Influence of Chiral Compounds on the Oxygen Evolution Reaction (OER) in the Water Splitting Process
Abstract
:1. Introduction
2. Results and Discussion
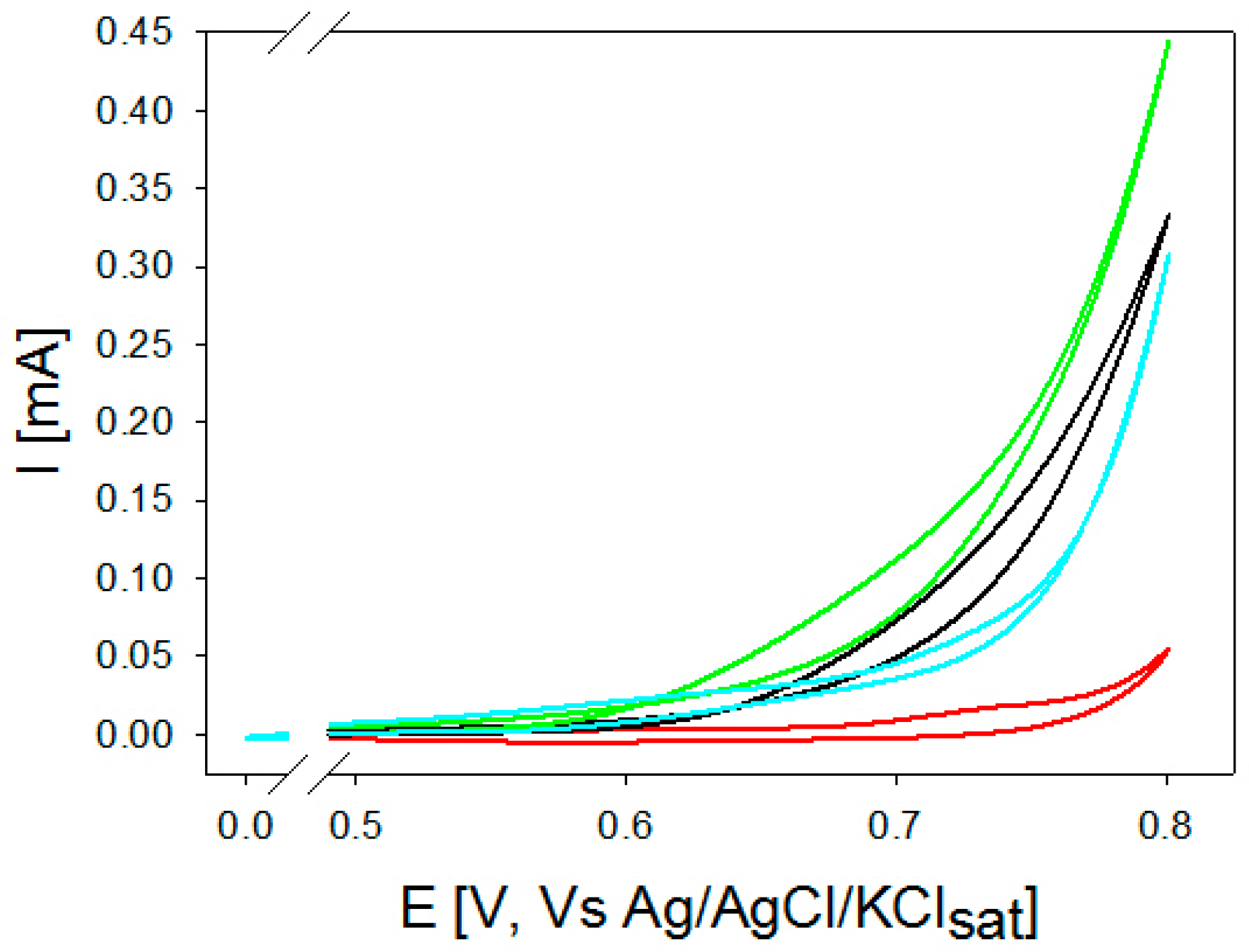
2.1. Steel AISI 316L
2.1.1. l-(+)-Tartaric Acid
2.1.2. l-(−)-Aspartic Acid
2.1.3. d-(+)-Glucose
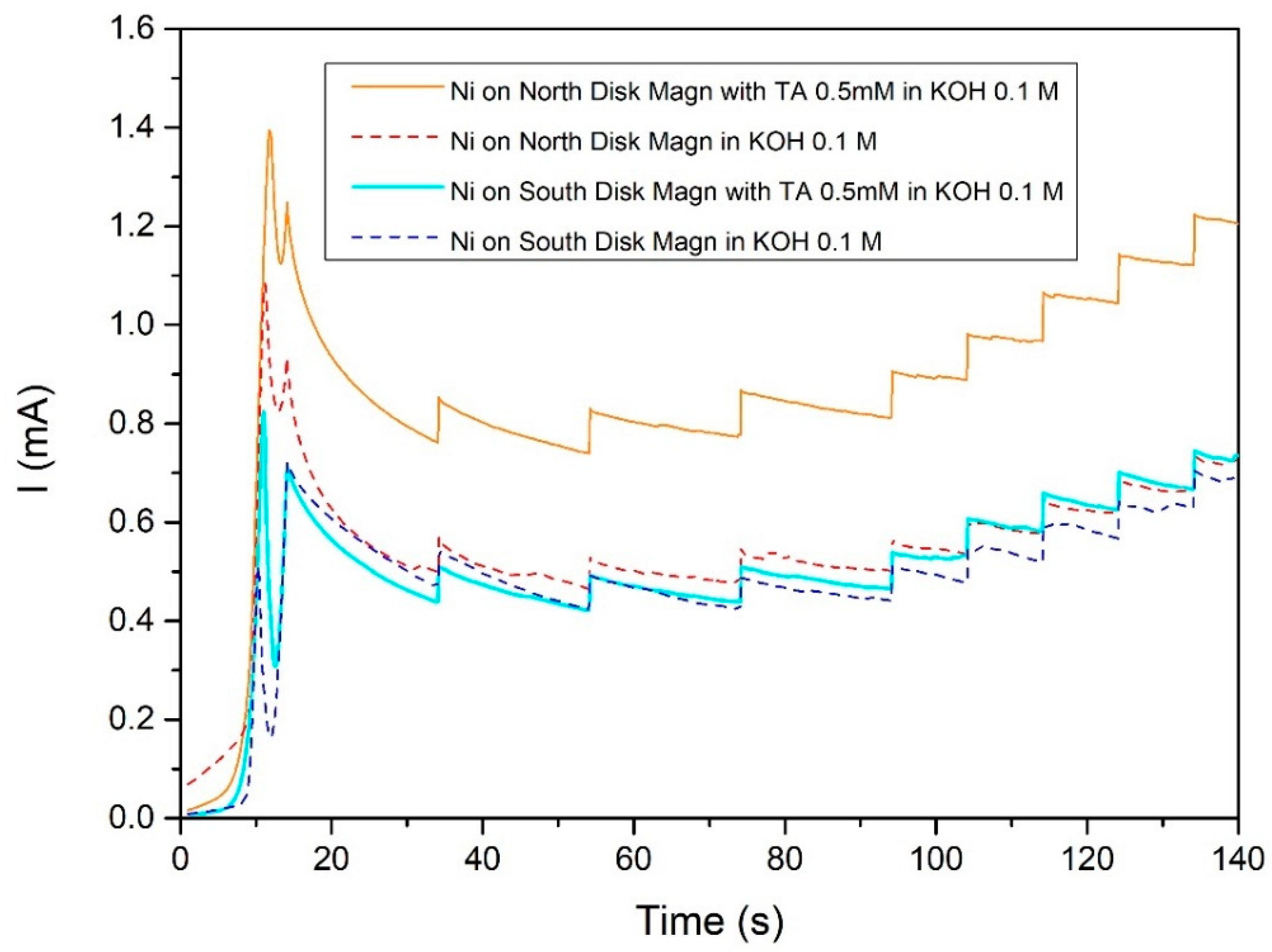
2.2. Nickel Electrodeposited on Magnet
d-(+)-Glucose
2.3. Step and Sweeps
2.3.1. Step and Sweeps on AISI 316 L
2.3.2. Step and Sweeps Ni-on-magnet
3. Experimental
3.1. Materials
3.2. Methods
3.3. Electrochemical System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beiter, P.; Tian, T. 2015 Renewable Energy Data Book; National Renewable Energy Lab (NREL): Golden, CO, USA, 2016.
- Sinsel, S.R.; Riemke, R.L.; Hoffmann, V.H. Challenges and solution technologies for the integration of variable renewable energy sources—A review. Renew. Energy 2020, 145, 2271–2285. [Google Scholar] [CrossRef]
- Global Energy Review 2019—Analysis. Available online: https://www.iea.org/reports/global-energy-review-2019 (accessed on 20 August 2020).
- BP Statistical Review of World Energy, 68th ed. 2019. Available online: www.bp.com (accessed on 29 August 2020).
- IEA. The Future of Hydrogen. 2019. Available online: https://www.iea.org/publications/reports/thefutureofhydrogen/ (accessed on 29 August 2020).
- AlRafea, K.; Fowler, M.; Elkamel, A.; Hajimiragha, A. Integration of renewable energy sources into combined cycle power plants through electrolysis generated hydrogen in a new designed energy hub. Int. J. Hydrogen Energy 2016, 41, 16718–16728. [Google Scholar] [CrossRef]
- Krajačić, G.; Martins, R.; Busuttil, A.; Duić, N.; da Graça Carvalho, M. Hydrogen as an energy vector in the islands’ energy supply. Int. J. Hydrogen Energy 2008, 33, 1091–1103. [Google Scholar] [CrossRef]
- Ntziachristos, L.; Kouridis, C.; Samaras, Z.; Pattas, K. A wind-power fuel-cell hybrid system study on the non-interconnected Aegean islands grid. Renew. Energy 2005, 30, 1471–1487. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Mtangi, W.; Kiran, V.; Fontanesi, C.; Naaman, R. Role of the electron spin polarization in water splitting. J. Phys. Chem. Lett. 2015, 6, 4916–4922. [Google Scholar] [CrossRef] [Green Version]
- Mtangi, W.; Tassinari, F.; Vankayala, K.; Vargas Jentzsch, A.; Adelizzi, B.; Palmans, A.R.A.; Fontanesi, C.; Meijer, E.W.; Naaman, R. Control of electrons’ spin eliminates hydrogen peroxide formation during water splitting. J. Am. Chem. Soc. 2017, 139, 2794–2798. [Google Scholar] [CrossRef]
- Fontanesi, C.; Naaman, R.; Mtangi, W. Water Splitting Method and System. WO/2016/056011, 14 April 2016. [Google Scholar]
- Liang, X.; Liu, J.; Zeng, D.; Li, C.; Chen, S.; Li, H. Hydrogen generation promoted by photocatalytic oxidation of ascorbate and glucose at a cadmium sulfide electrode. Electrochim. Acta 2016, 198, 40–48. [Google Scholar] [CrossRef]
- Garcés-Pineda, F.A.; Blasco-Ahicart, M.; Nieto-Castro, D.; López, N.; Galán-Mascarós, J.R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 2019, 4, 519–525. [Google Scholar] [CrossRef]
- Haschke, S.; Pankin, D.; Petrov, Y.; Bochmann, S.; Manshina, A.; Bachmann, J. Design rules for oxygen evolution catalysis at porous iron oxide electrodes: A 1000-fold current density increase. ChemSusChem 2017, 10, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Morvillo, P.; Parenti, F.; Diana, R.; Fontanesi, C.; Mucci, A.; Tassinari, F.; Schenetti, L. A novel copolymer from benzodithiophene and alkylsulfanyl-bithiophene: Synthesis, characterization and application in polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 104, 45–52. [Google Scholar] [CrossRef]
- Morvillo, P.; Diana, R.; Fontanesi, C.; Ricciardi, R.; Lanzi, M.; Mucci, A.; Tassinari, F.; Schenetti, L.; Minarini, C.; Parenti, F. Low band gap polymers for application in solar cells: Synthesis and characterization of thienothiophene-thiophene copolymers. Polym. Chem. 2013. [Google Scholar] [CrossRef]
- Appleyard, S.J. Simple photovoltaic cells for exploring solar energy concepts. Phys. Educ. 2006, 41, 409. [Google Scholar] [CrossRef]
- Mishra, D.; Markus, T.Z.; Naaman, R.; Kettner, M.; Göhler, B.; Zacharias, H.; Friedman, N.; Sheves, M.; Fontanesi, C. Spin-dependent electron transmission through bacteriorhodopsin embedded in purple membrane. Proc. Natl. Acad. Sci. USA 2013, 110, 14872–14876. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.; Ananthavel, S.P.; Waldeck, D.H.; Naaman, R. Asymmetric scattering of polarized electrons by organized organic films of chiral molecules. Science 1999, 283, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, I.; Kumar, K.S.; Heifler, O.; Carmeli, C.; Naaman, R. Spin selectivity in electron transfer in photosystem, I. Angew. Chem. Int. Ed. 2014, 53, 8953–8958. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Chiral-induced spin selectivity effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. [Google Scholar] [CrossRef]
- Tassinari, F.; Mathew, S.P.; Fontanesi, C.; Schenetti, L.; Naaman, R. Electric-field-driven alignment of chiral conductive polymer thin films. Langmuir 2014, 30, 4838–4843. [Google Scholar] [CrossRef]
- Rizzo, S.; Arnaboldi, S.; Mihali, V.; Cirilli, R.; Forni, A.; Gennaro, A.; Isse, A.A.; Pierini, M.; Mussini, P.R.; Sannicolò, F. “Inherently chiral” ionic-liquid media: Effective chiral electroanalysis on achiral electrodes. Angew. Chem. Int. Ed. 2017, 56, 2079–2082. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Sala, X.; Maji, S.; Bofill, R.; García-Antón, J.; Escriche, L.; Llobet, A. Molecular water oxidation mechanisms followed by transition metals: State of the art. Acc. Chem. Res. 2014, 47, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. Chem. Cat. Chem. 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Giovanardi, R.; Fontanesi, C.; Dallabarba, W. Adsorption of organic compounds at the aluminium oxide/aqueous solution interface during the aluminium anodizing process. Electrochim. Acta 2011, 56, 3128–3138. [Google Scholar] [CrossRef]
- Fontanesi, C.; Benassi, R.; Giovanardi, R.; Marcaccio, M.; Paolucci, F.; Roffia, S. Computational electrochemistry. Ab initio calculation of solvent effect in the multiple electroreduction of polypyridinic compounds. J. Mol. Struct. 2002, 612, 277–286. [Google Scholar] [CrossRef]
- Fontanesi, C. Theoretical study of the dissociative process of the 4-chlorotoluene radical anion. J. Mol. Struct. THEOCHEM 1997, 392, 87–94. [Google Scholar] [CrossRef]
- Marcaccio, M.; Paolucci, F.; Fontanesi, C.; Fioravanti, G.; Zanarini, S. Electrochemistry and spectroelectrochemistry of polypyridine ligands: A theoretical approach. Inorg. Chim. Acta 2007, 360, 1154–1162. [Google Scholar] [CrossRef]
- Pozio, A.; Masci, A.; Pasquali, M. Nickel-TiO2 nanotube anode for photo-electrolysers. Sol. Energy 2016, 136, 590–596. [Google Scholar] [CrossRef]
- Kuang, Y.; Yamada, T.; Domen, K. Surface and interface engineering for photoelectrochemical water oxidation. Joule 2017. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, M.; Stanescu, D.; Alvarez, J.; Foy, E.; Kleider, J.-P.; Magnan, H.; Minea, T.; Herlin-Boime, N.; Bouchet-Fabre, B.; Hugon, M.-C. The role of oxygen in magnetron-sputtered Ta3N5 thin films for the photoelectrolysis of water. Surf. Coat. Technol. 2017, 324, 620–625. [Google Scholar] [CrossRef]
- Gazzotti, M.; Arnaboldi, S.; Grecchi, S.; Giovanardi, R.; Cannio, M.; Pasquali, L.; Giacomino, A.; Abollino, O.; Fontanesi, C. Spin-dependent electrochemistry: Enantio-selectivity driven by chiral-induced spin selectivity effect. Electrochim. Acta 2018, 286, 271–278. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzotti, M.; Stefani, A.; Bonechi, M.; Giurlani, W.; Innocenti, M.; Fontanesi, C. Influence of Chiral Compounds on the Oxygen Evolution Reaction (OER) in the Water Splitting Process. Molecules 2020, 25, 3988. https://doi.org/10.3390/molecules25173988
Gazzotti M, Stefani A, Bonechi M, Giurlani W, Innocenti M, Fontanesi C. Influence of Chiral Compounds on the Oxygen Evolution Reaction (OER) in the Water Splitting Process. Molecules. 2020; 25(17):3988. https://doi.org/10.3390/molecules25173988
Chicago/Turabian StyleGazzotti, Mirko, Andrea Stefani, Marco Bonechi, Walter Giurlani, Massimo Innocenti, and Claudio Fontanesi. 2020. "Influence of Chiral Compounds on the Oxygen Evolution Reaction (OER) in the Water Splitting Process" Molecules 25, no. 17: 3988. https://doi.org/10.3390/molecules25173988
APA StyleGazzotti, M., Stefani, A., Bonechi, M., Giurlani, W., Innocenti, M., & Fontanesi, C. (2020). Influence of Chiral Compounds on the Oxygen Evolution Reaction (OER) in the Water Splitting Process. Molecules, 25(17), 3988. https://doi.org/10.3390/molecules25173988