Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations
2.1.1. SEM
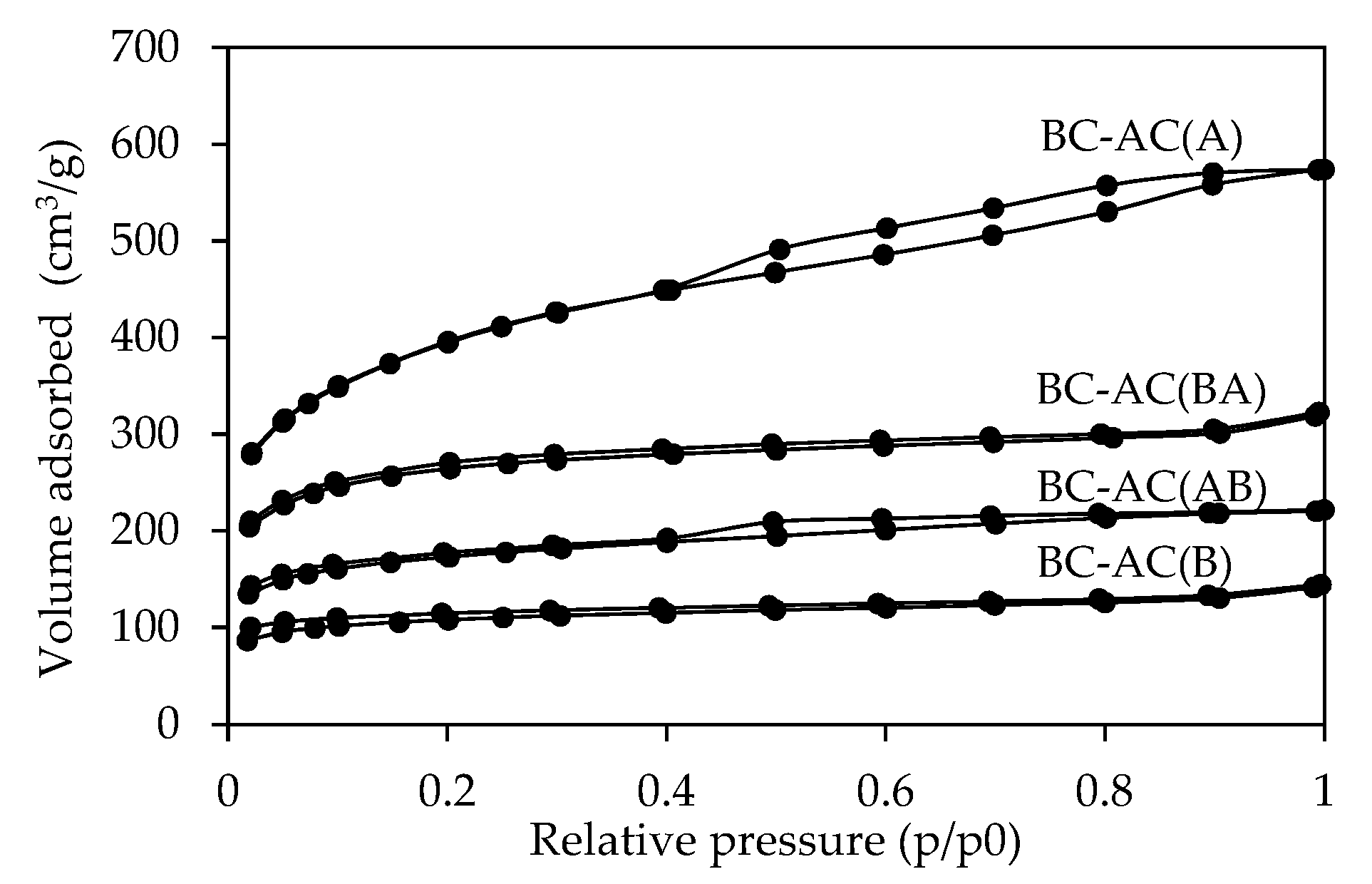
2.1.2. Pore Structure and Surface Area Analysis
2.1.3. Acidity
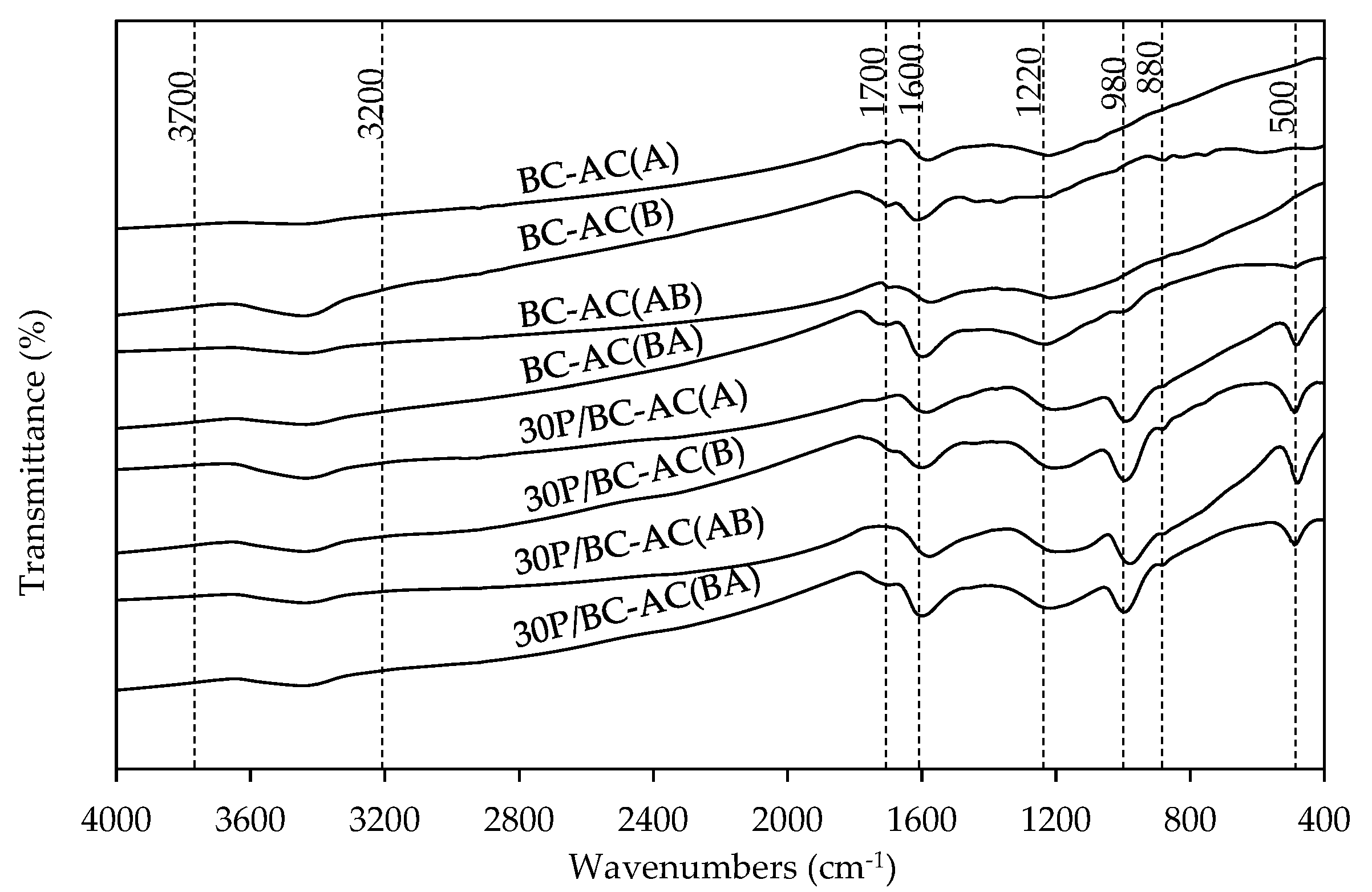
2.1.4. FTIR
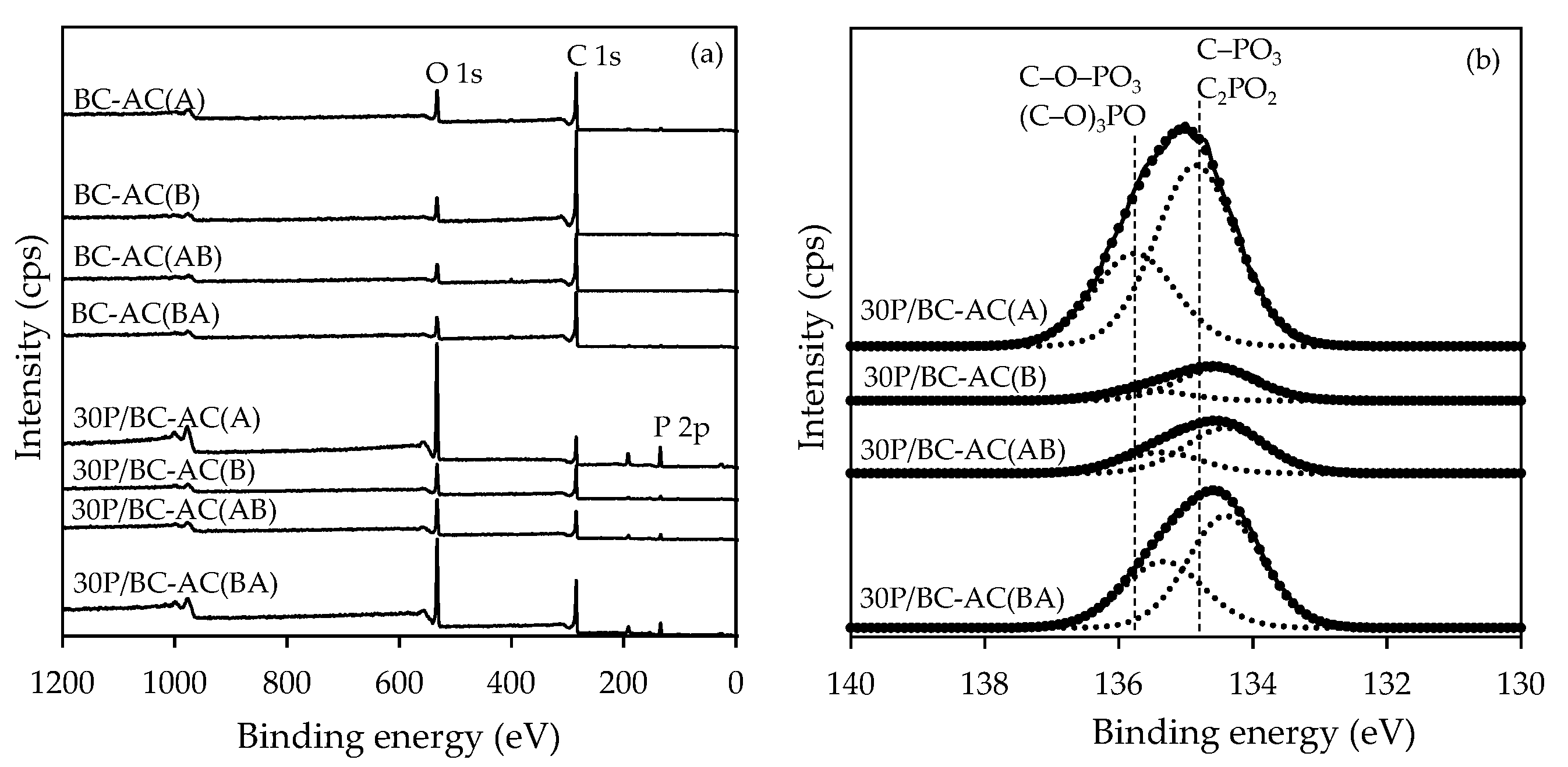
2.1.5. XPS
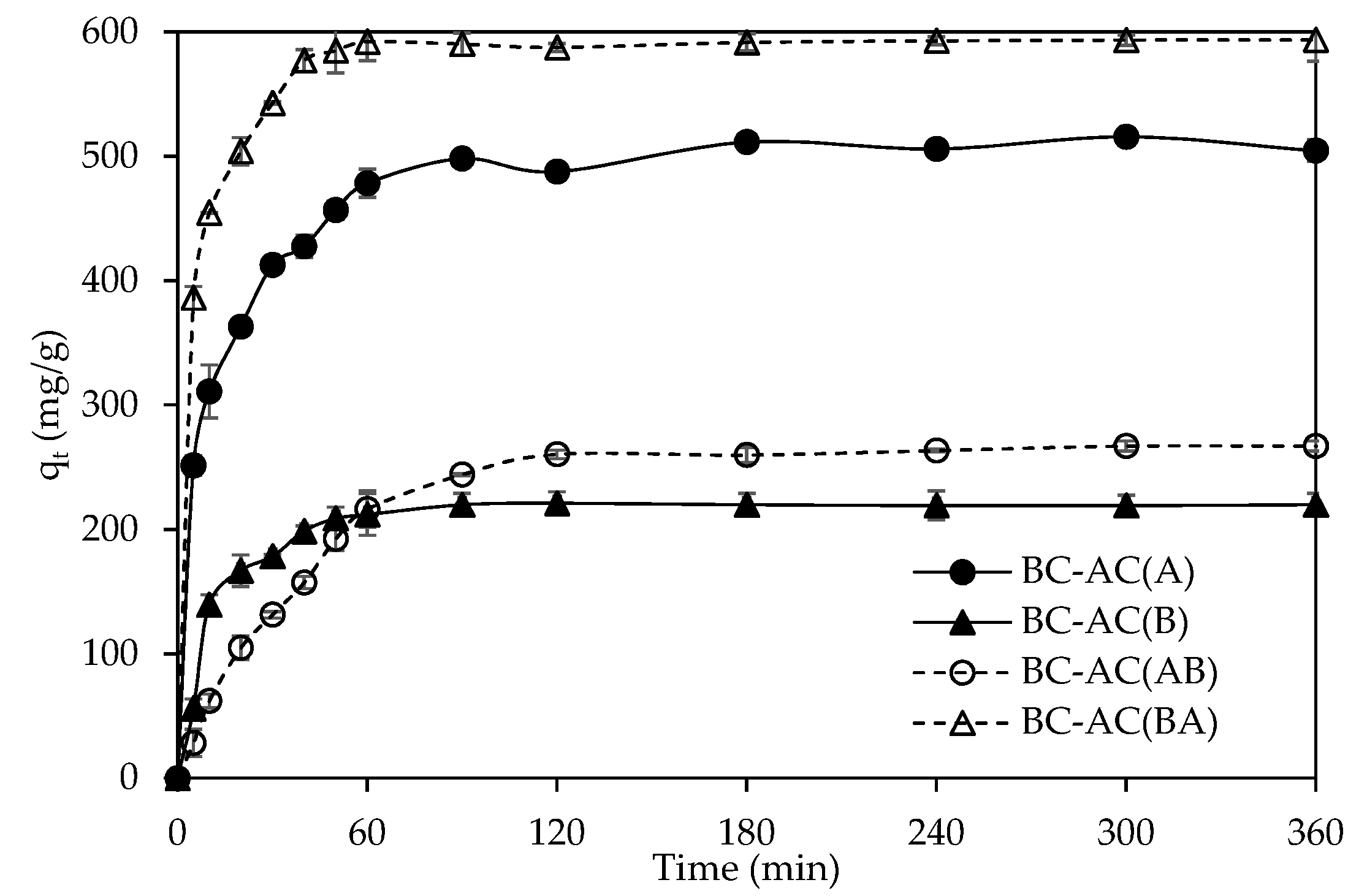
2.2. Mass Transfer and Adsorption Capacity
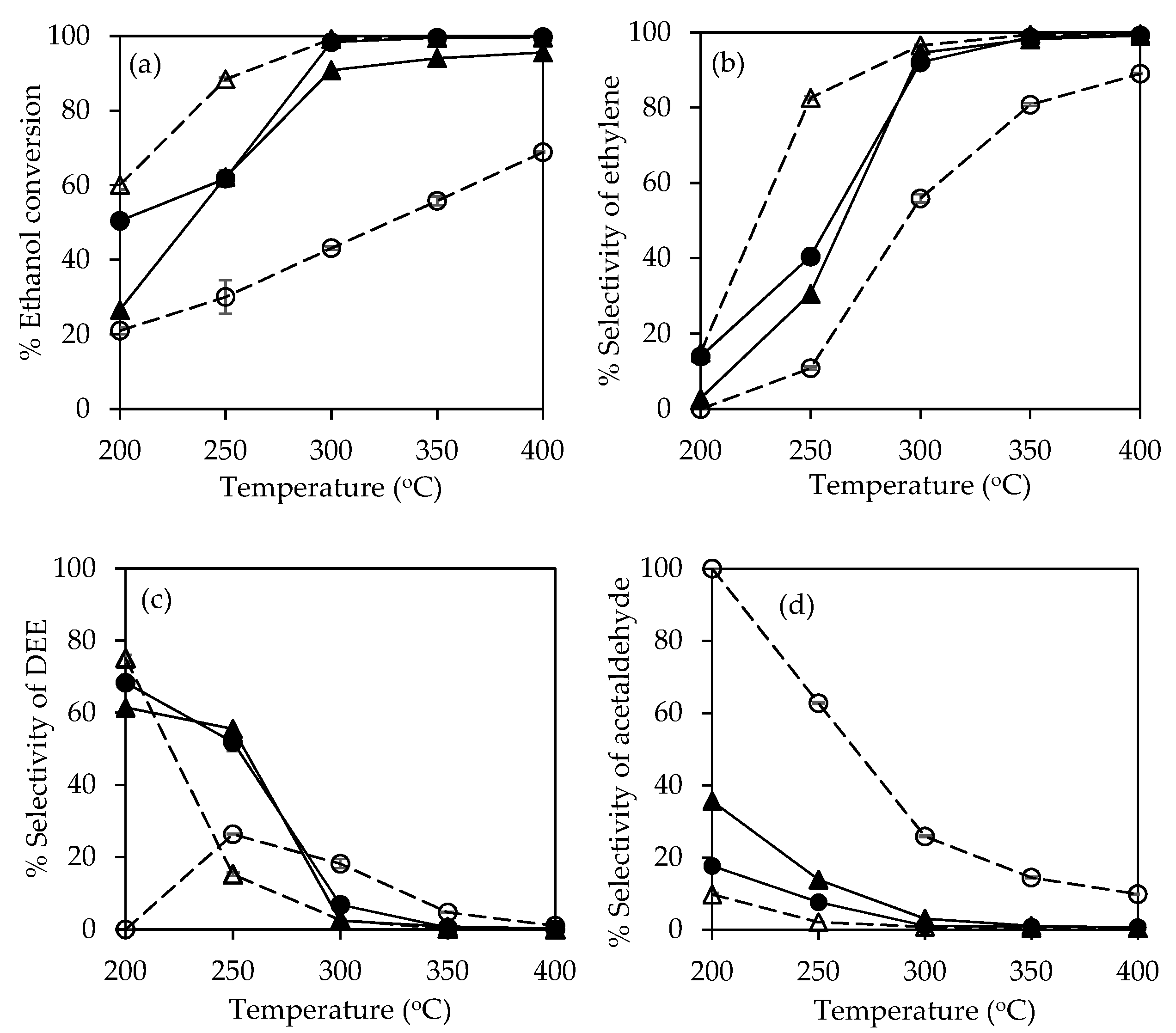
2.3. Catalytic Ethanol Dehydration Performance
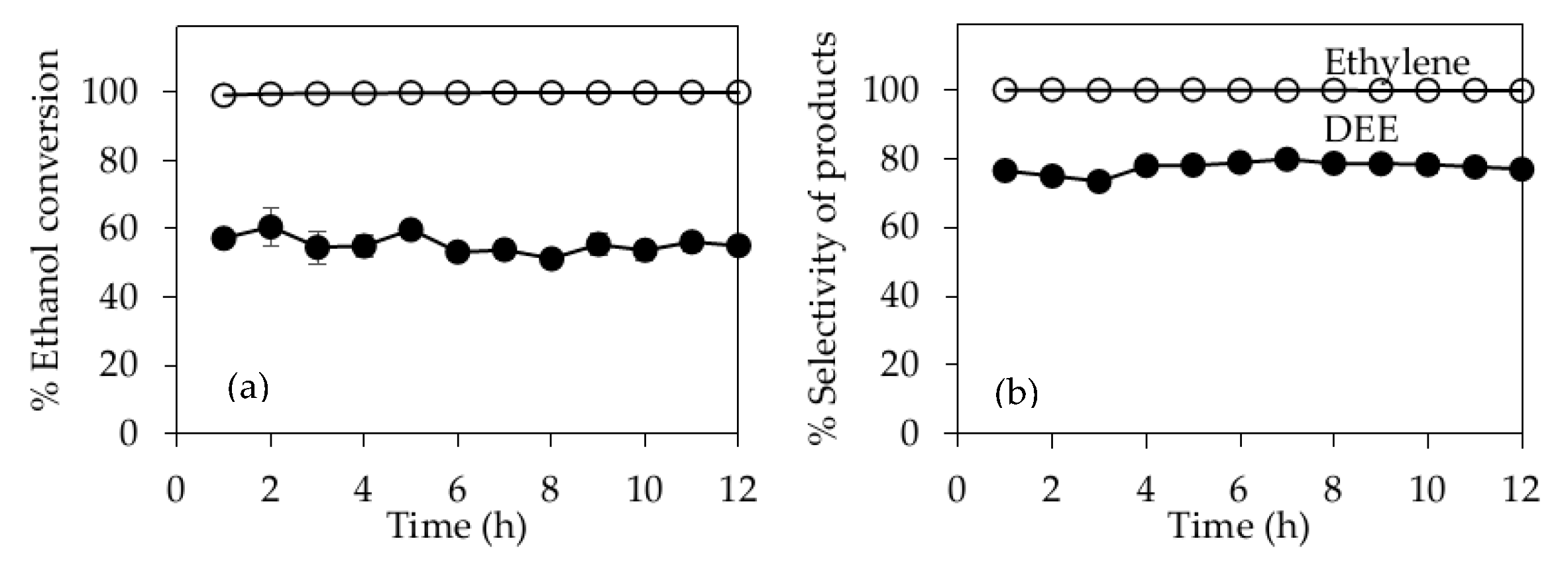
2.4. Stability Test
3. Materials and Methods
3.1. Materials
3.2. Activated Carbon and Catalyst Preparation
3.3. Characterizations
3.4. Mass Transfer and Adsorption Capacity
3.5. Catalytic Ethanol Dehydration Reaction and Catalytic Stability of Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharghi, H.; Aberi, M.; Doroodmand, M.M. Reusable Cobalt(III)-Salen Complex Supported on Activated Carbon as an Efficient Heterogeneous Catalyst for Synthesis of 2-Arylbenzimidazole Derivatives. Adv. Synth. Catal. 2008, 350, 2380–2390. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2on Molecular Sieves and Activated Carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, F.; Qi, J.; Zhao, L. Biodiesel Production from Sewage Sludge by Using Alkali Catalyst Catalyze. Procedia Environ. Sci. 2016, 31, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Kadirvelu, K. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.; Rao, M. Activated carbon for gas separation and storage. Carbon 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Jongsomjit, B.; Robison, J.; Phisalaphong, M. Activated carbon from bacterial cellulose as an effective adsorbent for removing dye from aqueous solution. Sep. Sci. Technol. 2018, 54, 2180–2193. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Phanthang, L.; Jongsomjit, B.; Phisalaphong, M.; Phathang, L. Activated carbon derived from bacterial cellulose and its use as catalyst support for ethanol conversion to ethylene. Catal. Commun. 2019, 129, 105750. [Google Scholar] [CrossRef]
- Taokaew, S.; Seetabhawang, S.; Siripong, P.; Phisalaphong, M. Biosynthesis and Characterization of Nanocellulose-Gelatin Films. Materials 2013, 6, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Bedia, J.; Barrionuevo, R.; Rodríguez-Mirasol, J.; Cordero, T. Ethanol dehydration to ethylene on acid carbon catalysts. Appl. Catal. B Environ. 2011, 103, 302–310. [Google Scholar] [CrossRef]
- Boongate, C.; Phisalaphong, M. Activated carbons from bacterial cellulose by chemical activation with potassium hydroxide. In Proceedings of the 2015 International Conference on Science and Technology (TICST), Pathum Thani, Thailand, 4–6 November 2015; pp. 144–147. [Google Scholar] [CrossRef]
- Kamiń, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and properties of homo- and heterogenously phosphorylated nanocellulose. Carbohydr. Polym. 2015, 125, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, G.; Wang, L.; Zhang, H. Activated carbons derived from hydrothermal impregnation of sucrose with phosphoric acid: Remarkable adsorbents for sulfamethoxazole removal. RSC Adv. 2019, 9, 17841–17851. [Google Scholar] [CrossRef] [Green Version]
- Villota, S.M.; Lei, H.; Villota, E.; Qian, M.; Lavarias, J.; Taylan, V.; Agulto, I.; Mateo, W.; Valentin, M.; Denson, M. Microwave-Assisted Activation of Waste Cocoa Pod Husk by H3PO4 and KOH—Comparative Insight into Textural Properties and Pore Development. ACS Omega 2019, 4, 7088–7095. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Yuan, L. A Langmuir-like desorption model for reflecting the inhomogeneous pore structure of coal and its experimental verification. RSC Adv. 2015, 5, 2434–2440. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thomas, M.; Patel, S.P.; Patel, A.V.; Pate, J.V. A comparative study on the efficiency of KOH and H3PO4 impregnated jackfruit leaf based carbon as adsorbent for removal of Cr (VI) from its aqueous solution. Int. J. Eng. Trends Technol. 2017, 45, 176–182. [Google Scholar] [CrossRef]
- Abdulwahab, M.I.; Khamkeaw, A.; Jongsomjit, B.; Phisalaphong, M. Bacterial Cellulose Supported Alumina Catalyst for Ethanol Dehydration. Catal. Lett. 2017, 147, 2462–2472. [Google Scholar] [CrossRef]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Bedia, J. Kinetic study of the decomposition of 2-butanol on carbon-based acid catalyst. AIChE J. 2009, 56, 1557–1568. [Google Scholar] [CrossRef]
- Wannaborworn, M.; Praserthdam, P.; Jongsomjit, B. A Comparative Study of Solvothermal and Sol-Gel-Derived Nanocrystalline Alumina Catalysts for Ethanol Dehydration. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yakout, S.M.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Gao, B. Acidified Activated Carbon with Enhanced Electrochemical Performance for Supercapacitors. Int. J. Electrochem. Sci. 2017, 12, 116–127. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorption of Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Ziatdinov, A. On the chemical structure of phosphorus compounds in phosphoric acid-activated carbon. Appl. Surf. Sci. 2006, 252, 8036–8038. [Google Scholar] [CrossRef]
- Aysan, H.; Edebali, S.; Ozdemir, C.; Karakaya, M.C.; Karakaya, N. Use of chabazite, a naturally abundant zeolite, for the investigation of the adsorption kinetics and mechanism of methylene blue dye. Microporous Mesoporous Mater. 2016, 235, 78–86. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Ma, G.; Kong, D.; Xiong, W.; Wang, J.; Zhu, Y.; Xia, Y. Heteroatom-doped porous carbons with enhanced carbon dioxide uptake and excellent methylene blue adsorption capacities. Microporous Mesoporous Mater. 2018, 257, 1–8. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Jiao, F.; Yuan, Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal. Today 2007, 125, 111–119. [Google Scholar] [CrossRef]
- Martins, L.; Cardoso, D.; Hammer, P.; Garetto, T.; Pulcinelli, S.H.; Santilli, C.V. Efficiency of ethanol conversion induced by controlled modification of pore structure and acidic properties of alumina catalysts. Appl. Catal. A Gen. 2011, 398, 59–65. [Google Scholar] [CrossRef]
- Ramesh, K.; Hui, L.M.; Han, Y.; Borgna, A. Structure and reactivity of phosphorous modified H-ZSM-5 catalysts for ethanol dehydration. Catal. Commun. 2009, 10, 567–571. [Google Scholar] [CrossRef]
- Limlamthong, M.; Chitpong, N.; Jongsomjit, B. Influence of Phosphoric Acid Modification on Catalytic Properties of γ-χ Al2O3 Catalysts for Dehydration of Ethanol to Diethyl Ether. Bull. Chem. React. Eng. Catal. 2019, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Etilena, P.; Pendehidratan, D.; Dengan, E.; Serium, M. Production of ethylene from ethanol dehydration over H3PO4-modified cerium oxide catalysts. Malaysian J. Anal. Sci. 2018, 21, 839–848. [Google Scholar] [CrossRef]
- Chaichana, E.; Wiwatthanodom, W.; Jongsomjit, B. Carbon-Based Catalyst from Pyrolysis of Waste Tire for Catalytic Ethanol Dehydration to Ethylene and Diethyl Ether. Int. J. Chem. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Phung, T.K.; Hernández, L.P.; Busca, G. Conversion of ethanol over transition metal oxide catalysts: Effect of tungsta addition on catalytic behaviour of titania and zirconia. Appl. Catal. A Gen. 2015, 489, 180–187. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Activated Carbons | BET Surface Area a (m2/g) | Pore Volume (cm3/g) | Average Pore Size Diameter (nm) | Porosity b (%) | |||
|---|---|---|---|---|---|---|---|
| a Micro | a Meso | b Macro | a Micro-Meso | b Macro | |||
| BC-AC(A) | 1316 | 0.17 | 0.71 | 1.00 | 2.4 | 54 | 65.0 |
| BC-AC(B) | 340 | 0.13 | 0.09 | 3.30 | 2.6 | 1730 | 79.7 |
| BC-AC(AB) | 551 | 0.15 | 0.19 | 1.60 | 2.7 | 265 | 71.3 |
| BC-AC(BA) | 833 | 0.23 | 0.26 | 4.40 | 2.5 | 2,704 | 91.2 |
| Samples | Acid Sites a (µmol NH3/g Catalyst) | |||
|---|---|---|---|---|
| Weak | Moderate to Strong | Total | Weak/Moderate to Strong | |
| BC-AC(A) | 206 | 109 | 282 | 1.9 |
| BC-AC(B) | 94 | 49 | 143 | 1.9 |
| BC-AC(AB) | 110 | 54 | 164 | 2.0 |
| BC-AC(BA) | 130 | 68 | 198 | 1.9 |
| 30P/BC-AC(A) | 1141 | 294 | 1436 | 3.9 |
| 30P/BC-AC(B) | 528 | 173 | 701 | 3.1 |
| 30P/BC-AC(AB) | 609 | 209 | 818 | 2.9 |
| 30P/BC-AC(BA) | 837 | 299 | 1136 | 2.8 |
| Samples | C (%) | O (%) | P (%) | N (%) | Si (%) |
|---|---|---|---|---|---|
| BC-AC(A) | 74.3 | 20.7 | 3.1 | 1.9 | - |
| BC-AC(B) | 87.7 | 11.4 | - | - | 0.9 |
| BC-AC(AB) | 83.4 | 14.9 | - | 1.7 | - |
| BC-AC(BA) | 77.6 | 15.6 | 2.4 | 3.2 | 1.3 |
| 30P/BC-AC(A) | 24.5 | 52.1 | 22.5 | - | 0.9 |
| 30P/BC-AC(B) | 63.1 | 27.2 | 6.0 | 1.4 | 2.4 |
| 30P/BC-AC(AB) | 55.9 | 30.5 | 11.5 | - | 2.2 |
| 30P/BC-AC(BA) | 48.6 | 37.0 | 12.6 | - | 1.8 |
| Samples | C–O–PO3 and (C–O)3PO (%) | C–PO3 and C2PO2 (%) |
|---|---|---|
| 30P/BC-AC(A) | 33.9 | 66.1 |
| 30P/BC-AC(B) | 24.1 | 75.9 |
| 30P/BC-AC(AB) | 30.3 | 69.7 |
| 30P/BC-AC(BA) | 38.9 | 61.1 |
| Catalysts | 200 °C | 250 °C | 300 °C | 400 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene | DEE | MeCHO | Ethylene | DEE | MeCHO | Ethylene | DEE | MeCHO | Ethylene | DEE | MeCHO | |
| 30P/BC-AC(A) | 7.1 | 34.5 | 2.5 | 24.9 | 32.1 | 4.7 | 90.5 | 6.8 | 1.1 | 100.0 | 0.0 | 0.0 |
| 30P/BC-AC(B) | 0.7 | 16.4 | 1.0 | 19.0 | 34.5 | 8.6 | 85.9 | 2.2 | 2.8 | 94.8 | 0.3 | 0.6 |
| 30P/BC-AC(AB) | 0.0 | 0.0 | 21.0 | 3.3 | 7.9 | 18.8 | 24.1 | 7.9 | 11.2 | 61.3 | 0.7 | 6.8 |
| 30P/BC-AC(BA) | 9.0 | 45.2 | 1.5 | 73.0 | 13.5 | 1.9 | 95.8 | 2.6 | 0.8 | 100.0 | 0.0 | 0.5 |
| Catalysts | Reaction Temperature (°C) | Ethanol Conversion (%) | Ethylene Selectivity (%) | DEE Selectivity (%) | References |
|---|---|---|---|---|---|
| 30P/BC-AC(BA) | 200–400 | 60.1–100 | 15.0–100 | 75.2–0.0 | This work |
| 30P/BC-AC500 | 200–400 | 50.5–100 | 13.9–100 | 68.4–0.0 | [7] |
| 20HP-ZSM-5 | 250–400 | 25.0–99.0 | 3.0–99.0 | 96–0.0 | [31] |
| 5P/Al2O3 | 200–400 | 9.1–86.1 | 0.0–94.0 | 100–6.0 | [32] |
| 30PA-CeO2 | 400–500 | 16.0–59.7 | 96.1–99.2 | 0.2–0.1 | [33] |
| 25Al/BC-FD | 200–400 | 41.3–65.7 | 1.1–43.3 | 40.0–43.3 | [19] |
| HA2-800 | 200–350 | 2.0–100 | 34.0–100 | 65.0–0 | [9] |
| AC_H420 | 400 | 39.1 | 69.4 | 29.9 | [34] |
| SiO2 | 200–400 | 0.0–8.6 | 0.0–48.8 | 0.0–31.8 | [35] |
| ZrO2 | 200–400 | 0.0–100.0 | 0.0–75.4 | 0.0–1.5 | [35] |
| TiO2 | 200–400 | 0.6–100.0 | 0.0–29.7 | 83.8–0.0 | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkeaw, A.; Asavamongkolkul, T.; Perngyai, T.; Jongsomjit, B.; Phisalaphong, M. Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance. Molecules 2020, 25, 4063. https://doi.org/10.3390/molecules25184063
Khamkeaw A, Asavamongkolkul T, Perngyai T, Jongsomjit B, Phisalaphong M. Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance. Molecules. 2020; 25(18):4063. https://doi.org/10.3390/molecules25184063
Chicago/Turabian StyleKhamkeaw, Arnon, Tatdanai Asavamongkolkul, Tianpichet Perngyai, Bunjerd Jongsomjit, and Muenduen Phisalaphong. 2020. "Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance" Molecules 25, no. 18: 4063. https://doi.org/10.3390/molecules25184063







