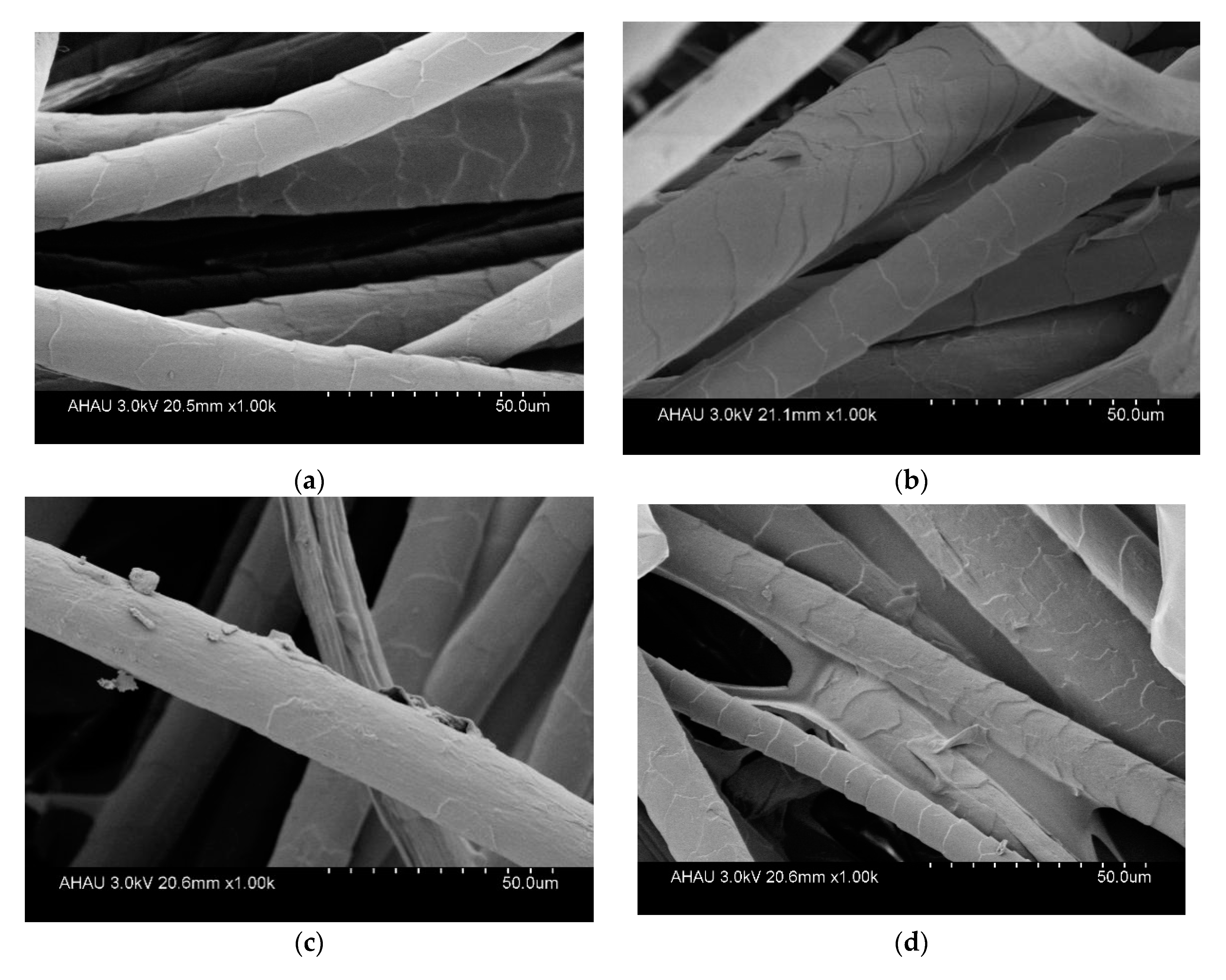
3.7. Morphology observation of OCGCF.
3.8. Textile Properties of Cashmere Fabric by OCGCF
3.9. Antibacterial Activity of OCGCF
4. Conclusions
- A facile method was developed to prepare oxidized chitosan by HNO3/H3PO4–NaNO2-mediated oxidation system and significantly expand its application for grafting oxidized chitosan onto fibers. The oxidation degree of chitosan approximately reached to 55% under the following oxidation conditions: total acid volume, 15 mL; initiator sodium nitrite in total acids volume, 1.4% (w/v); reaction time, 3 h. The reaction of oxidized chitosan with cashmere fibers afforded OCGCF, which exhibited a new absorption band of C=O at 1733 cm−1 in the infrared spectrum and a new peak at approximately 175 ppm in the 13C NMR spectrum, indicating that the C6 primary hydroxyl in the chitosan glucopyranose units selectively oxidized to carboxyl group.
- The surface of cashmere fiber was successfully modified through the grafting reaction between the carboxyl group of the oxidized chitosan and the amino group of cashmere fiber surface via the amide formation reaction. The FT-IR spectrum revealed that the carboxyl groups in the oxidized chitosan reacted with the amino groups in the cashmere fiber. With increasing graft add-on of chitosan onto the cashmere fibers, the resulting new adsorption peak was significantly high in intensity, indicating the occurrence of amide reaction. An optimum balance between the graft add-on of chitosan and the properties of resulting OCGCFs was obtained under the following conditions: grafting reaction time, 2 h; pH, in the range 4–4.5; chitosan aqueous solution, 2% (w/w) for grafting cashmere fibers. The grafted cashmere fibers displayed increased crease resistance and moisture regain, compared to those of the original cashmere fibers. The oxidized chitosan endows cashmere fibers with durable antibacterial property against S. aureus and E. coli, and the bacterial reduction rates against both exceeded 96%.
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Zhang, Y.; Wang, J.; Yang, Y.; Miao, C.; Guo, Y.; Zhang, Z.; Cao, Q.; Shui, W. Combining Untargeted and Targeted Proteomic Strategies for Discrimination and Quantification of Cashmere Fibers. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Daoud, W.A. Mechanical energy harvester based on cashmere fibers. J. Mater. Chem. A 2018, 6, 11198–11204. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Marelli, B.; Omenetto, F.G. Cashmere-derived keratin for device manufacturing on the micro- and nanoscale. J. Mater. Chem. C 2015, 3, 2783–2787. [Google Scholar] [CrossRef]
- Popescu, C.; Höcker, H. Hair—The most sophisticated biological composite material. Chem. Soc. Rev. 2007, 36, 1282–1291. [Google Scholar] [CrossRef]
- Raja, A.S.M.; Shakyawar, D.B.; Pareek, P.K.; Temani, P.; Sofi, A.H. A Novel Chemical Finishing Process for Cashmere/PVA-Blended Yarn-Made Cashmere Fabric. J. Nat. Fibers 2013, 10, 381–389. [Google Scholar] [CrossRef]
- McGregor, B.A.; Doughty, A.; Thompson, J.; Naebe, M.; Tester, D. Effects of variation in wool fiber curvature and yarn hairiness on sensorial assessment of knitted fabrics. Text. Res. J. 2015, 85, 1153–1166. [Google Scholar] [CrossRef]
- Asad, R.A.M.; Yu, W.; Zheng, Y.-H.; He, Y. Characterization of prickle tactile discomfort properties of different textile single fibers using an axial fiber-compression-bending analyzer (FICBA). Text. Res. J. 2015, 85, 512–523. [Google Scholar] [CrossRef]
- Li, L.; Jiang, F.; Jia, G.; Wang, W. Anti-felting Oxidation Treatment of Cashmere Fibers. J. Eng. Fibers Fabr. 2012, 7, 111–117. [Google Scholar] [CrossRef]
- Li, L.; Zhou, W. Analysis on the pilling factors of cashmere knitted fabric. Fibers Polym. 2006, 7, 213–216. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, C.; Wei, X.; Yan, T.; Li, H.; He, J.; Huang, Y. Preparation and Characterization of 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-Oxidized Cellulose Nanocrystal/Alginate Biodegradable Composite Dressing for Hemostasis Applications. ACS Sustain. Chem. Eng. 2017, 5, 3819–3828. [Google Scholar] [CrossRef]
- Liu, L.; Borghei, M.; Wang, Z.; Xu, J.; Fan, Y.; Rojas, O.J. Salt-Induced Colloidal Destabilization, Separation, Drying, and Redispersion in Aqueous Phase of Cationic and Anionic Nanochitins. J. Agric. Food Chem. 2018, 66, 9189–9198. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Preparing water-soluble 2, 3-dialdehyde cellulose as a bio-origin cross-linker of chitosan. Cellulose 2018, 25, 987–998. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Chen, G.; Xu, W.; Fu, Z.; Jiang, G.; Wu, J.; Liu, Z. Polyelectrolytes Tailored Enzyme Cascades with Enhanced Stability and Activity for One-pot Synthesis. Chemcatchem 2018, 10, 5391–5396. [Google Scholar] [CrossRef]
- Wang, B.; Kang, I.-J. Modified Chitosan Membrane for Adsorption of GABA. J. Chitin Chitosan 2018, 23, 23–28. [Google Scholar] [CrossRef]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. Part A 2019, 107, 742–754. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Yan, L.; Huang, G.; Dong, Z. Effect of oxidization and chitosan on the surface activity of soy protein isolate. Carbohydr. Polym. 2016, 151, 700–706. [Google Scholar] [CrossRef]
- Chen, C.-H.; Dai, Y.-F. Effect of chitosan on interfacial polymerization of aniline. Carbohydr. Polym. 2011, 84, 840–843. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-Oxidized Nanocellulose Participating as Crosslinking Aid for Alginate-Based Sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, R.J. In Situ Preparation of Stabilized Iron Sulfide Nanoparticle-Impregnated Alginate Composite for Selenite Remediation. Environ. Sci. Technol. 2018, 52, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, W.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. A carbon dot-based ratiometric fluorometric and colorimetric method for determination of ascorbic acid and of the activity of ascorbic acid oxidase. Microchim. Acta 2019, 186, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, H.; Yang, Y.; Huang, Y.; Chen, X. Oxidized Cellulose Enhanced Collagen Hydrogels. Chem. J. Chin. Univ. -Chin. 2015, 36, 2040–2046. [Google Scholar]
- Xu, Y.; Chen, D.; Du, Z.; Li, J.; Wang, Y.; Yang, Z.; Peng, F. Structure and properties of silk fibroin grafted carboxylic cotton fabric via amide covalent modification. Carbohydr. Polym. 2017, 161, 99–108. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Liu, X.; Tan, J.; Zhu, H. Influence of HNO3/H3PO4-NANO(2) mediated oxidation on the structure and properties of cellulose fibers. Carbohydr. Polym. 2014, 111, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiu, C.; Zhang, X.; Zhang, W. Crosslinking chitosan into H3PO4/HNO3-NANO(2) oxidized cellulose fabrics as antibacterial-finished material. Carbohydr. Polym. 2014, 112, 186–194. [Google Scholar] [CrossRef]
- Xu, T.-T.; Jiang, T.-S.; Han, X.-L.; Xu, Y.-H.; Qiao, J.-P. Modular synthesis of (E)-cinnamaldehydes directly from allylarenes via a metal-free DDQ-mediated oxidative process. Org. Biomol. Chem. 2018, 16, 5350–5358. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, C.; Wang, X. Characterization and controlled release aloe extract of collagen protein modified cotton fiber. Carbohydr. Polym. 2013, 92, 982–988. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Hu, X.; Feng, H.; Xiong, W.; Guo, W.; Zhou, J.; Mosa, A.; Peng, Y. Multicavity triethylenetetramine-chitosan/alginate composite beads for enhanced Cr(VI) removal. J. Clean. Prod. 2019, 231, 733–745. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.-F.; Deng, R.-H.; Xia, G.-Q.; Shang, X.-F.; Zhou, P.-H. Construction of chitosan/ZnO nanocomposite film by in situ precipitation. Int. J. Biol. Macromol. 2019, 122, 82–87. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Shi, Q.; Zhang, W.; Song, S. Utilization of N-carboxymethyl chitosan as a selective depressant for talc in flotation of chalcopyrite. Physicochem. Probl. Miner. Process. 2019, 55, 108–115. [Google Scholar]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiao, Y.; Luo, P.; Fan, L. Preparation and characterization of C-phycocyanin peptide grafted N-succinyl chitosan by enzyme method. Int. J. Biol. Macromol. 2018, 113, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int. J. Biol. Macromol. 2018, 107, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Deng, P.; Zhou, C.; Hou, Y.; Chen, R.; Liang, F.; Liao, L. Preparation of a novel antibacterial chitosan-poly(ethylene glycol) cryogel/silver nanoparticles composites. J. Biomater. Sci. Polym. Ed. 2017, 28, 1324–1337. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Z.; Hu, L.; Shi, X.; Guan, W.; Du, Y. Synthesis of chitosan-stabilized gold nanoparticles by atmospheric plasma. Carbohydr. Polym. 2013, 91, 152–156. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J. Transformation of complex internal structures of poly(ethylene oxide)/chitosan oligosaccharide electrospun nanofibers. Polym. Int. 2012, 61, 135–140. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, L.; Xu, D.; Cai, J.; Huang, L.; Zhou, J.; Zhang, L. Distinctive Construction of Chitin-Derived Hierarchically Porous Carbon Microspheres/Polyaniline for High-Rate Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 28918–28927. [Google Scholar] [CrossRef]
- Pei, X.; Deng, Y.; Li, Y.; Huang, Y.; Yuan, K.; Lee, J.-F.; Chan, T.-S.; Zhou, J.; Lei, A.; Zhang, L. Size-controllable ultrafine palladium nanoparticles immobilized on calcined chitin microspheres as efficient and recyclable catalysts for hydrogenation. Nanoscale 2018, 10, 14719–14725. [Google Scholar] [CrossRef]
- Min, L.; Liu, M.; Liu, L.; Rao, Z.; Zhu, C.; Fan, L. Enzymatic synthesis of quaternary ammonium chitosan-silk fibroin peptide copolymer and its characterization. Int. J. Biol. Macromol. 2018, 109, 1125–1131. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan—Collagen peptide/oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, Y.; Xiao, L.; Deng, H.; Du, Y.; Chen, Y.; Shi, X. Electrodeposition to construct free-standing chitosan/layered double hydroxides hydro-membrane for electrically triggered protein release. Colloids Surf. B-Biointerfaces 2017, 158, 474–479. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Y.; Zou, W.; Duan, J.; Chen, Y. Preparation and Characterization Magnetic Chitosan Microcapsules. J. Chem. 2013, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Li, C.; Zheng, H.; Song, H.; Xiong, F.; Qiu, T.; Yang, J. Cisplatin-crosslinked glutathione-sensitive micelles loaded with doxorubicin for combination and targeted therapy of tumors. Carbohydr. Polym. 2017, 155, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.-W.; Yu, A.-X.; Zhu, S.-B.; Zhou, M.; Wu, G. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-beta 1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp. Biol. Med. 2013, 238, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xu, L. Adsorption of Acid red onto chitosan/rectorite composites from aqueous solution. Rsc. Adv. 2013, 3, 21685–21690. [Google Scholar] [CrossRef]
- He, M.; Zhang, X.; Yao, W.; Wang, C.; Shi, L.; Zhou, P. Construction of alternate layered chitosanialginate composite hydrogels and their properties. Mater. Lett. 2017, 200, 43–46. [Google Scholar] [CrossRef]
- Huang, P.; Ma, K.; Cai, X.; Huang, D.; Yang, X.; Ran, J.; Wang, F.; Jiang, T. Enhanced antibacterial activity and biocompatibility of zinc-incorporated organic-inorganic nanocomposite coatings via electrophoretic deposition. Colloids Surf. B-Biointerfaces 2017, 160, 628–638. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, R.; Duan, B.; Liu, M.; Lu, A.; Zhang, L. Recyclable Universal Solvents for Chitin to Chitosan with Various Degrees of Acetylation and Construction of Robust Hydrogels. ACS Sustain. Chem. Eng. 2017, 5, 2725–2733. [Google Scholar] [CrossRef]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of cellulose/nanosilver sponge materials and their antibacterial activities for infected wounds healing. Cellulose 2016, 23, 749–763. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, H.; Yang, J.; Tong, J.; Yi, J.; Hu, Z.; Hu, J.; Wang, T.; Fan, L. Antibacterial activity of chitosan grafting nisin: Preparation and characterization. React. Funct. Polym. 2015, 91–92, 71–76. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Zhang, X.; Wang, H.; Deng, H.; Li, Y.; Xu, X.; Huang, R.; Li, X. Antibacterial and hemostatic performance of chitosan-organic rectorite/alginate composite sponge. Rsc. Adv. 2015, 5, 50523–50531. [Google Scholar] [CrossRef]
Sample Availability: Samples of CF, CS, oxidized chitosan and OCGCF are available from the authors. |


| Samples | Graft Add-On (%) | Tensile Strength (N/m) | WRA (°) | Moisture Regain (%) | Yellow Index |
|---|---|---|---|---|---|
| Untreated CF | 0 | 551 ± 5.64 | 141.23 ± 3.43 | 9.1 ± 3.78 | 69.8 ± 0.34 |
| OCGCF-I | 4.34 | 512 ± 3.57 | 183.65 ± 4.35 | 11.2 ± 4.12 | 58.0 ± 0.21 |
| OCGCF-II | 9.51 | 603 ± 4.37 | 202.87 ± 4.31 | 13.3 ± 4.01 | 47.6 ± 0.35 |
| Samples | Graft Add-On (%) | Inhibition after Washing Times | |||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 30 | |||||
| S. Aureus | E. Coli | S. Aureus | E. Coli | S. Aureus | E. Coli | ||
| CS | / | 99.2% | 95.6% | 99.2% | 95.6% | 99.2% | 95.6% |
| CF | / | 0% | 0% | 0% | 0% | 0% | 0% |
| OCGCF-Ⅰ | 4.34 | 92.6% | 89.6% | 91.3% | 86.7% | 90.4% | 85.2% |
| OCGCF-Ⅱ | 9.51 | 98.1% | 94.6% | 97.1% | 93.5% | 94.4% | 91.8% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fang, T.; Yan, W.; Zhang, F.; Xu, Y.; Du, Z. Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification. Molecules 2020, 25, 3812. https://doi.org/10.3390/molecules25173812
Li J, Fang T, Yan W, Zhang F, Xu Y, Du Z. Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification. Molecules. 2020; 25(17):3812. https://doi.org/10.3390/molecules25173812
Chicago/Turabian StyleLi, Jifeng, Ting Fang, Wenjing Yan, Fei Zhang, Yunhui Xu, and Zhaofang Du. 2020. "Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification" Molecules 25, no. 17: 3812. https://doi.org/10.3390/molecules25173812
APA StyleLi, J., Fang, T., Yan, W., Zhang, F., Xu, Y., & Du, Z. (2020). Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification. Molecules, 25(17), 3812. https://doi.org/10.3390/molecules25173812






