Chiral Mesoporous Silica Materials: A Review on Synthetic Strategies and Applications
Abstract
:1. Introduction
2. Synthesis of Chiral Mesoporous Silica (CMS) Nanoparticles
2.1. CMS Templated by Chiral Anionic Surfactants
2.2. CMS Templated by Chiral Cationic Surfactants and Gelators
2.3. CMS Templated by Achiral Templates
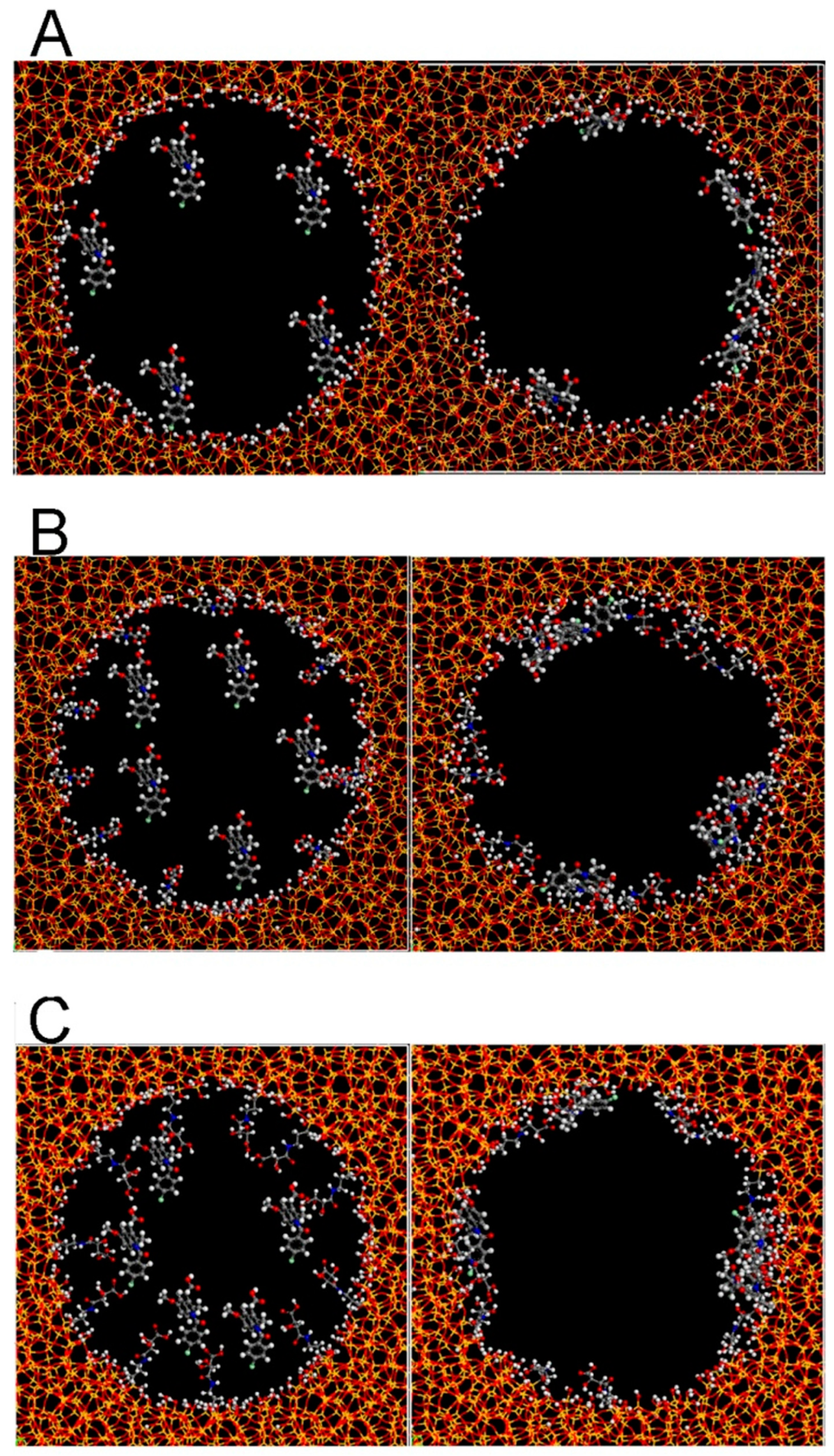
2.4. CMS with Molecular Chirality
3. Applications of Chiral Mesoporous Silica
3.1. Enantioselective Resolution and Adsorption
3.2. Chromatographic Separations
3.3. Chiral Drug Delivery
3.4. Achiral Drug Delivery
3.5. Asymmetric Catalysis
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.; Cheng, C.; Shan, W.; Zhang, Y.; Ren, N.; Yang, W.; Tang, Y. Chiral mesostructured silica nanofibers of MCM-41. Angew. Chem. Int. Ed. Engl. 2006, 45, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Che, S. Chiral mesoporous silica: Chiral construction and imprinting via cooperative self-assembly of amphiphiles and silica precursors. Chem. Soc. Rev. 2011, 40, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Mceleney, K.; Crudden, C.M. Enantioselective catalysis with a chiral, phosphane-containing PMO material. Chem. Commun. 2012, 48, 6369–6371. [Google Scholar] [CrossRef] [PubMed]
- García, R.A.; Morales, V.; Garcés, T. One-step synthesis of a thioester chiral PMO and its use as a catalyst in asymmetric oxidation reactions. J. Mater. Chem. 2012, 22, 2607–2615. [Google Scholar]
- Motealleh, A.; Dorri, P.; Kehr, N.S. Self-assembled monolayers of chiral periodic mesoporous organosilica as a stimuli responsive local drug delivery system. J. Mater. Chem. B 2019, 7, 2362–2371. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Hanabusa, K.; Shinkai, S. Creation of both right-handed and left-handed silica structures by sol-gel transcription of organogel fibers comprised of chiral diaminocyclohexane derivatives. J. Am. Chem. Soc. 2000, 122, 5008–5009. [Google Scholar] [CrossRef]
- Narushima, T.; Okamoto, H. Strong Nanoscale Optical Activity Localized in Two-Dimensional Chiral Metal Nanostructures. J. Phys. Chem. C 2013, 117, 23964–23969. [Google Scholar] [CrossRef]
- Collins, J.T.; Kuppe, C.; Hooper, D.C.; Sibilia, C.; Centini, M.; Ventsislav, V.K. Chirality and Chiroptical Effects in Metal Nanostructures: Fundamentals and Current Trends. Adv. Opt. Mater. 2017, 5, 1700182–1700227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, L. Towards chirality-pure carbon nanotubes. Nanoscale 2010, 2, 1919–1929. [Google Scholar] [CrossRef]
- Sanchez-Valencia, J.R.; Dienel, T.; Groning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.; Ruffieux, P.; Fasel, R. Controlled synthesis of single-chirality carbon nanotubes. Nature 2014, 512, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Magnin, Y.; Amara, H.; Ducastelle, F.; Loiseau, A.; Bichara, C. Entropy-driven stability of chiral single-walled carbon nanotubes. Science 2018, 362, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moloney, M.P.; Gun’ko, Y.K.; Kelly, J.M. Chiral highly luminescent CdS quantum dots. Chem. Commun. 2007, 3900–3902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, M.; Sun, K.; Tang, Z.; Kotov, N.A. Similar topological origin of chiral centers in organic and nanoscale inorganic structures: Effect of stabilizer chirality on optical isomerism and growth of CdTe nanocrystals. J. Am. Chem. Soc. 2010, 132, 6006–6013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bu, X.; Zhang, J.; Wu, T.; Feng, P. Chiral semiconductor frameworks from cadmium sulfide clusters. J. Am. Chem. Soc. 2007, 129, 8412–8413. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Simeon, F.; Sande, J.B.S.; Hatton, T.A. Formation of magnetic nanotubes by the cooperative self-assembly of chiral amphiphilic molecules and Fe3O4 nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 11938–11943. [Google Scholar] [CrossRef]
- Nakashima, T.; Kobayashi, Y.; Kawai, T. Optical Activity and Chiral Memory of Thiol-Capped CdTe Nanocrystals. J. Am. Chem. Soc. 2009, 131, 10342–10343. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Wang, Y.; Chen, H. Emerging chirality in nanoscience. Chem. Soc. Rev. 2013, 42, 2930–2962. [Google Scholar] [CrossRef]
- Fijneman, A.J.; Högblom, J.; Palmlöf, M.; With, G.; Persson, M.; Friedrich, H. Multiscale Colloidal Assembly of Silica Nanoparticles into Microspheres with Tunable Mesopores. Adv. Funct. Mater. 2020, 2002725–2002732. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W.; Skrzypek, I.; Pikus, S.; Kozak, M. Effect of addition of pore expanding agent on changes of structure characteristics of ordered mesoporous silicas. Appl. Surf. Sci. 2008, 255, 2851–2858. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Coté, M.F.; Gaudreault, R.C.; Fortin, M.A.; Kleitz, F. Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.P.H.; Mohammadpour, R.; Ghandehari, H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 311–312, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, S. Surface pH buffering to promote degradation of mesoporous silica nanoparticles under a physiological condition. J. Colloid Interface Sci. 2018, 533, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634–1604684. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhang, W.; Kan, O.; Chen, L.; Xie, L.; Cui, M.; Xi, Z.; Xi, Y.; Li, S.; Xu, L. Preparation and application of mesoporous core-shell nanosilica using leucine derivative as template in effective drug delivery. Chin. Chem. Lett. 2020, 31, 1165–1167. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, N.; Chen, L.; Xie, L.; Cui, M.; Li, S.; Xu, L. Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin. Pharmaceutics 2018, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yu, M.R.; Xi, Z.Y.; Nie, D.; Dai, Z.; Wang, J.; Qian, K.; Weng, H.X.; Gan, Y.; Xu, L. Cancer cell membrane-camouflaged nanorods with endoplasmic reticulum targeting for improved antitumor therapy. ACS Appl. Mater. Interfaces 2019, 11, 46614–46625. [Google Scholar] [CrossRef]
- Che, S.; Liu, Z.; Ohsuna, T.; Sakamoto, K.; Terasaki, O.; Tatsumi, T. Synthesis and characterization of chiral mesoporous silica. Nature 2004, 429, 281–284. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bing, N. Chiral mesoporous silica synthesized with the presence of different anionic acids. Mater. Chem. Phys. 2011, 127, 409–412. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Z.; Ohsuna, T.; Terasaki, O.; Inoue, Y.; Sakamoto, K.; Nakanishi, T.; Ariga, K.; Che, S. Control of Morphology and Helicity of Chiral Mesoporous Silica. Adv. Mater. 2006, 18, 593–596. [Google Scholar] [CrossRef]
- Guo, Z.; Du, Y.; Chen, Y.; Ng, S.C.; Yang, Y. Understanding the mechanism of chirality transfer in the formation of a chiral MCM-41 mesoporous silica. J. Phys. Chem. C 2010, 114, 14353–14361. [Google Scholar] [CrossRef]
- Yokoi, T.; Yamataka, Y.; Ara, Y.; Sato, S.; Kubota, Y.; Tatsumi, T. Synthesis of chiral mesoporous silica by using chiral anionic surfactants. Microporous Mesoporous Mater. 2007, 103, 20–28. [Google Scholar] [CrossRef]
- Yokoi, T.; Ogawa, K.; Lu, D.; Kondo, J.N.; Kubota, Y.; Tatsumi, T. Preparation of chiral mesoporous materials with helicity perfectly controlled. Chem. Mater. 2011, 23, 2014–2016. [Google Scholar] [CrossRef]
- Qiu, H.; Che, S. Tighter packing leads to higher enantiopurity: Effect of basicity on the enantiopurity of N-acylamino acid-templated chiral mesoporous silica. Chem. Lett. 2010, 39, 70–71. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, S.; Zhang, W.; Sakamoto, K.; Terasaki, O.; Inoue, Y.; Che, S. Steric and temperature control of enantiopurity of chiral mesoporous silica. J. Phys. Chem. C 2008, 112, 1871–1877. [Google Scholar] [CrossRef]
- Yang, Y.; Suzuki, M.; Owa, S.; Shirai, H.; Hanabusa, K. Preparation of helical nanostructures using chiral cationic surfactants. Chem. Commun. 2005, 4462–4464. [Google Scholar] [CrossRef]
- Yang, Y.; Suzuki, M.; Owa, S.; Shirai, H.; Hanabusa, K. Control of helical silica nanostructures using a chiral surfactant. J. Mater. Chem. 2006, 16, 1644–1650. [Google Scholar] [CrossRef]
- Wan, X.; Pei, X.; Zhao, H.; Chen, Y.; Guo, Y.; Li, B.; Hanabusa, K.; Yang, Y. The formation of helical mesoporous silica nanotubes. Nanotechnology 2008, 19, 315602–315606. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, J.; Wang, S.; Chen, Y.; Wu, X.; Li, Y.; Li, B.; Yang, Y. Organization of helical mesoporous silica nanotubes. J. Sol-Gel Sci. Technol. 2009, 50, 397–402. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Whitman, C.M.; Lin, S.Y. Morphological control of room-temperature ionic liquid templated mesoporous silica nanoparticles for controlled release of antibacterial agents. Nano Lett. 2004, 4, 2139–2143. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, L.; Ying, J.Y. Entropy-driven helical mesostructure formation with achiral cationic surfactant templates. Adv. Mater. 2007, 19, 2454–2459. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, L.; Yu, C.; Zhou, X.; Tang, J.; Yuan, P.; Chen, D.; Zhao, D. On the origin of helical mesostructures. J. Am. Chem. Soc. 2006, 128, 10460–10466. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Li, Y.; Zhuang, W.; Zhang, M.; Li, B.Z.; Yang, Y.C. Preparation and characterizations of helical mesoporous silica nanorods using CTAB and alcohols. Chin. Chem. Lett. 2012, 23, 1201–1204. [Google Scholar] [CrossRef]
- Cui, M.; Xie, L.; Zhang, S.; Chen, L.; Xi, Y.; Wang, Y.; Guo, Y.; Xu, L. Chiral mesoporous silica based LOFL delivery systems using achiral alcohols as co-structure-directing agents: Construction, characterization, sustained release and antibacterial activity. Colloids Surf. B Biointerfaces 2019, 184, 110483–110489. [Google Scholar] [CrossRef]
- Gao, Z.; Lv, X.; Fu, Y.; Zang, X.; Sun, Y.; Li, S.; Zhang, H.; Qiao, S.; Wang, L.; Sun, Y. Chiral mesoporous silica synthesized by a facile strategy for loading and releasing poorly water-soluble drug. Drug Dev. Ind. Pharm. 2020, 46, 1177–1184. [Google Scholar] [CrossRef]
- Lacasta, S.; Sebastián, V.; Casado, C.; Mayoral, A.; Romero, P.; Larrea, A.; Vispe, E.; Lopez-Ram-de-Viu, P.; Uriel, S.; Coronas, J. Chiral imprinting with amino acids of ordered mesoporous silica exhibiting enantioselectivity after calcination. Chem. Mater. 2011, 23, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Su, M.X.; Liu, Z.Y.; Chen, J.L.; Cheng, L.F.; Li, B.; Yan, F.; Di, B. Stereoselective adsorption utilizing l-phenylalanine imprinting chiral ordered mesoporous silica. RSC Adv. 2014, 4, 54998–55002. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, F.; Li, J.; Wang, Y. Concealed body mesoporous silica nanoparticles for orally delivering indometacin with chiral recognition function. Mater. Sci. Eng. C 2018, 90, 314–324. [Google Scholar] [CrossRef]
- Fan, N.; Liu, R.; Ma, P.; Wang, X.; Li, C.; Li, J. The on-off chiral mesoporous silica nanoparticles for delivering achiral drug in chiral environment. Colloids Surf. B. Biointerfaces 2019, 176, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, L.; Gou, K.; Wang, Y.; Hu, B.; Pang, Y.; Li, S.; Li, H. Functional mesoporous silica nanoparticles for delivering nimesulide with chiral recognition performance. Microporous Mesoporous Mater. 2020, 294, 109862–109872. [Google Scholar] [CrossRef]
- Guo, Y.; Gou, K.; Yang, B.; Wang, Y.; Pu, X.; Li, S.; Li, H. Enlarged pore size chiral mesoporous silica nanoparticles loaded poorly water-soluble drug perform superior delivery effect. Molecules 2019, 24, 3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Li, Y.; Wang, S.; Xu, Z.; Chen, Y.; Li, B.; Zhu, X.; Zhu, G.; Yang, Y. Artificial frustule prepared through a single-templating approach. Chem. Commun. Camb. 2010, 46, 8410–8412. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhao, H.; Pei, X.; Bi, L.; Hanabusa, K.; Yang, Y. From branched self-assemblies to branched mesoporous silica nanoribbons. Chem. Commun. 2008, 47, 6366–6368. [Google Scholar] [CrossRef]
- Snir, Y.; Kamien, R.D. Entropically driven helix formation. Science 2005, 5712, 1067. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Wang, X.; Lin, X.; Wu, X.; Xie, Z. A molecularly imprinted monolith for the fast chiral separation of antiparasitic drugs by pressurized CEC. J. Sep. Sci. 2010, 33, 2123–2130. [Google Scholar] [CrossRef]
- Kulsing, C.; Knob, R.; Macka, M.; Junor, P.; Boysen, R.I.; Hearn, M.T.W. Molecular imprinted polymeric porous layers in open tubular capillaries for chiral separations. J. Chromatogr. A 2014, 1354, 85–91. [Google Scholar] [CrossRef]
- Huang, Q.; Li, H.; Guo, T.; Li, S.; Shen, G.; Ban, C.; Liu, J. Chiral separation of (d, l)-lactic acid through molecularly imprinted cellulose acetate composite membrane. Cellulose 2018, 25, 3435–3448. [Google Scholar] [CrossRef]
- Alatawi, R.A.; Monier, M.; Elsayed, N.H. Chiral separation of (±)-methamphetamine racemate using molecularly imprinted sulfonic acid functionalized resin. J. Colloid Interface Sci. 2018, 531, 654–663. [Google Scholar] [CrossRef]
- Suedee, R.; Jantarat, C.; Lindner, W.; Viernstein, H.; Songkro, S.; Srichana, T. Development of a pH-responsive drug delivery system for enantioselective-controlled delivery of racemic drugs. J. Control. Release 2010, 142, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Ihara, M.; Kuroda, S.; Tsuzuki, H.; Ueda, H. Open-sandwich molecular imprinting: Making a recognition matrix with antigen-imprinted antibody fragments. Bioconjugate Chem. 2012, 23, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Rangel, P.X.M.; Moroni, E.; Merlier, F.; Gheber, L.A.; Vago, R.; Bui, B.T.S.; Haupt, K. Chemical antibody mimics inhibit cadherin-mediated cell–cell adhesion: A promising strategy for cancer therapy. Angew. Chem. Int. Ed. 2020, 59, 2816–2822. [Google Scholar] [CrossRef]
- Xu, J.; Miao, H.; Wang, J.; Pan, G. Molecularly imprinted synthetic antibodies: From chemical design to biomedical applications. Small 2020, 16, 1906644–1906664. [Google Scholar] [CrossRef]
- Shukla, N.; Bartel, M.A.; Gellman, A.J. Enantioselective separation on chiral Au nanoparticles. J. Am. Chem. Soc. 2010, 132, 8575–8580. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Cardenas, S.; Simonet, B.M.; Valcarcel, M. Selective quantification of carnitine enantiomers using chiral cysteine-capped CdSe (ZnS) quantum dots. Anal. Chem. 2009, 81, 4730–4733. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Ma, W.; Wang, L.; Kuang, H.; Xu, C.; Kotov, N.A. Shell-engineered chiroplasmonic assemblies of nanoparticles for zeptomolar DNA detection. Nano Lett. 2014, 14, 3908–3913. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Ma, W.; Wu, X.; Sun, M.; Kuang, H.; Wang, L.; Kotov, N.A.; Xu, C. Dual-mode ultrasensitive quantification of microRNA in living cells by chiroplasmonic nanopyramids self-assembled from gold and upconversion nanoparticles. J. Am. Chem. Soc. 2016, 138, 306–312. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly imprinted polymers for the identification and separation of chiral drugs and biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef]
- Banerjee-Ghosh, K.; Dor, O.B.; Tassinari, F.; Capua, E.; Yochelis, S.; Capua, A.; Yang, S.H.; Parkin, S.S.; Sarkar, S.; Kronik, L. Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. Science 2018, 360, 1331–1334. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.L.; Yang, C.X.; Yan, X.P. Bottom-up synthesis of chiral covalent organic frameworks and their bound capillaries for chiral separation. Nat. Commun. 2016, 7, 12104–12110. [Google Scholar] [CrossRef] [PubMed]
- Di, B.; Cheng, L.; Jiang, Q.; Su, M.; Hao, W. Anionic surfactant templated chiral nanospheres and their enantioselective adsorption. New J. Chem. 2013, 37, 1603–1609. [Google Scholar] [CrossRef]
- Casado, C.; Castan, J.; Gracia, I.; Yus, M.; Mayoral, A.; Sebastian, V.; Lopez-Ram-de-Viu, P.; Uriel, S.; Coronas, J. l- and d-proline adsorption by chiral ordered mesoporous silica. Langmuir 2012, 28, 6638–6644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, P.; Gedanken, A.; Mastai, Y. Enantioselective separation using chiral mesoporous spherical silica prepared by templating of chiral block copolymers. ACS Appl. Mater. Interfaces 2009, 1, 1834–1842. [Google Scholar] [CrossRef]
- Paik, P.; Gedanken, A.; Mastai, Y. Chiral separation abilities: Aspartic acid block copolymer-imprinted mesoporous silica. Microporous Mesoporous Mater. 2010, 129, 82–89. [Google Scholar] [CrossRef]
- Fireman-Shoresh, S.; Marx, S.; Avnir, D. Enantioselective sol–gel materials obtained by either doping or imprinting with a chiral surfactant. Adv. Mater. 2007, 19, 2145–2150. [Google Scholar] [CrossRef]
- Marx, S.; Avnir, D. The induction of chirality in sol–gel materials. Acc. Chem. Res. 2007, 40, 768–776. [Google Scholar] [CrossRef]
- Huang, Y.; Vidal, X.; Garcia-Bennett, A.E. Chiral resolution using supramolecular-templated mesostructured materials. Angew. Chem. Int. Ed. Engl. 2019, 58, 10859–10862. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, G.; Wang, D.; Liang, G.; Chen, Y. Chiral separation and enantiomeric purity determination of pazufloxacin mesilate by HPLC using chiral mobile phase additives. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 813–827. [Google Scholar] [CrossRef]
- Bhushan, R.; Tanwar, S. Different approaches of impregnation for resolution of enantiomers of atenolol, propranolol and salbutamol using Cu (II)-l-amino acid complexes for ligand exchange on commercial thin layer chromatographic plates. J. Chromatogr. A 2010, 1217, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.M.; Yuan, L.M. Recent progress of chiral stationary phases for separation of enantiomers in gas chromatography. J. Sep. Sci. 2017, 40, 124–137. [Google Scholar] [CrossRef]
- Fanali, C.; Fanali, S.; Chankvetadze, B. HPLC separation of enantiomers of some flavanone derivatives using polysaccharide-based chiral selectors covalently immobilized on silica. Chromatographia 2016, 79, 119–124. [Google Scholar] [CrossRef]
- Senra, J.D.; Malta, L.F.B.; Ceva-Antunes, P.; Corrêa, R.J.; Antunes, O. Elucidation of chiral Recognition mechanism of alpha-amino acids using ligand exchange high performance liquid chromatography. J. Braz. Chem. Soc. 2007, 18, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hu, X.; Wang, Q.; He, P. Investigation of hydroxypropyl-β-cyclodextrin-based synergistic system with chiral nematic mesoporous silica as chiral stationary phase for enantiomeric separation in microchip electrophoresis. Talanta 2020, 218, 121121–121127. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.M.; Zhang, M.; Zi, M.; He, P.G.; Yuan, L.M. Novel inorganic mesoporous material with chiral nematic structure derived from nanocrystalline cellulose for high-resolution gas chromatographic separations. Anal. Chem. 2014, 86, 9595–9602. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, M.; Xie, S.M.; He, P.G.; Yuan, L.M. A novel inorganic mesoporous material with a nematic structure derived from nanocrystalline cellulose as the stationary phase for high-performance liquid chromatography. Anal. Methods 2015, 7, 3448–3453. [Google Scholar] [CrossRef]
- Li, Y.X.; Fu, S.G.; Zhang, J.H.; Xie, S.M.; Li, L.; He, Y.Y.; Zi, M.; Yuan, L.M. A highly ordered chiral inorganic mesoporous material used as stationary phase for high-resolution gas chromatographic separations. J. Chromatogr. A 2018, 1557, 99–106. [Google Scholar] [CrossRef]
- He, Y.Y.; Zhang, J.H.; Pu, Q.; Xie, S.M.; Li, Y.X.; Luo, L.; Chen, X.X.; Yuan, L.M. A novel chiral inorganic mesoporous silica used as a stationary phase in GC. Chirality 2019, 31, 1053–1059. [Google Scholar] [CrossRef]
- Wu, X.; Jin, H.; Liu, Z.; Ohsuna, T.; Terasaki, O.; Sakamoto, K.; Che, S. Racemic Helical Mesoporous Silica Formation by Achiral Anionic Surfactant. Chem. Mater. 2006, 18, 241–243. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Pan, W.; Yu, Z.; Yang, L.; Tang, B. Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 2123–2129. [Google Scholar] [CrossRef]
- De Oliveira Freitas, L.B.; De Melo Corgosinho, L.; Faria, J.A.Q.A.; Dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; De Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Ramila, A.; Del Real, R.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, J.; Liu, Y.; Huang, L.; Qiao, J.; Liu, X.; Wei, J.; Guan, Q. Effects of degree of substitution on stearic acid-modified Bletilla striata polysaccharides nanoparticles and interactions between nanoparticles and bovine serum albumin. Chin. Chem. Lett. 2018, 29, 1861–1864. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, P.; Chen, Z. Dialysis preparation of smart redox and acidity dual responsive tea polyphenol functionalized calcium phosphate nanospheres as anticancer drug carriers. Molecules 2020, 25, 1221. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Zhu, T.; Huang, X.; Sun, M.; Li, H.; Zhu, X.; Liu, M.; Xie, Y.; Huang, W.; Yan, D. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: Light-triggered size-reducing and enhanced tumor penetration. Biomaterials 2019, 211, 68–80. [Google Scholar] [CrossRef]
- Yao, Y.; Saw, P.E.; Nie, Y.; Wong, P.P.; Jiang, L.; Ye, X.; Chen, J.; Ding, T.; Xu, L.; Yao, H. Multifunctional sharp pH-responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J. Mater. Chem. B 2019, 7, 576–585. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Zimmerman, D.; Smits, S.; Hynes, M.; Cantrell, B.; Leander, J.; Mendelsohn, L.; Nickander, R. Picenadol. Drug Alcohol Depend. 1985, 14, 381–401. [Google Scholar] [CrossRef]
- Guo, Z.; Du, Y.; Liu, X.; Ng, S.C.; Chen, Y.; Yang, Y. Enantioselectively controlled release of chiral drug (metoprolol) using chiral mesoporous silica materials. Nanotechnology 2010, 21, 165103–165113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, W.; Liu, T.; Xu, L.; Guo, Y.; Ke, J.; Li, S.; Li, H. Design and preparation of mesoporous silica carriers with chiral structures for drug release differentiation. Mater. Sci. Eng. C 2019, 103, 109737–109744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ke, J.; Gou, K.; Guo, Y.; Xu, X.; Li, S.; Li, H. Amino functionalized mesoporous silica with twisted rod-like shapes: Synthetic design, in vitro and in vivo evaluation for ibuprofen delivery. Microporous Mesoporous Mater. 2020, 294, 109896–109926. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, S.; Jin, Y.; Cheng, L.; Yan, Z.; Lu, G.Q. Hydrophobic functional group initiated helical mesostructured silica for controlled drug release. Adv. Funct. Mater. 2008, 18, 3834–3842. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Fan, N.; Li, J.; Zhang, H.; Shang, L.; He, Z.; Sun, J. Amino functionalized chiral mesoporous silica nanoparticles for improved loading and release of poorly water-soluble drug. Asian J. Pharm. Sci. 2019, 14, 405–412. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wei, C.; Ke, J.; Li, J.; Xu, L.; Liu, H.; Yang, Y.; Li, S.; Yang, M. Biomimetic synthesis and evaluation of histidine-derivative templated chiral mesoporous silica for improved oral delivery of the poorly water-soluble drug, nimodipine. Eur. J. Pharm. Sci. 2018, 117, 321–330. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Li, J.; Li, S.; Li, H. Superiority of l-tartaric acid modified chiral mesoporous silica nanoparticle as a drug carrier: Structure, wettability, degradation, bio-adhesion and biocompatibility. Int. J. Nanomed. 2020, 15, 601–618. [Google Scholar] [CrossRef] [Green Version]
- Noyori, R. Asymmetric catalysis: Science and opportunities (Nobel lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022. [Google Scholar] [CrossRef]
- Kawasaki, T.; Araki, Y.; Hatase, K.; Suzuki, K.; Matsumoto, A.; Yokoi, T.; Kubota, Y.; Tatsumi, T.; Soai, K. Helical mesoporous silica as an inorganic heterogeneous chiral trigger for asymmetric autocatalysis with amplification of enantiomeric excess. Chem. Commun. 2015, 51, 8742–8744. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Synthesis Method | Morphology | Textural Properties | References | ||
|---|---|---|---|---|---|
| Specific Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) | |||
| Chiral anionic surfactant C14-l-AlaS as a template, TMAPS, or APS as CSDA. | Twisted hexagonal rod-like | 600 | 2.20 | 0.37 | [30] |
| Chiral anionic amphiphilic molecules (N-acyl-l-alanine) as template, TMAPS as CSDA, and various acids including HCl, HBr, HNO3, or H2SO4. | Twisted hexagonal rod-like | 577–974 | 2.40–2.96 | 0.43–0.90 | [31] |
| Chiral anionic surfactant C14-l-AlaS as a template, APTES as CSDA | Twisted-ribbon-like and various twisted-rod-like | 400 | 3.60 | 0.50 | [32] |
| Achiral CTAB as template and a chiral metal complex Λ-[Co(+)(chxn)3]I3 as contemplate. | Twisted hexagonal rod-like | 1047 | 2.60 | 1.90 | [33] |
| Chiral anionic surfactants C14-l/d-AlaA as template. | Twisted hexagonal rod-like | 603; 735 | 2.20 | N/A | [34] |
| Chiral anionic surfactant, C14-l/d-AlaA, and an organosilane compound, BTEE, in the presence of l/d-arginine. | Twisted hexagonal rod-like | 853 | 2.10 | 0.66 | [35] |
| Chiral anionic surfactant C16-l-Ala, C16-l-Val C16-l-Ile, and C16-l-Phe as templates and TMAPS as CSDA. | Twisted hexagonal rod-like | N/A | N/A | N/A | [36] |
| Chiral anionic surfactant N-acylamino acid with a bulky substituent as template and TMAPS as CSDA. | Twisted hexagonal rod-like | 496–884 | 2.10–3.20 | 0.23–0.99 | [37] |
| Chiral cationic surfactant l-4PyCl as template by sol-gel transcription in different solvents. | Helical | 730 | 3.80 | 1.56 | [38,39] |
| Chiral cationic gelators l-PhePyBr, l-ValPyBr, and l-IlePyBr as template by sol-gel transcription. | Helical, balls | 354; 628 | 5.20; 5.00 | N/A | [40,41] |
| Room-temperature ionic liquids 1-octadecyl-3-methylimidazolium bromide as template | Helical | 893 | 3.270 | 0.99 | [42] |
| Achiral cationic surfactant CTAB as template. | Helical | 998 | 2.80 | N/A | [43] |
| Achiral cationic surfactant CTAB and perfluorooctanoic acid as co-templates. | Helical rod-like | 462–635 | 2.60 | 0.59–0.73 | [44] |
| Achiral cationic surfactant CTAB as template and achiral alcohols as CSDA. | Helical | 459–842 | 2.00–4.20 | N/A | [45] |
| CTAB as a template and either n-heptanol or n-nonanol as CSDA. | Twisted hexagonal rod-like | 930; 1329 | 6.83; 6.97 | 1.40; 2.30 | [46] |
| Achiral cationic surfactant CTAB as template. | Helical rod-like | 1027 | 3.40 | 1.02 | [47] |
| Achiral cationic surfactant C18-TMS as a template in the presence of arginine, histidine, isoleucine, and proline in basic media. | Pores with an “eight-like” morphology | 840–1130 | 2.80–3.30 | 0.80–1.13 | [48] |
| Tetraethyl orthosilicate and quaternized aminosilane as silica sources together with l-phenylalanine as a chiral imprinted reagent | Irregular | 730 | 2.30–2.50 | 0.47 | [49] |
| Achiral cationic surfactant CTAB as template together with APTTES-l or APTTES-d (synthesized with APTES and l/d-tartaric acid) as chiral silica coupling agent. | Spherical | 565; 387 | 3.30; 3.20 | 0.40; 0.30 | [50] |
| Achiral cationic surfactant CTAB as template together with APTTES-l or APTTES-d (synthesized with APTES and l/d-tartaric acid) as chiral silica coupling agent. | Spherical | 578; 441 | 3.47; 2.98 | 0.50; 0.33 | [51] |
| Achiral cationic surfactant STAB as template together with APTTES-l or APTTES-d (synthesized with APTES and l/d-tartaric acid) as chiral silica coupling agent. | Spherical | 571; 585 | 2.71; 2.74 | 0.69; 0.75 | [52] |
| Achiral cationic surfactant STAB as template together with APTTES-d (synthesized with APTES and d-tartaric acid) as chiral silica coupling agent, and TMB as pore-enlarging agent. | Spherical | 441; 474 | 2.20; 4.30 | 0.36; 1.10 | [53] |
| Applications | Compound or Drug | Key Points | Reference |
|---|---|---|---|
| Enantioselective adsorption | l- and d-alanine | With a chiral selectivity factor of 3.15 | [74] |
| Enantioselective adsorption | l- and d-proline | l/d-proline shows different adsorption preference on CMS prepared with l-proline or d-proline | [75] |
| Enantioselective adsorption | Racemic valine | With a chiral selectivity factor of 5.22 | [76] |
| Enantioselective resolution | Racemic valine and alanine | With a chiral selectivity factor of 7.52 for alanine and high enantiomeric excess of ca. 45% for valine | [77] |
| Enantioselective resolution | Racemic proline, isoleucine, trans-4-hydroxyproline, pipecolic acid, valine, leucine, and phenylglycine | The CMS prepared via chiral imprinting still exhibits enantioselectivity for racemic mixtures after calcination. | [48] |
| Stereoselective adsorption | d,l phenylalanine, d,l alanine, d,l tryptophan, d,l lysine, racemic naproxen, and racemic chlorpheniramine maleate | l-Phenylalanine imprinting exhibits enantioselective adsorption for l- and d-phenylalanine with a chiral selectivity factor of 3.24, and desirable stereoselective adsorption capacity for other racemic mixtures | [49] |
| Enantiomeric adsorption | d-(−)-Tartaric acid and l-(+)-Tartaric acid, l-valine, and d-Valine, as well as (+)-a-Pinene and (−)-a-Pinene | Supramolecular templated materials prepared with guanosine monophosphate (NGM-1) and folic acid have opposite enantiomeric selectivity for enantiomeric pairs | [80] |
| Chromatographic separations | Isomers, PAHs, linear alkanes, long-chain alkanes, Grob’stest mixture, aromatic hydrocarbons, and chiral compounds | As stationary phase for high-resolution gas chromatography separations | [89] |
| Chromatographic separations | Phenylalanine, tryptophan, glutamic, alanine, serine, aspartic acid, cysteine, methionine, tyrosine, and histidine | As chiral stationary phase, with hydroxypropyl-β-cyclodextrin (HP-β-CD) as the chiral selector for enantioseparation using MCE | [86] |
| Chiral drug delivery | Levofloxacin | In vitro sustained drug release and antibacterial activity of levofloxacin | [46] |
| Chiral drug delivery | Metoprolol | In vitro enantioselective controlled release | [103] |
| Chiral drug delivery | Ibuprofen | Release differentiation, controlled release in vitro, favorable oral bioavailability, elimination half-life, and anti-inflammatory effect in vivo. | [104,105] |
| Achiral drug delivery | Aspirin | In vitro controlled release | [106] |
| Achiral drug delivery | Indomethacin | Improved in vitro dissolution of poorly water-soluble drug | [107] |
| Achiral drug delivery | Curcumin | Improved in vitro dissolution of poorly water-soluble drug | [47] |
| Achiral drug delivery | Nimodipine | Improved in vitro dissolution; enhanced bioavailability, therapeutic effect, and brain distribution in vivo | [108] |
| Achiral drug delivery | Indometacin | Improved in vitro dissolution; enhanced in vivo oral bioavailability and anti-inflammatory effect | [50] |
| Achiral drug delivery | Indometacin | Different chiral recognition functions in the in-vitro chiral dissolution medium | [51] |
| Achiral drug delivery | Nimesulide | Different chiral recognition functions in the in-vitro chiral dissolution medium; enhanced in vivo oral bioavailability and anti-inflammatory effect | [52] |
| Achiral drug delivery | Nimesulide | Improved in vitro dissolution; enhanced bioavailability and anti-inflammatory effect in vivo | [53] |
| Asymmetric catalysis | Diisopropylzinc, pyrimidine-5-carbaldehyde 1 | A chiral inorganic trigger of asymmetric autocatalysis | [111] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Zhang, W.; Xie, L.; Chen, L.; Xu, L. Chiral Mesoporous Silica Materials: A Review on Synthetic Strategies and Applications. Molecules 2020, 25, 3899. https://doi.org/10.3390/molecules25173899
Cui M, Zhang W, Xie L, Chen L, Xu L. Chiral Mesoporous Silica Materials: A Review on Synthetic Strategies and Applications. Molecules. 2020; 25(17):3899. https://doi.org/10.3390/molecules25173899
Chicago/Turabian StyleCui, Mingshu, Wei Zhang, Luyao Xie, Lu Chen, and Lu Xu. 2020. "Chiral Mesoporous Silica Materials: A Review on Synthetic Strategies and Applications" Molecules 25, no. 17: 3899. https://doi.org/10.3390/molecules25173899






