Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp.
Abstract
:1. Introduction
2. Results
2.1. Double Distilled Water with pH 7 Was Suitable for Total Phycobiliproteins Extraction from Arthrospira sp.
2.2. A 0.50% w/v Biomass/Solvent Ratio Was Recommended for Cyanobacterium Arthrospira sp. for Maximum Total Phycobiliproteins
2.3. Optimum Freezing–Thawing Temperature in Extracting Total Phycobiliproteins from Cyanobacteria Was Freezing at −80 °C and Thawing at 25 °C
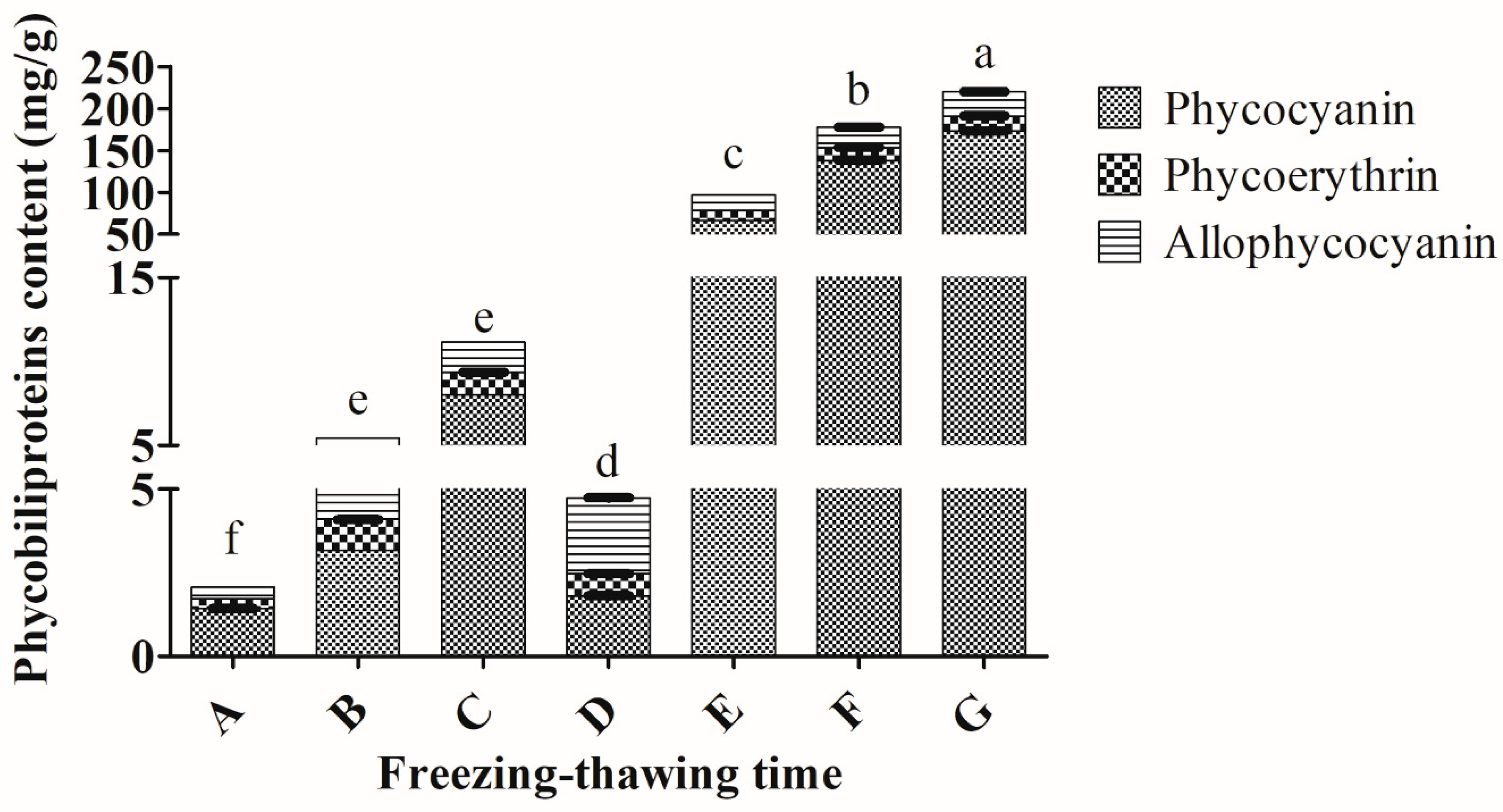
2.4. Freezing for 2 h and Thawing for 24 h Were Suggested to Maximize the Extraction of Total Phycobiliproteins from Arthrospira sp.
2.5. One Cycle of Freezing and Thawing Was Sufficient to Obtain the Highest Amount of Phycobiliproteins from Arthrospira sp.
2.6. Amount of Extracted Total Phycobiliproteins from Arthrospira sp. Was Reduced over 24 h
3. Discussion
4. Materials and Methods
4.1. Cyanobacteria Culture
4.2. Preparation of Starting Material
4.3. Optimization of Freezing–Thawing Extraction Method (Phycobiliprotein Extraction Method)
4.4. Estimation of Phycobiliproteins Extracted
4.5. Stability of Phycobiliproteins
4.6. Purity of Phycobiliproteins
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khattar, J.I.S.; Kaur, S.; Kaushal, S.; Singh, Y.; Singh, D.P.; Rana, S.; Gulati, A. Hyperproduction of Phycobiliproteins by the Cyanobacterium Anabaena fertilissima PUPCCC 410.5 under Optimized Culture Conditions. Algal Res. 2015, 12, 463–469. [Google Scholar] [CrossRef]
- Ani Azaman, S.N.; Nagao, N.; Yusoff, F.M.; Tan, S.W.; Yeap, S.K. A Comparison of the Morphological and Biochemical Characteristics of Chlorella sorokiniana and Chlorella zofingiensis Cultured under Photoautotrophic and Mixotrophic Conditions. PeerJ 2017, 2017, e3473. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.; Issa, A.A.; Fawzy, M. Abiotic Stress Induced Production of β-Carotene, Allophycocyanin and Total Lipids in Spirulina sp. J. Biol. Earth Sci. 2013, 3, 54–64. [Google Scholar]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [Green Version]
- Lage-Yusty, M.A.; Caramés-Adán, P.; López-Hernández, J. Determination of Phycobiliproteins by Constant-Wavelength Synchronous Spectrofluorimetry Method in Red Algae. CYTA J. Food 2013, 11, 243–247. [Google Scholar] [CrossRef]
- Galetovic, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Nuñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Kim, Y.R.; Do, J.M.; Kim, K.H.; Stoica, A.R.; Jo, S.W.; Kim, U.K.; Yoon, H.S. C-Phycocyanin from Limnothrix Species KNUA002 Alleviates Cisplatin-Induced Ototoxicity by Blocking the Mitochondrial Apoptotic Pathway in Auditory Cells. Mar. Drugs 2019, 17, 235. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, C.; Hou, Y.; Chen, S.; Xiao, D.; Zhang, J.; Chen, F. Isolation and Characterization of a Marine Microalga for Biofuel Production with Astaxanthin as a Co-Product. Energies 2013, 6, 2759–2772. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.F.; Wang, G.C.; Tseng, C.K. Method for Large-Scale Isolation and Purification of R-Phycoerythrin from Red Alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Banerjee, S.; Yusoff, F.M.; Shariff, M. Effects of Salinity on the Growth and Proximate Composition of Selected Tropical Marine Periphytic Diatoms and Cyanobacteria. Aquac. Res. 2010, 41, 1348–1355. [Google Scholar] [CrossRef]
- Mehta, K.K.; Evitt, N.H.; Swartz, J.R. Chemical Lysis of Cyanobacteria. J. Biol. Eng. 2015, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, B.Z.; Siegel, S.M. The Chemical Composition of Algal Cell Walls. Crit. Rev. Microbiol. 1973, 3, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Yang, H.; Chen, Y.T.; Li, P.P. Biosynthesis of Fluorescent β Subunits of C-Phycocyanin from Spirulina subsalsa in Escherichia coli, and Their Antioxidant Properties. Molecules 2018, 23, 1369. [Google Scholar] [CrossRef] [Green Version]
- Vernès, L.; Granvillain, P.; Chemat, F.; Vian, M. Phycocyanin from Arthrospira platensis. Production, Extraction and Analysis. Curr. Biotechnol. 2015, 4, 481–491. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid Green Extractions of C-Phycocyanin from Arthrospira maxima for Functional Applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Shivaprakash, M.K. Evaluation of Three Spirulina Species Grown under Similar Conditions for Their Growth and Biochemicals. J. Sci. Food Agric. 2005, 85, 333–336. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the Edible Microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef]
- Horváth, H.; Kovács, A.W.; Riddick, C.; Présing, M. Extraction Methods for Phycocyanin Determination in Freshwater Filamentous Cyanobacteria and Their Application in a Shallow Lake. Eur. J. Phycol. 2013, 48, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Sivasankari, S.; Ravindran, D. Comparison of Different Extraction Methods for Phycocyanin Extraction. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 904–909. [Google Scholar]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemlata, G.; Fareha, B. Studies on Anabaena sp. NCCU-9 with Special Reference to Phycocyanin. J. Algal Biomass Util. 2011, 2, 30–51. [Google Scholar]
- Thoisen, C.; Hansen, B.W.; Nielsen, S.L. A Simple and Fast Method for Extraction and Quantification of Cryptophyte Phycoerythrin. MethodsX 2017, 4, 209–213. [Google Scholar] [CrossRef]
- Maurya, S.S.; Maurya, J.N.; Pandey, V.D. Factors Regulating Phycobiliprotein Production in Cyanobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 764–771. [Google Scholar]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-Phycocyanin Extraction from Spirulina platensis Wet Biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Jin, Y.; Zeng, X.; Chen, J.; Lu, Y.; Jing, K. Fed-Batch Strategy for Enhancing Cell Growth and C-Phycocyanin Production of Arthrospira (Spirulina) platensis under Phototrophic Cultivation. Bioresour. Technol. 2015, 180, 281–287. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and Characterization of Phycocyanin from the Marine Cyanobacterium Synechococcus sp. IO9201. Plant. Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of Phycocyanin Extraction from Spirulina platensis Using Factorial Design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of Phycocyanin Extraction from Spirulina platensis Using Different Techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Doke, J.M. An Improved and Efficient Method for the Extraction of Phycocyanin from Spirulina sp. Int. J. Food Eng. 2005, 1, 1–13. [Google Scholar] [CrossRef]
- Soni, B.; Kalavadia, B.; Trivedi, U.; Madamwar, D. Extraction, Purification and Characterization of Phycocyanin from Oscillatoria quadripunctulata-Isolated from the Rocky Shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006, 41, 2017–2023. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Physical Extraction and Extrusion Entrapment of C-Phycocyanin from Arthrospira platensis. J. King Saud Univ.Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Suman, S.; Puri, N.; Jasuja, N.D.; Kumar, M.; Sharma, G. Optimization, Purification and Characterization of Phycocyanin from Spirulina platensis. Int. J. Appl. Pure Sci. Agric. 2016, 2, 15–20. [Google Scholar]
- Mogany, T.; Kumari, S.; Swalaha, F.M.; Bux, F. Extraction and Characterisation of Analytical Grade C-Phycocyanin from Euhalothece sp. J. Appl. Phycol. 2019, 31, 1661–1674. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote Sensing of the Cyanobacterial Pigment Phycocyanin in Turbid Inland Water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp.: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and Efficient Method for Extraction of C-Phycocyanin from Dry Biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- De Morais, M.G.; Prates, F.; Moreira, J.B.; Duarte, J.H.; Alberto, J.; Costa, V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Nuez, L.; Amador, D. Practical Application of Aqueous Two-Phase Systems for the Development of a Prototype Process for c-Phycocyanin Recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G. Methods for Extraction, Isolation and Purification of C-Phycocyanin: 50 Years of Research in Review. Int. J. Food Nutr. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Lawrenz, E.; Fedewa, E.J.; Richardson, T.L. Extraction Protocols for the Quantification of Phycobilins in Aqueous Phytoplankton Extracts. J. Appl. Phycol. 2011, 23, 865–871. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR Primers to Amplify 16S rRNA Genes from Cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [Green Version]
- Seddek, N.H.; Fawzy, M.A.; El-Said, W.A.; Ahmed, M.M.R. Evaluation of Antimicrobial, Antioxidant and Cytotoxic Activities and Characterization of Bioactive Substances from Freshwater Blue-Green Algae. Glob. Nest J. 2019, 21, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Khaw, Y.S.; Khong, N.M.H.; Shaharuddin, N.A.; Yusoff, F.M. A Simple 18S rDNA Approach for the Identification of Cultured Eukaryotic Microalgae with an Emphasis on Primers. J. Microbiol. Methods 2020, 172, 105890. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-Phycocyanin Extraction from Two Freshwater Cyanobacteria by Freeze Thaw and Pulsed Electric Field Techniques to Improve Extraction Efficiency and Purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. https://doi.org/10.3390/molecules25173894
Tan HT, Khong NMH, Khaw YS, Ahmad SA, Yusoff FM. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules. 2020; 25(17):3894. https://doi.org/10.3390/molecules25173894
Chicago/Turabian StyleTan, Hui Teng, Nicholas M. H. Khong, Yam Sim Khaw, Siti Aqlima Ahmad, and Fatimah M. Yusoff. 2020. "Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp." Molecules 25, no. 17: 3894. https://doi.org/10.3390/molecules25173894






