Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals
Abstract
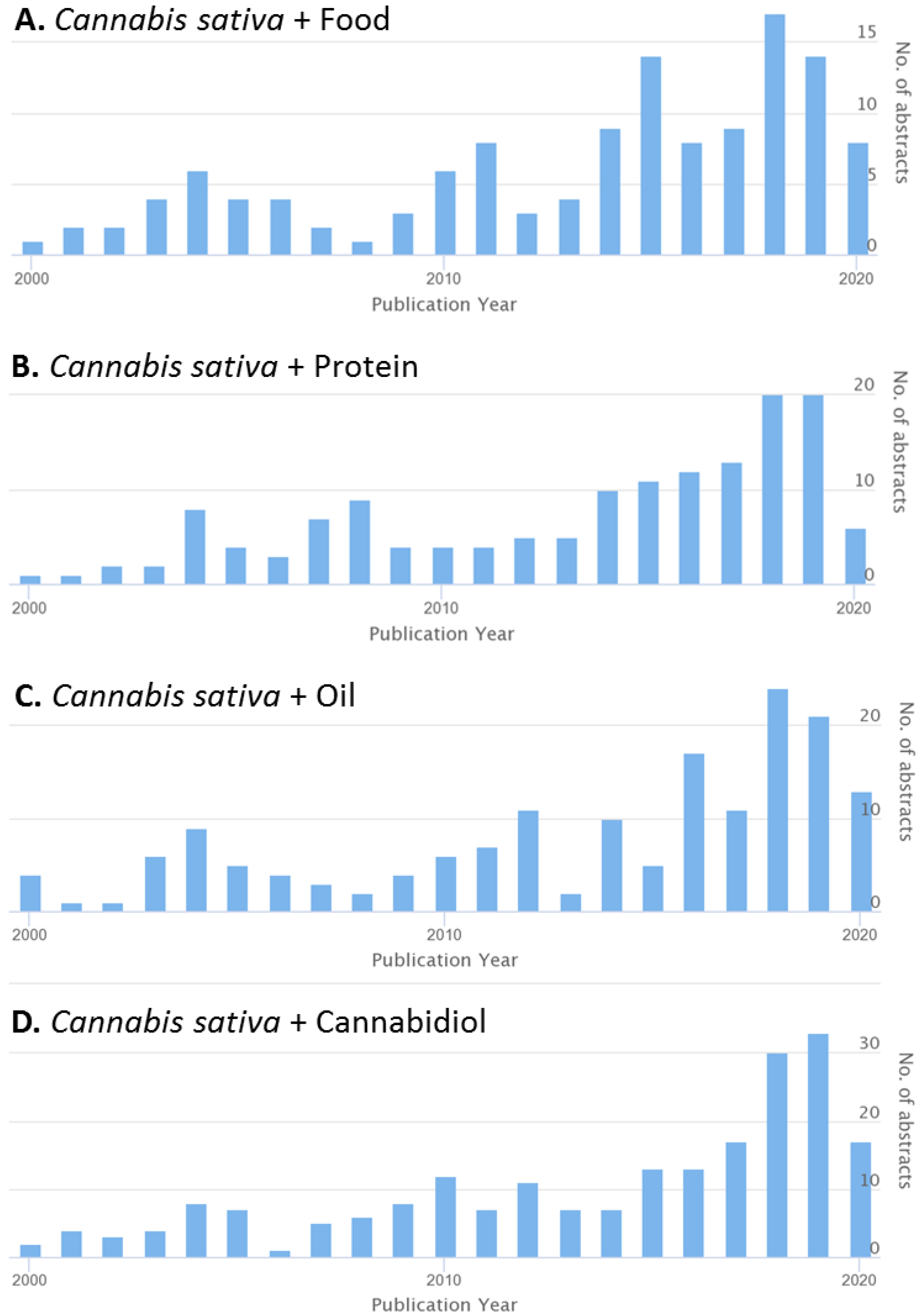
1. Introduction
1.1. Botany
1.2. Sex Expression
1.3. The Genetic Basis of the Difference between Hemp and Medical Cannabis
2. Hemp Industrial Products
2.1. Crop Production
2.2. History of Hemp Production
2.3. Industrial Hemp Market
3. Hemp Seed Composition
3.1. Nutrients
3.2. Phytocannabinoids and Endocannabinoid System
3.3. Hemp Seed Oil
4. Potential Health Benefits
4.1. Cardiovascular Health
4.2. Cancers
4.3. Diseases of the Central Nervous System
4.4. Rheumatoid Arthritis
4.5. Dermatitis and Skin Diseases
4.6. Mental Health and Sleep Disorders
4.7. Additional Health Benefits
5. Food and Nutraceutical Applications
5.1. Hemp Seed in Food Products
5.2. Advancement in the Extraction of Oil and Cannabinoids from Hemp Seed
5.3. Methods of Enhancing Oxidative Stability of Hemp Seed Oil
5.4. Microencapsulation Technologies
6. Future Prospects and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Composit. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lip. Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Sawler, J.; Stout, J.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The genetic structure of marijuana and hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef]
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet. Resour. Crop Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop Evol. 2005, 52, 161–180. [Google Scholar] [CrossRef]
- Faux, A.M.; Berhin, A.; Dauguet, N.; Bertin, P. Sex chromosomes and quantitative sex expression in monoecious hemp (Cannabis sativa L.). Euphytica 2014, 196, 183–197. [Google Scholar] [CrossRef]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Bot. Museum Leaflets Harvard Univ. 1974, 23, 337–367. Available online: https://www.biodiversitylibrary.org/page/7467406#page/359/mode/1up (accessed on 15 July 2020).
- Razumova, O.V.; Alexandrov, O.S.; Divashuk, M.G.; Sukhorada, T.I.; Karlov, G.I. Molecular cytogenetic analysis of monoecious hemp (Cannabis sativa L.) cultivars reveals its karyotype variations and sex chromosomes constitution. Protoplasma 2016, 253, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Small, E. Cannabis: A Complete Guide; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- De Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [PubMed]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodiver. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Datwyler, S.L.; Weiblen, G.D. Genetic variation in hemp and marijuana (Cannabis sativa L.) according to amplified fragment length polymorphisms. J. Forensic Sci. 2006, 51, 371–375. [Google Scholar] [CrossRef]
- Hakki, E.E.; Kayis, S.A.; Pinarkara, E.; Sag, A. Inter simple sequence repeats separate efficiently hemp from marijuana (Cannabis sativa L.). Electron. J. Biotechnol. 2007, 10. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity. Available online: https://fas.org/sgp/crs/misc/RL32725.pdf (accessed on 22 June 2018).
- Mass, E. Hemp: The new, old fiber makes a comeback for clothes, fabrics, and home furnishings. Nat. Life 2009, 127, 36. [Google Scholar]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A review on the current state of knowledge of growing conditions, agronomic soil health practices and utilities of hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Canada’s Industrial Hemp Industry. 2016. Available online: http://www4.agr.gc.ca (accessed on 10 January 2020).
- West, D.P. Hemp and Marijuana: Myths & Realities. 1998. Available online: https://www.votehemp.com/PDF/myths_facts.pdf (accessed on 10 January 2020).
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites—A review. J. Comp. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Deitch, R. Hemp: American History Revisited; Algora Publishing: New York, NY, USA, 2003. [Google Scholar]
- Robinson, B.B.; Evans, R. Hemp for Victory [Motion Picture]; United States Department of Agriculture: Washington, DC, USA, 1942.
- Agricultural Act of 2014. (U.S.C. 2014, c.7). § 5940. Available online: https://www.congress.gov/bill/113th-congress/house-bill/2642/text (accessed on 10 January 2020).
- United States Department of Agriculture. Hemp and Farm Bill Programs. 2019. Available online: https://www.farmers.gov/manage/hemp?utm_medium=email&utm_source=govdelivery (accessed on 10 January 2020).
- Health Canada. Cultivation Licenses. 2011. Available online: http://www.hc-sc.gc.ca/ (accessed on 10 January 2020).
- Health Canada. Industrial Hemp Licensing Statistics for 2018. 2019. Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/producing-selling-hemp/about-hemp-canada-hemp-industry/statistics-reports-fact-sheets-hemp.html (accessed on 10 January 2020).
- Mark, T.; Shepherd, J.; Olson, D.; Snell, W.; Proper, S.; Thornsbury, S. Economic Viability of Industrial Hemp in the United States: A Review of State Pilot Programs. Available online: https://www.ers.usda.gov/webdocs/publications/95930/eib-217.pdf?v=4149.6 (accessed on 10 January 2020).
- Deferne, J.L.; Pate, D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp Assoc. 1996, 3, 4–7. [Google Scholar]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Silversides, F.G.; Lefrancois, M.R. The effect of feeding hemp seed meal to laying hens. Br. Poult. Sci. 2005, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ditrói, K.; Kleiner, D.; Böszörményi, A.; Szentmihályi, K.; Fébel, H. The alimentary impact of the hemp seed. Acta Aliment. 2013, 42, 410–416. [Google Scholar] [CrossRef]
- Schultz, C.J.; Lim, W.L.; Khor, S.F.; Neumann, K.A.; Schulz, J.M.; Ansari, O.; Skewes, M.A.; Burton, R.A. Consumer and health-related traits of seed from selected commercial and breeding lines of industrial hemp, Cannabis sativa L. J. Agric. Food Res. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Dimić, E.; Romanić, R.; Vujasinović, V. Essential fatty acids, nutritive value and oxidative stability of cold pressed hempseed (Cannabis sativa L.) oil from different varieties. Acta Aliment. 2009, 38, 229–236. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Alberta Agriculture and Forestry. Industrial Hemp Enterprise. 2015. Available online: www.agriculture.alberta.ca/publications (accessed on 12 May 2020).
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceut. Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- Petrović, M.; Debeljak, Ž.; Kezić, N.; Džidara, P. Relationship between cannabinoids content and composition of fatty acids in hempseed oils. Food Chem. 2015, 170, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic compounds and antioxidant activity of cold-pressed seed oil from finola cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Grof, C.P. Cannabis, from plant to pill. Br. J. Clin. Pharmacol. 2018, 84, 2463–2467. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef]
- Corroon, J.; Felice, J.F. The endocannabinoid system and its modulation by cannabidiol (CBD). Alter. Ther. Health Med. 2019, 25, 6–14. [Google Scholar]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Mastinu, A.; Premoli, M.; Ferrari-Toninelli, G.; Tambaro, S.; Maccarinelli, G.; Memo, M.; Bonini, S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm. Mol. Biol. Clin. Investig. 2018, 36, 1–15. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; de Ceballos, M.L.; Del Pulgar, T.G.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Cajal, S.R.Y.; Guzmán, M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. Available online: https://cancerres.aacrjournals.org/content/61/15/5784 (accessed on 12 May 2020). [PubMed]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Della Pina, S.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorgan. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Schwab, U.S.; Callaway, J.C.; Erkkilä, A.T.; Gynther, J.; Uusitupa, M.I.; Järvinen, T. Effects of hemp seed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. Eur. J. Nutr. 2006, 45, 457–470. [Google Scholar] [CrossRef]
- Al-Khalifa, A.; Maddaford, T.G.; Chahine, M.N.; Austria, J.A.; Edel, A.L.; Richard, M.N.; Ander, B.P.; Gavel, N.; Kopilas, M.; Ganguly, R.; et al. Effect of dietary hempseed intake on cardiac ischemia-reperfusion injury. Am. J. Physiology. Integr. Comp. Physiol. 2007, 292, R1198–R1203. [Google Scholar] [CrossRef]
- Richard, M.N.; Ganguly, R.; Steigerwald, S.N.; Al-Khalifa, A.; Pierce, G.N. Dietary hemp seed reduces platelet aggregation. J. Thromb. Haemost. 2007, 5, 424–425. [Google Scholar] [CrossRef]
- Prociuk, M.A.; Edel, A.L.; Richard, M.N.; Gavel, N.T.; Ander, B.P.; Dupasquier, C.M.; Pierce, G.N. Cholesterol-induced stimulation of platelet aggregation is prevented by a hempseed-enriched diet. Can. J. Physiol. Pharmacol. 2008, 86, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer Lett. 2009, 285, 6–12. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef]
- Vaccani, A.; Massi, P.; Colombo, A.; Rubino, T.; Parolaro, D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br. J. Pharmacol. 2005, 144, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in glioblastoma therapy: New applications for old drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernández-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzmán, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzman, M.; Velasco, G.; Iovanna, J.L. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnellic, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizán, E.M.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Therap. 2010, 332, 569–577. [Google Scholar] [CrossRef]
- Jones, N.A.; Glyn, S.E.; Akiyama, S.; Hill, T.D.; Hill, A.J.; Weston, S.E.; Burnett, M.D.; Yamasaki, Y.; Stephens, G.J.; Whalley, B.J.; et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 2012, 21, 344–352. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Bebin, E.M.; Cutter, G.; DeWolfe, J.; Dure, L.S.; Gaston, T.E.; Kankirawatana, P.; Liu, Y.; Singh, R.; Standaert, D.G.; et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018, 87, 131–136. [Google Scholar] [CrossRef]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. J. 2004, 10, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Vaney, C.; Heinzel-Gutenbrunner, M.; Jobin, P.; Tschopp, F.; Gattlen, B.; Hagen, U.; Schnelle, M.; Reif, M. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled, crossover study. Mult. Scler. J. 2004, 10, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Junior, N.C.; Campos, A.C.; Guimarães, F.S.; Del-Bel, E.; Zimmermann, P.M.D.R.; Junior, L.B.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Brum, L. Biological bases for a possible effect of cannabidiol in Parkinson’s disease. Bras. J. Psychiatry 2020, 42, 218–224. [Google Scholar] [CrossRef]
- Cho, H. Dongeubogam—Naekyeong and Oehyeong; Yeo Gang Publishing Company: Seoul, Korea, 2005; pp. 1–1279. [Google Scholar]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. S5), v3–v11. [Google Scholar] [CrossRef]
- Jeong, M.; Cho, J.; Shin, J.-I.; Jeon, Y.-J.; Kim, J.-H.; Lee, S.-J.; Kim, E.-S.; Lee, K.-H. Hempseed oil induces reactive oxygen species- and C/EBP homologous protein-mediated apoptosis in MH7A human rheumatoid arthritis fibroblast-like synovial cells. J. Ethnopharmacol. 2014, 154, 745–752. [Google Scholar] [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Harbridge, L.S. Dietary n-6 and n-3 fatty acids in immunity and autoimmune disease. Proc. Nutr. Soc. 1998, 57, 555–562. [Google Scholar] [CrossRef]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Kobayashi, T.; Watanabe, S. Dietary fatty acids –the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog. Lipid Res. 1996, 35, 409–457. [Google Scholar] [CrossRef]
- Whiting, P.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.H.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Wright, M.; Di Ciano, P.; Brands, B. Use of cannabidiol for the treatment of anxiety: A short synthesis of preclinical and clinical evidence. Cannabis Cannabinoid Res. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Bitencourt, R.M.; Takahashi, R.N. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. Front. Neurosci. 2018, 12, 502. [Google Scholar] [CrossRef]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wilson, R.; Appiah-Kusi, E.; O’Neill, A.; Brammer, M.; Perez, J.; Murray, R.M.; Allen, P.; Bossong, M.G.; McGuire, P.K. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: A randomized clinical trial. JAMA Psychiatry 2018, 75, 1107. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in anxiety and sleep: A large case series. Perman. J. 2019, 23, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E.; Sarro-Ramírez, A.; Sánchez, D.; Mijangos-Moreno, S.; Tejeda-Padrón, A.; Poot-Aké, A.; Guzmán, K.; Pacheco-Pantoja, E.; Arias-Carrión, O. Potential effects of cannabidiol as a wake-promoting agent. Curr. Neuropharmacol. 2014, 12, 269–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crippa, J.A.; Zuardi, A.W.; Martín-Santos, R.; Bhattacharyya, S.; Atakan, Z.; McGuire, P.; Fusar-Poli, P. Cannabis and anxiety: A critical review of the evidence. Hum. Psychopharmacol. 2009, 24, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.; Cata, R.; Jutras-Aswad, D. Cannabidiol as an intervention for addictive behaviors: A systematic review of the evidence. Subst. Abuse Res. Treat. 2015, 9, 33–38. [Google Scholar] [CrossRef]
- Cheng, C.W.; Bian, Z.X.; Zhu, L.X.; Wu, J.C.Y.; Sung, J.J.Y. Efficacy of a Chinese herbal proprietary medicine (hemp seed pill) for functional constipation. Am. J. Gastroenterol. 2011, 106, 120–129. [Google Scholar] [CrossRef]
- Lin, Z.M.; Chen, L.M.; Liang, Z.M.; Xia, X. The effect of different extractants from hemp seed in mice with Alzheimer’s disease. Pharmacol. Clin. Chin. Materia Med. 2016, 32, 130–134. [Google Scholar] [CrossRef]
- Luo, J.; Yin, J.H.; Wu, H.Z.; Wei, Q. Extract from Fructus cannabis activating calcineurin improved learning and memory in mice with chemical drug-induced dysmnesia. Acta Pharmacol. Sin. 2003, 24, 1137–1142. Available online: https://pubmed.ncbi.nlm.nih.gov/14627499/ (accessed on 12 May 2020).
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.; Fisher, N.B.; Tugwell, B.; Szczesniak, A.; Kelly, M.; Zhou, J. Experimental cannabidiol treatment reduces early pancreatic inflammation in type 1 diabetes. Clin. Hemorheol. Microcir. 2016, 64, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Ji, J.; Lou, H.; Fan, P. Hemp (Cannabis sativa L.) seed phenylpropionamides composition and effects on memory dysfunction and biomarkers of neuroinflammation induced by lipopolysaccharide in mice. ACS Omega 2018, 3, 15988–15995. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Clinical endocannabinoid deficiency reconsidered: Current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res. 2016, 1, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, Headache, and pain: An update on current evidence and cannabis science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and pain: New insights from old molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J. Food Sci. 2006, 68, 1240–1243. [Google Scholar] [CrossRef]
- Uluata, S.; Özdemir, N. Antioxidant activities and oxidative stabilities of some unconventional oilseeds. J. Am. Oil Chem. Soc. 2012, 89, 551–559. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D. Hemp: A new crop with new uses for North America. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 284–326. [Google Scholar]
- Shim, J.S. Manufacturing Method of Bread Containing Blue Ginseng Seed. South Korea Patent No. 100927544 B1, 2009. Available online: https://patents.google.com/patent/KR100927544B1/en (accessed on 10 January 2020).
- Shim, J.S. Method for Manufacturing Confectionery Containing Blue Ginseng. South Korea Patent No. 100969163 B1, 2010. Available online: https://patents.google.com/patent/KR100969163B1/en (accessed on 10 January 2020).
- Guang, H.; Wenwei, C. Application of Powder of Whole Cannabis Sativa Seeds for Preparing Functional Food with Adjuvant Therapy of Lowering Blood Fat. China Patent No. 100998414 B, 2010. Available online: https://patents.google.com/patent/CN100998414B/en (accessed on 12 May 2020).
- Metz, M.; Selg-Mann, K. Production of a Food Seasoning, e.g., Useful as a Substitute for Soy Sauce, by Two-Stage Fermentation of Hemp Seeds. Germany Patent No. 10002389 A1, 2005. Available online: https://patents.google.com/patent/DE10002389A1/en (accessed on 12 May 2020).
- Steinbach, W. Hemp Pralines. Germany Patent No. 19746830 C1, 1999. Available online: https://patents.google.com/patent/DE19746830C1/en (accessed on 12 May 2020).
- Berghofer, E.; Pollmann, K.; Traby, M.; Frenkenberger, C. Method for Producing Hemp Milk. Canada Patent No. 2505350 C, 25 September 2012. Available online: https://patents.google.com/patent/CA2505350C/en (accessed on 12 May 2020).
- Ge, Z.; Wu, R. Hempseed Protein Drink and Producing Method Thereof. China Patent No. 1054034 C, 2000. Available online: https://patents.google.com/patent/CN1054034C/en (accessed on 12 May 2020).
- Guang, H.; Hua, Y. Hempseed Protein Powder and Its Preparing Method and Use. China Patent No. 1294833 C, 2007. Available online: https://patents.google.com/patent/CN1294833C/en (accessed on 12 May 2020).
- Guang, H.; Wenwei, C. Application of Fructus Cannabis Protein in Preparation of Health Food for Relieving Nutritional Anemia. China Patent No. 100433990 C, 2008. Available online: https://patents.google.com/patent/CN100433990C/en (accessed on 12 May 2020).
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Bisterfeld von Merr, G. Method of Obtaining Hemp Plant Juice and Use of Same for the Production of Beverages. USA Patent No. 8778418 B2, 15 July 2014. Available online: https://patents.google.com/patent/US8778418B2/en (accessed on 12 May 2020).
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive quality of Romanian hemp varieties (Cannabis sativa L.) with special focus on oil and metal contents of seeds. Chem. Central J. 2012, 6, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannaflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lypoxygenase. Pharm. Nutr. 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Subratti, A.; Lalgee, L.J.; Jalsa, N.K. Liquified dimethyl ether (DME): A green solvent for the extraction of hemp (Cannabis sativa L.) seed oil. Sustain. Chem. Pharm. 2019, 12, 100144. [Google Scholar] [CrossRef]
- Bomgardner, M. Entrepreneurs get in on the ground floor with CBD from hemp. Chem. Eng. News 2018, 96, 32. [Google Scholar]
- Grijó, D.R.; Piva, G.K.; Osorio, I.V.; Cardozo-Filho, L. Hemp (Cannabis sativa L.) seed oil extraction with pressurized n-propane and supercritical carbon dioxide. J. Supercrit. Fluids 2019, 143, 268–274. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Da Porto, C.; Voinovich, D.; Decorti, D.; Natolino, A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Quitain, A.T.; Sasaki, M.; Fukuzato, R.; Goto, M. Extraction and solubility evaluation of functional seed oil in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 79, 109–113. [Google Scholar] [CrossRef]
- Zongbing, P. Be Rich in the Industrial Hemp Extraction of Essential Oil Equipment of Cannabidiol. China Patent No. 204111719 U, 2015. Available online: https://patents.google.com/patent/CN204111719U/en (accessed on 17 June 2020).
- Mueller, A. Method for Producing an Extract from Cannabis Plant Matter, Containing a Tetrahydrocannabinol and a Cannabidiol and Cannabis Extracts. USA Patent No. 8895078 B2, 2014. Available online: https://patents.google.com/patent/US8895078B2/en (accessed on 17 June 2020).
- Keliang, L.; Qingtao, T.; Yazhong, G.; Zhonghua, M.; Huisen, L. Ultrasonic Assisted Supercritical CO2 Hemp Seed Oil Extraction Method. China Patent No. 102676290 B, 2013. Available online: https://patents.google.com/patent/CN102676290B/en (accessed on 17 June 2020).
- Horska, I. Transport and Absorption Agents and the Method of Its Manufacture from Hems. Germany Patent No. 602004007164 T2, 2008. Available online: https://patents.google.com/patent/DE602004007164T2/en (accessed on 17 June 2020).
- Webster, G.R.B.; Sarna, L.P. Cannabinoid Extraction Method. USA Patent No. 6403126 B1, 11 June 2002. Available online: https://patents.google.com/patent/US6403126B1/en (accessed on 17 June 2020).
- Raderman, J.M. Method for Modifying THC Content in a Lipid-Based Extract of Cannabis. USA Patent No. 9259449 B2, 2016. Available online: https://patents.google.com/patent/US9259449B2/en (accessed on 17 June 2020).
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, E.J. The use of microwave and pulsed electric field as a pretreatment step in ultrasonic extraction of polyphenols from defatted hemp seed cake (Cannabis sativa) using response surface methodology. Food Bioprocess Technol. 2014, 7, 3064–3076. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, Y.T. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical characterization of hempseed (seed of Cannabis sativa L.) oil from eight regions in China. J. Diet. Suppl. 2010, 7, 117–129. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Pierce, D.; Brooks, G. Quaternized Hemp Seed Oil. USA Patent No. 6063369, 2000. Available online: https://patents.google.com/patent/US6063369A/en (accessed on 17 June 2020).
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT—Food Sci. Technol. 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Anwar, S.H.; Weissbrodt, J.; Kunz, B. Microencapsulation of fish oil by spray granulation and fluid bed film coating. J. Food Sci. 2010, 75, E359–E371. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Microencapsulation of chia seed oil using chia seed protein isolate-chia seed gum complex coacervates. Int. J. Biol. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin–sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Vojvodic, A.; Mršić, G.; Komes, D. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and their applications in food nanotechnology. J. Lip. Res. 2008, 18, 309–327. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Ayub, M.A.Z. Probiotics production and alternative encapsulation methodologies to improve their viabilities under adverse environmental conditions. Int. J. Food Sci. Nutr. 2016, 67, 929–943. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Contr. 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Vojvodić, A.; Bušić, A.; Keppler, J.; Steffen-Heins, A.; Komes, D. Encapsulation templated approach to valorization of cocoa husk, poppy and hemp macrostructural and bioactive constituents. Ind. Crops Prod. 2018, 112, 402–411. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluka, R.E. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innovat. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]


| Product | Compound | Content | References |
|---|---|---|---|
| Hemp seed | Carbohydrate | 20–30 †; 27.6 † | [3,35] |
| Crude fat | 25–35 †; 33.2 †; 30.4 †; 31.1 † | [2,35,36,37] | |
| Crude protein | 20–25 †; 24.8 †; 24.9 †; 24.0 †; 27.3 † | [2,3,35,36,37] | |
| Neutral detergent fiber | 37.2 †; 32.1 †; 38.1 † | [2,36,37] | |
| Acid detergent fiber | 23.5 †; 29.6 † | [2,36] | |
| Ash | 5.6 †; 5.8 †; 4.8 †; 5.9 † | [2,3,36,37] | |
| Hemp seed oil | Cannabidiol (CBD) | 10 ‡; 4.18–243.68 ‡ | [43,44] |
| Linoleic acid (omega-6 PUFA) | 52–62 §; 53.4 §; 16.84 †; 56.2 ¶; 56.07 § | [2,41,43,44,45] | |
| Alpha-linolenic acid (omega-3 PUFA) | 12–23 §; 15.1 §; 6.8 †; 17.2 ¶; 15.98 § | [2,41,43,44,45] | |
| Beta-tocopherol | 6 ‡; 1.6 ‡; 0.64 ‡ | [41,45,46] | |
| Gamma-tocopherol | 733 ‡; 216.8 ‡; 91.57 ‡ | [41,45,46] | |
| Alpha-tocopherol | 34 ‡; 18.2 ‡; 19.74 ‡ | [41,45,46] | |
| Delta-tocopherol | 25 ‡; 12.0 ‡; 2.09 ‡ | [41,45,46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. https://doi.org/10.3390/molecules25184078
Rupasinghe HPV, Davis A, Kumar SK, Murray B, Zheljazkov VD. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules. 2020; 25(18):4078. https://doi.org/10.3390/molecules25184078
Chicago/Turabian StyleRupasinghe, H. P. Vasantha, Amy Davis, Shanthanu K. Kumar, Beth Murray, and Valtcho D. Zheljazkov. 2020. "Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals" Molecules 25, no. 18: 4078. https://doi.org/10.3390/molecules25184078
APA StyleRupasinghe, H. P. V., Davis, A., Kumar, S. K., Murray, B., & Zheljazkov, V. D. (2020). Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules, 25(18), 4078. https://doi.org/10.3390/molecules25184078








