Optimization of Extraction Conditions for Gracilaria gracilis Extracts and Their Antioxidative Stability as Part of Microfiber Food Coating Additives
Abstract
1. Introduction
2. Results and Discussion
2.1. Selective Optimization of Extraction Conditions
2.2. Results of Electrospinning Technique
2.3. Antioxidant Stability of Electrospun Coatings
3. Materials and Methods
3.1. Materials
3.2. Selective Optimization of Extraction Conditions
3.3. Antioxidant Activity Assays
3.4. Electrospinning Technique
3.5. Antioxidant Stability of Electrospun Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schumann, B.; Schmid, M. Packaging concepts for fresh and processed meat—Recent progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Silva, L.T.; de Souza, C.O.; da Silva, J.R.; Albuquerque, E.C.C.; Druzian, J.I. Coffee-cocoa additives for bio-based antioxidant packaging. Food Packag. Shelf Life 2018, 18, 37–41. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Salmieri, S.; Lacroix, M. Physicochemical properties of alginate/polycaprolactone-based films containing essential oils. J. Agric. Food Chem. 2006, 54, 10205–10214. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Zepeda, E.; Freile-Pelegrín, Y.; Robledo, D. Nutraceutical assessment of Solieria filiformis and Gracilaria cornea (Rhodophyta) under light quality modulation in culture. J. Appl. Phycol. 2020, 1–11. [Google Scholar] [CrossRef]
- Greiner, A.; Wendroff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. ChemInform 2007, 38, 5670–5703. [Google Scholar] [CrossRef]
- Abd El-aziz, A.M.; El-Maghraby, A.; Taha, N.A. Comparison between polyvinyl alcohol (PVA) nanofiber and polyvinyl alcohol (PVA) nanofiber/hydroxyapatite (HA) for removal of Zn2+ ions from wastewater. Arab. J. Chem. 2017, 10, 1052–1060. [Google Scholar] [CrossRef]
- Yeum, J.H.; Yang, S.B.; Sabina, Y. Fabrication of Highly Aligned Poly(Vinyl Alcohol) Nanofibers and its Yarn by Electrospinning. In Electrospinning-Material, Techniques, and Biomedical Applications; Haider, S., Haider, A., Eds.; InTech: London, UK, 2016; pp. 47–65. [Google Scholar]
- Nishinari, K.; Fang, Y. Relation between structure and rheological/thermal properties of agar. A mini-review on the effect of alkali treatment and the role of agaropectin. Food Struct. 2017, 13, 24–34. [Google Scholar] [CrossRef]
- Melo, M.R.S.; Feitosa, J.P.A.; Freitas, A.L.P.; De Paula, R.C.M. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491–498. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, A.V.; Sendón, R.; Abad, M.J.; González-Rodríguez, M.V.; Barros-Velázquez, J.; Aubourg, S.P.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quiros, A. Migration kinetics of sorbic acid from polylactic acid and seaweed based films into food simulants. Lwt-Food Sci. Technol. 2016, 65, 630–636. [Google Scholar]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. Extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Déléris, P.; Fleurence, J.; Nguyen-Le, C.T.; Vo, K.H.; Dumay, J. Purification of R-phycoerythrin from a marine macroalga Gracilaria gracilis by anion-exchange chromatography. J. Appl. Phycol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
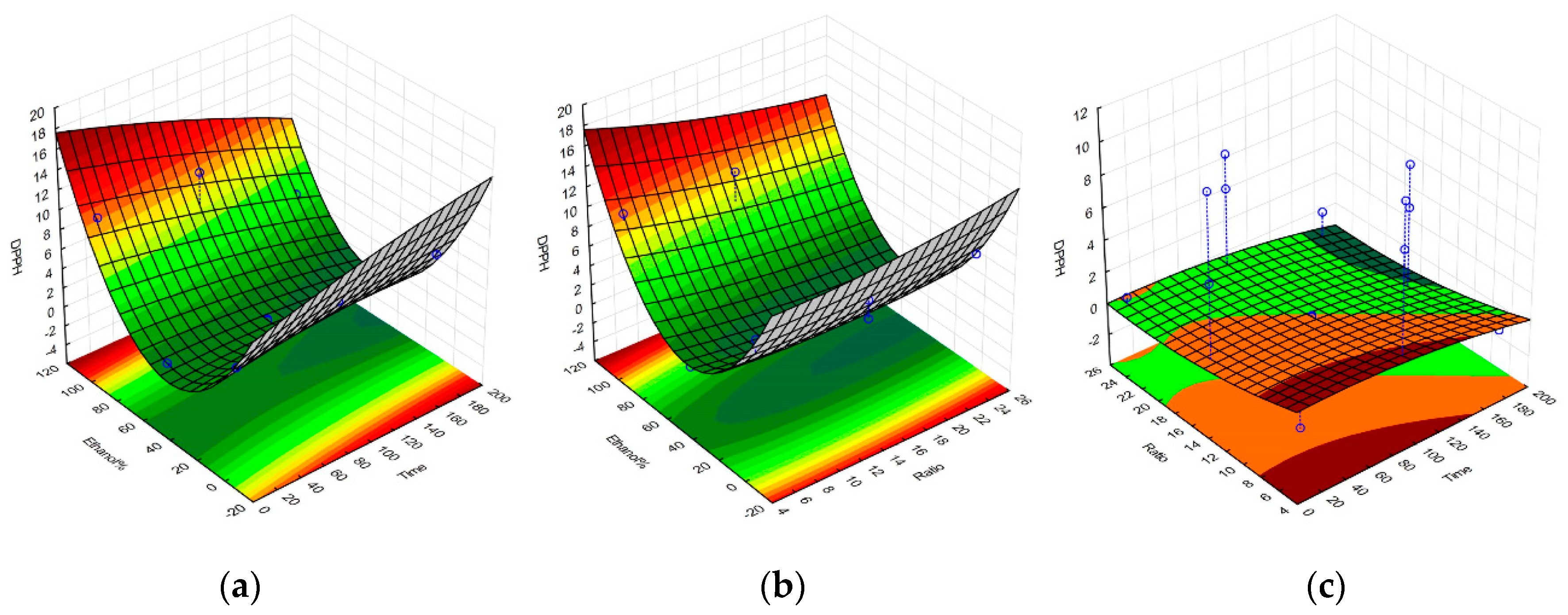

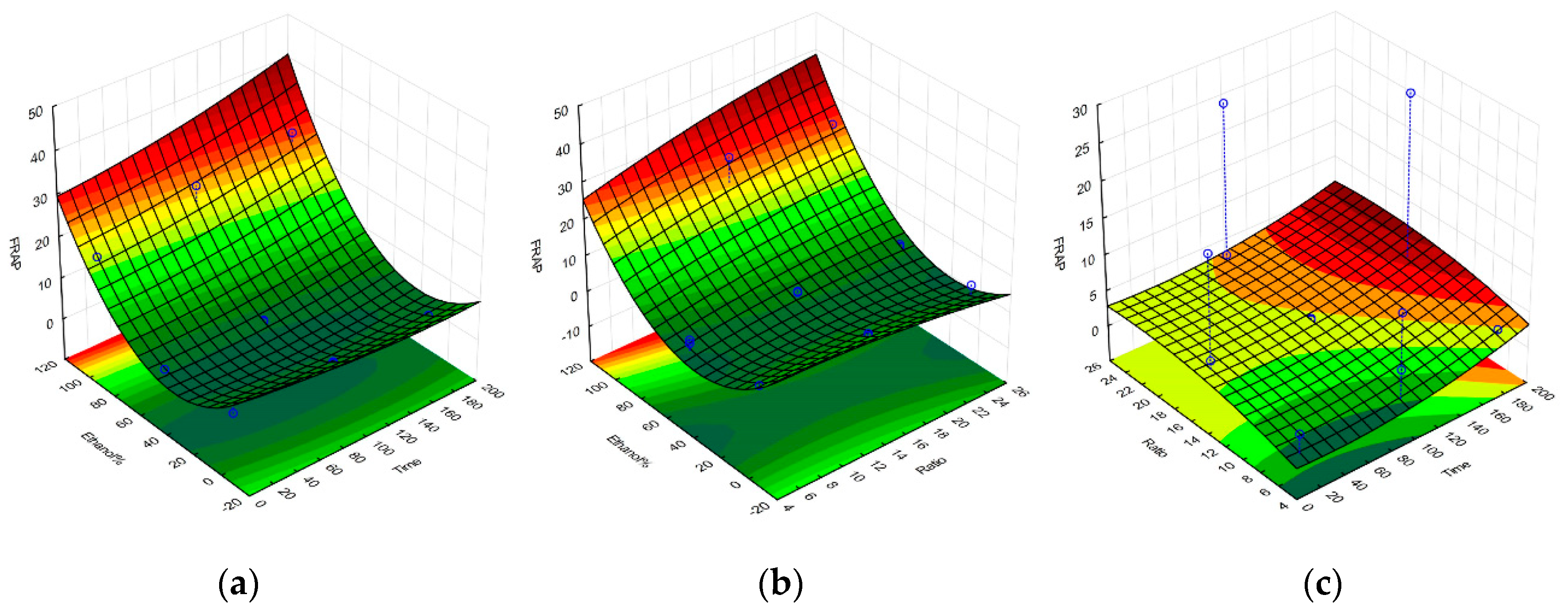
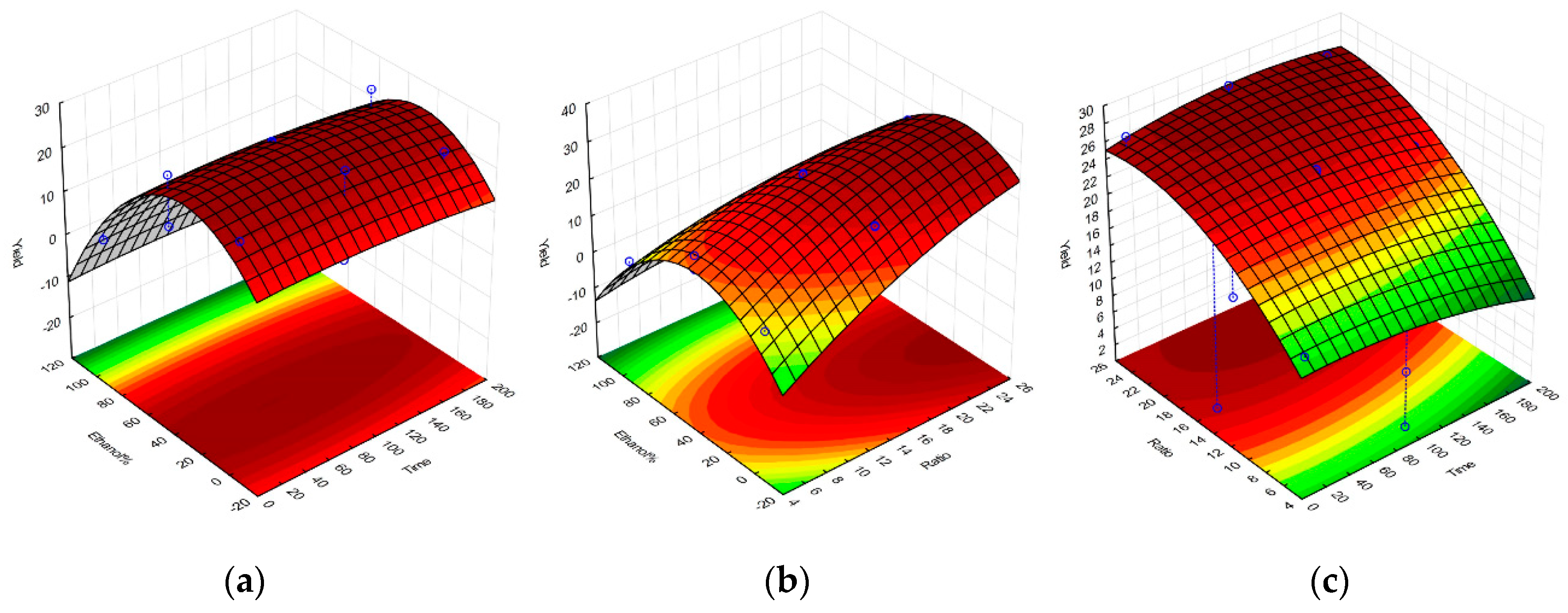
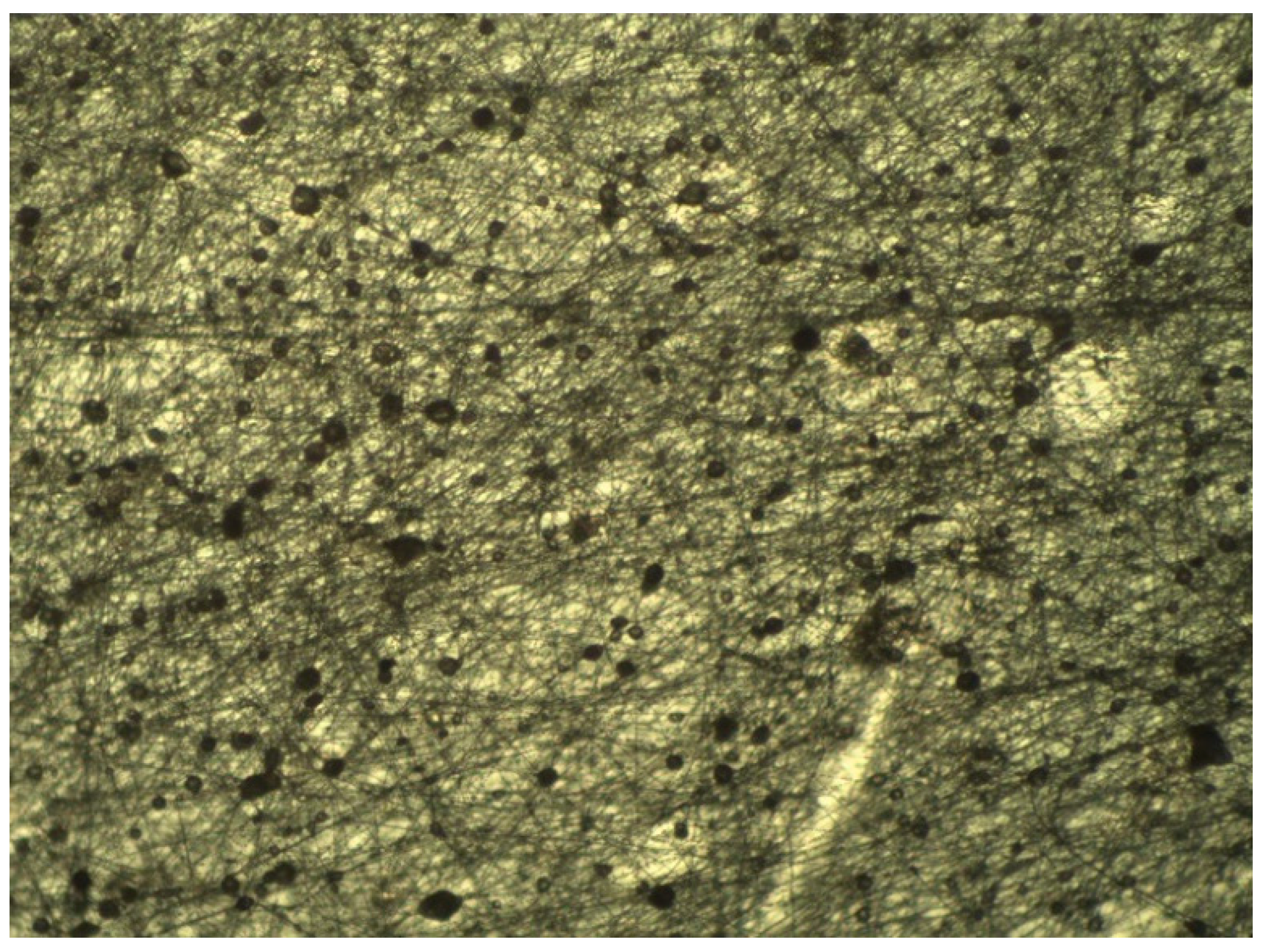
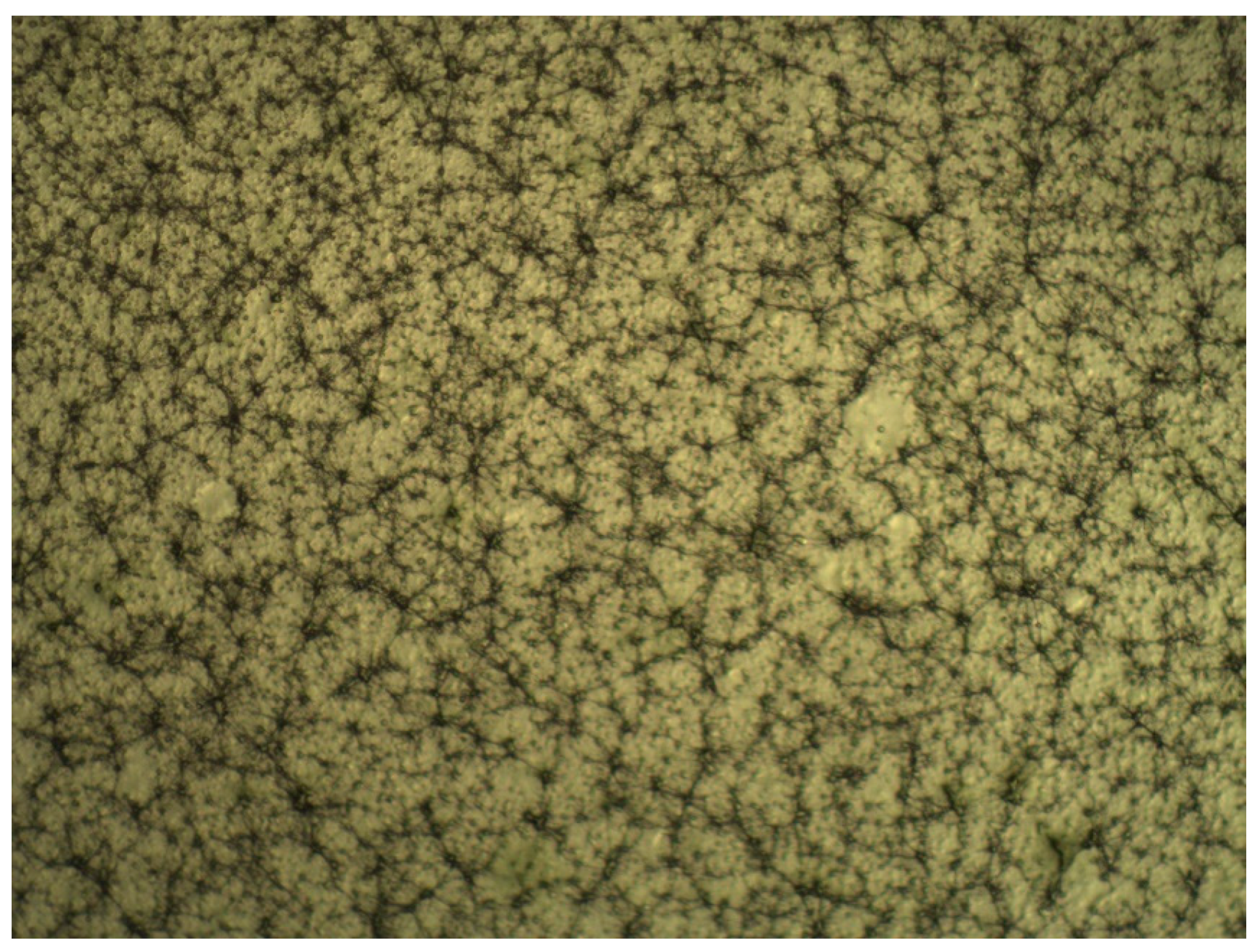

| Regression Coefficient β | DPPH | TPC | FRAP | Yield |
|---|---|---|---|---|
| Intercept | 9.057 | 6.642 | −1.220 | 5.077 |
| Linear | ||||
| Time | 0.43 | 0.006 | −0.256 | 0.012 |
| SLR | −0.200 | −0.827 | 0.156 | 1.392 ** |
| Ethanol% | −1.744 ** | −2.917 * | −1.594 ** | 1.452 *** |
| Quadratic | ||||
| Time2 | −0.152 | −0.448 | 0.208 | −0.166 |
| SLR2 | 0.298 | 0.509 | −0.310 | −0.717 * |
| Ethanol%2 | 2.768 *** | 1.706 | 1.766 ** | −1.702 *** |
| CrossProduct | ||||
| Time*SLR | −0.005 | −0.018 | −0.014 | 0.137 |
| Time*Ethanol% | −0.645 * | 1.137 | 0.326 | 0.014 |
| SLR*Ethanol% | −0.463 | 0.366 | 0.556 | −0.653 ** |
| R2 | 0.861 | 0.270 | 0.890 | 0.972 |
| p value of lack of fit | 0.0011 | 0.0436 | 0.0018 | 0.0090 |
| p value of the models | 0.0091 | 0.3207 | 0.0052 | 0.0002 |
| DPPH | TPC | FRAP | |
|---|---|---|---|
| Aqueous; 0.3 mL/h, 14 kV, 10 cm dist. 47% RH; 22 °C | 78.61 ± 6.6 | 63.37 ± 4.2 | 88.89 ± 6.9 |
| 50% Ethanolic; 0.4 mL/h, 11 kV; 11 cm dist., 46% RH, 21 °C | 100 * | 78.61 ± 2.3 | 80.36 ± 1.1 |
| 100% Ethanolic; 0.5 mL/h; 11 kV; 11 cm dist., 40% RH, 21 °C | 76.27 ± 2.8 | 90.93 ± 1.1 | 89.9 ± 3.8 |
| X1 | X2 | X3 | Ethanol% | Time (min) | SLR (g/mL) |
|---|---|---|---|---|---|
| −1 | −1 | 0 | 0 | 10 | 1/55 |
| −1 | 0 | −1 | 0 | 95 | 1/10 |
| −1 | 0 | 1 | 0 | 95 | 1/100 |
| −1 | 1 | 0 | 0 | 180 | 1/55 |
| 1 | −1 | 0 | 100 | 10 | 1/55 |
| 1 | 0 | −1 | 100 | 95 | 1/10 |
| 1 | 0 | 1 | 100 | 95 | 1/100 |
| 1 | 1 | 0 | 100 | 180 | 1/55 |
| 0 | −1 | −1 | 50 | 10 | 1/10 |
| 0 | −1 | 1 | 50 | 10 | 1/100 |
| 0 | 0 | 0 | 50 | 95 | 1/55 |
| 0 | 0 | 0 | 50 | 95 | 1/55 |
| 0 | 0 | 0 | 50 | 95 | 1/55 |
| 0 | 1 | −1 | 50 | 180 | 1/10 |
| 0 | 1 | 1 | 50 | 180 | 1/100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboleira, J.; Ganhão, R.; Mendes, S.; Adão, P.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Sousa, D.; Mateus, A.; Bernardino, S. Optimization of Extraction Conditions for Gracilaria gracilis Extracts and Their Antioxidative Stability as Part of Microfiber Food Coating Additives. Molecules 2020, 25, 4060. https://doi.org/10.3390/molecules25184060
Reboleira J, Ganhão R, Mendes S, Adão P, Andrade M, Vilarinho F, Sanches-Silva A, Sousa D, Mateus A, Bernardino S. Optimization of Extraction Conditions for Gracilaria gracilis Extracts and Their Antioxidative Stability as Part of Microfiber Food Coating Additives. Molecules. 2020; 25(18):4060. https://doi.org/10.3390/molecules25184060
Chicago/Turabian StyleReboleira, João, Rui Ganhão, Susana Mendes, Pedro Adão, Mariana Andrade, Fernanda Vilarinho, Ana Sanches-Silva, Dora Sousa, Artur Mateus, and Susana Bernardino. 2020. "Optimization of Extraction Conditions for Gracilaria gracilis Extracts and Their Antioxidative Stability as Part of Microfiber Food Coating Additives" Molecules 25, no. 18: 4060. https://doi.org/10.3390/molecules25184060
APA StyleReboleira, J., Ganhão, R., Mendes, S., Adão, P., Andrade, M., Vilarinho, F., Sanches-Silva, A., Sousa, D., Mateus, A., & Bernardino, S. (2020). Optimization of Extraction Conditions for Gracilaria gracilis Extracts and Their Antioxidative Stability as Part of Microfiber Food Coating Additives. Molecules, 25(18), 4060. https://doi.org/10.3390/molecules25184060









