The Effects of Temperature and Ethanol on Proanthocyanidin Adsorption to Grape Cell Wall Material in the Presence of Anthocyanins
Abstract
1. Introduction
2. Results
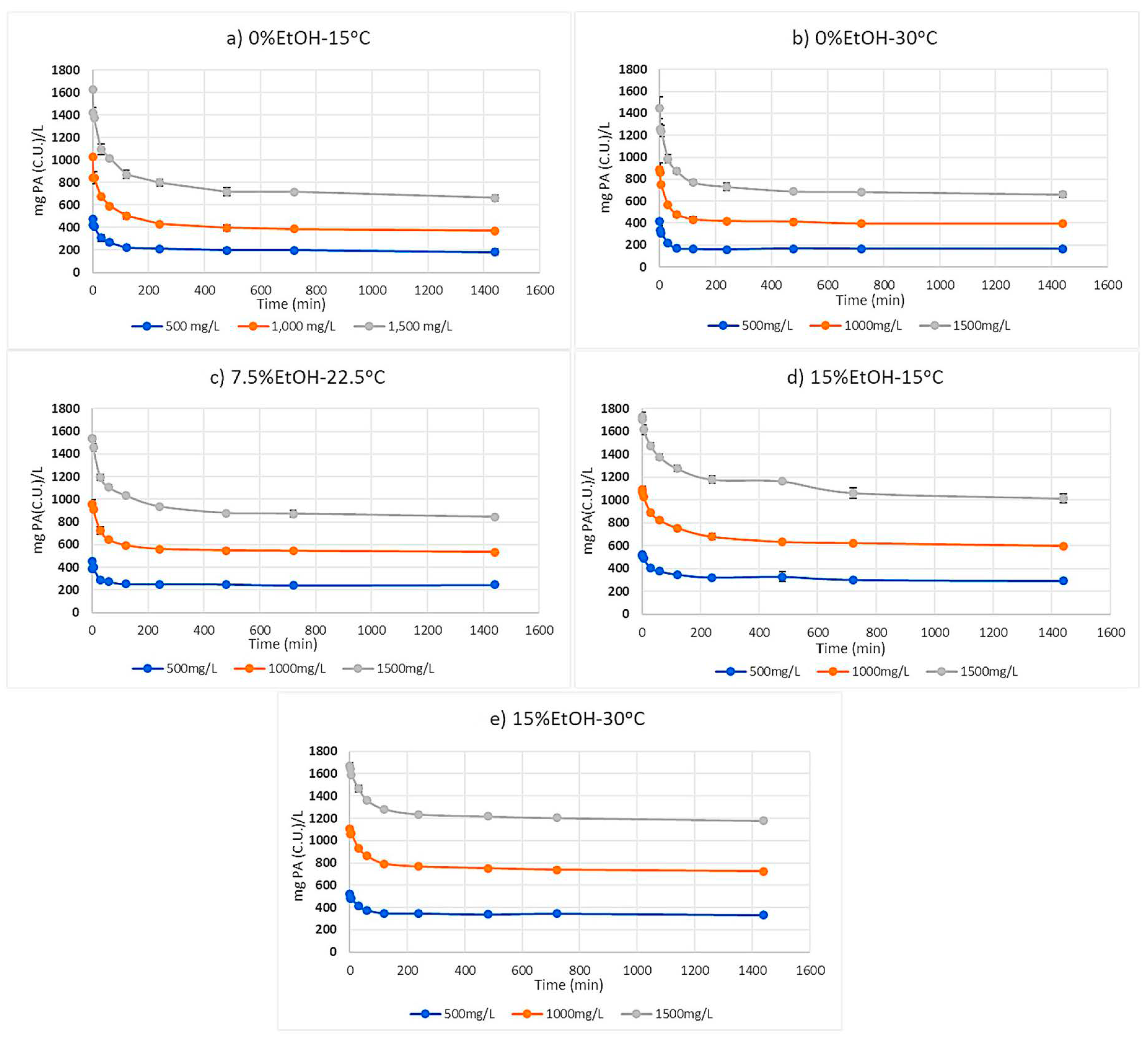
2.1. Proanthocyanidin Progress Curves
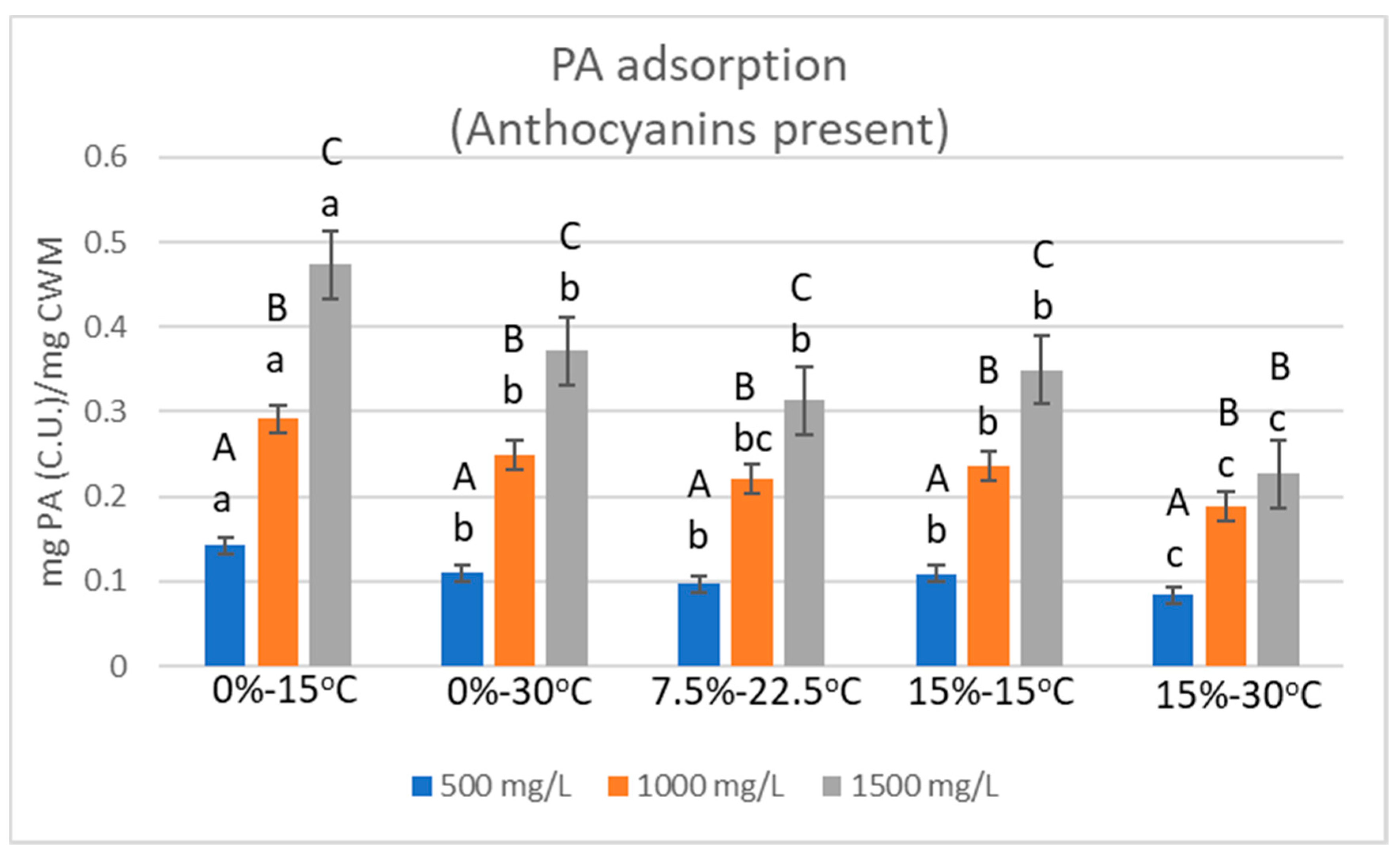
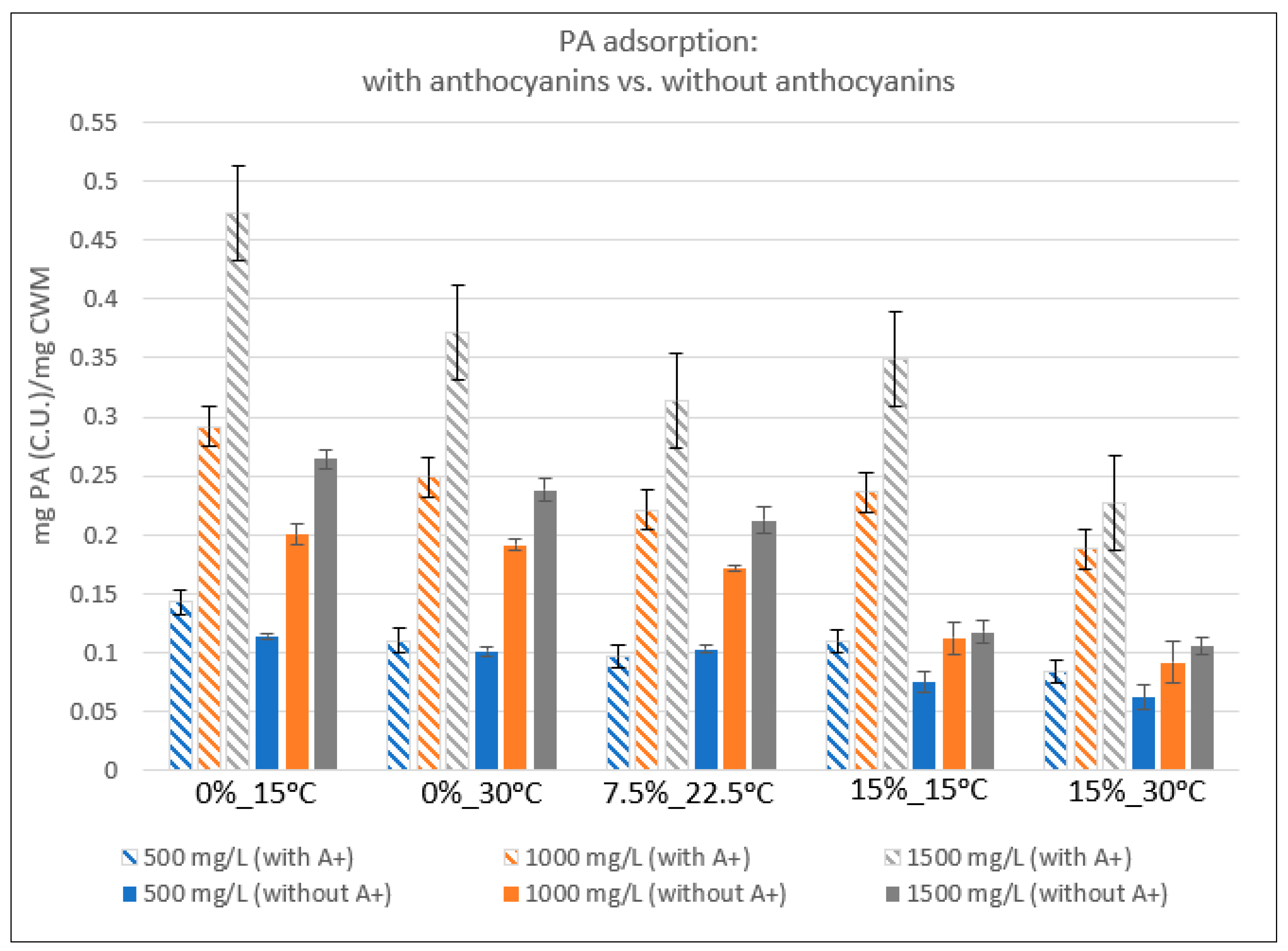
2.2. Relative Proanthocyanidin Adsorption
2.3. Adsorption Isotherms and Models
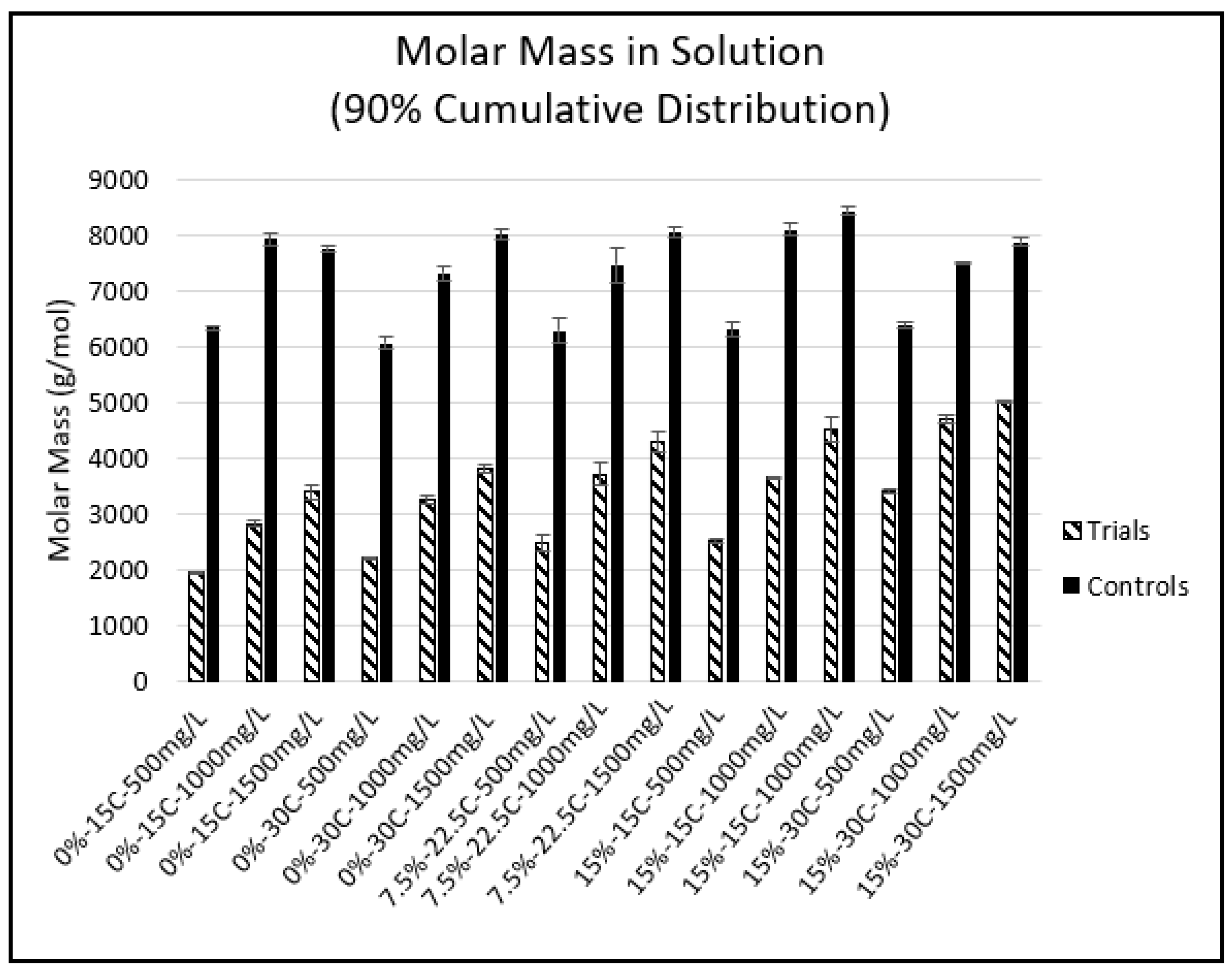
2.4. Phloroglucinolysis and GPC
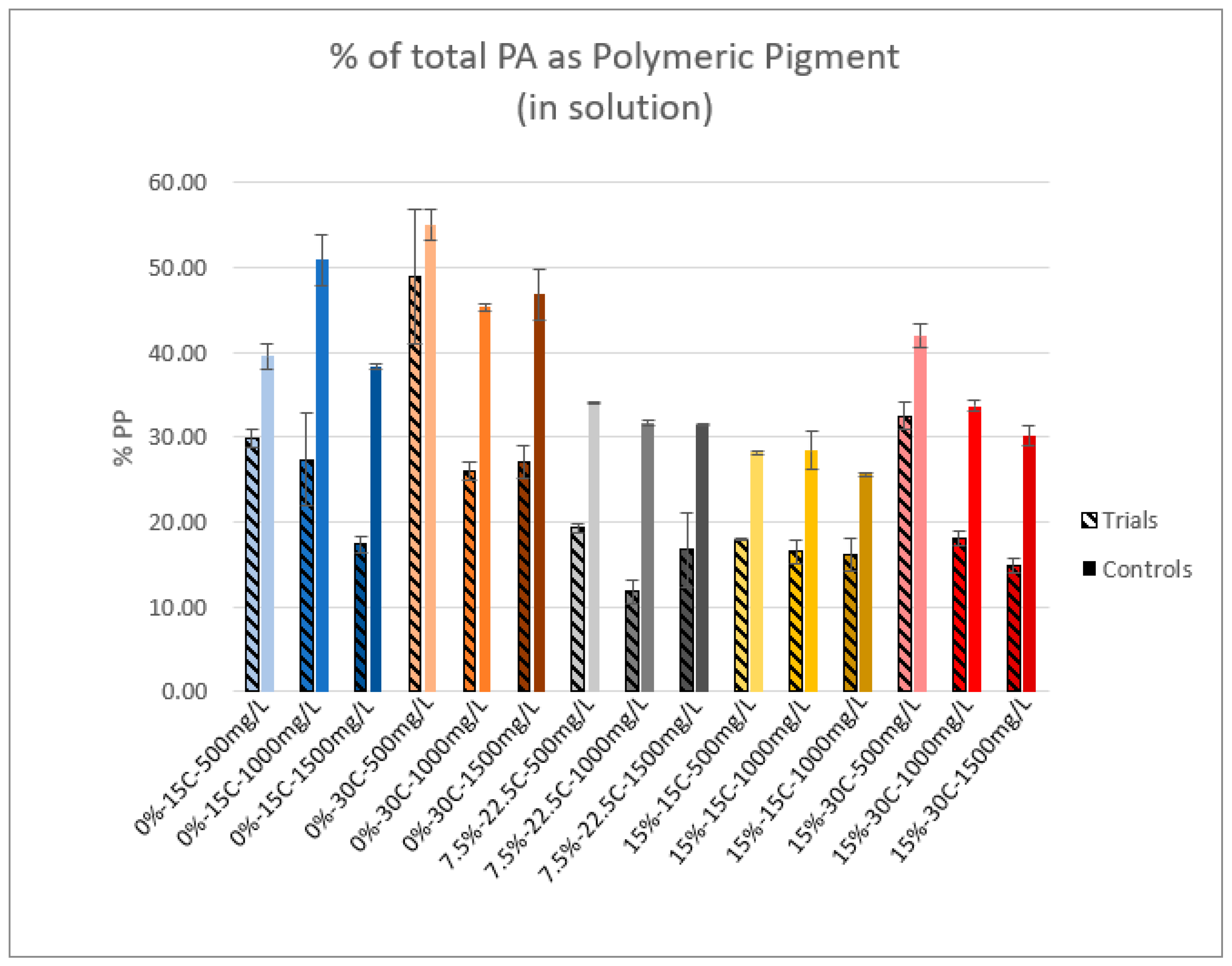
2.5. Polymeric Pigment
3. Discussion
3.1. Proanthocyanidin Composition
3.2. Polymeric Pigment
3.3. Proanthocyanidin Adsorption: Presence vs. Absence of Anthocyanins
4. Materials and Methods
4.1. Model Wine Preparation
4.2. Instrumentation and Software
4.3. Statistical Analysis
4.4. Isolation of Grape Skin Cell Wall Material
4.5. Isolation of Proanthocyanidins
4.6. Isolation of Anthocyanins
4.7. In Vitro Adsorption Experiments
4.8. Quantitative Analysis of Proanthocyanidins
4.9. Adsorption Isotherms
- qPA = mass of proanthocyanidin adsorbed per mass of CWM at equilibrium (mg PA (C.U.)/mg CWM);
- CPA = the concentration of proanthocyanidins in solution at equilibrium (mg PA (C.U.)/L);
- Keq = the equilibrium constant of the system (L/mg PA (C.U.));
- SCWM = the saturation point of the CWM (mg PA (C.U.)/mg CWM).
4.10. Qualitative Analysis of Proanthocyanidin–Anthocyanin Solutions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | proanthocyanidin(s) |
| CWM | cell wall material |
| EtOH | ethanol |
| C.U. | catechin units |
| mDP | mean degree of polymerization |
| aMW | average molecular weight |
| PP | polymeric pigment |
Appendix A
| Experimental Condition | Starting PA Concentration (mg/L) | mDP | mDP StDev | %Galloylation | %Galloylation StDev | aMW | aMW StDev |
|---|---|---|---|---|---|---|---|
| 0%-15 °C Control | 500 | 7.97 | 0.25 | 65.32 | 1.57 | 3084.35 | 82.04 |
| 0%-15 °C Trial | 500 | 3.48 * | 0.20 | 57.17 * | 0.93 | 1305.49 * | 77.72 |
| 0%-15 °C Control | 1000 | 11.82 | 0.85 | 61.35 | 1.84 | 4505.64 | 340.39 |
| 0%-15 °C Trial | 1000 | 5.21 * | 0.40 | 58.44 | 4.9 | 1961.52 * | 121.34 |
| 0%-15 °C Control | 1500 | 13.99 | 1.37 | 62.92 | 0.85 | 5365.04 | 537.82 |
| 0%-15 °C Trial | 1500 | 7.74 * | 0.28 | 55.04 * | 0.45 | 2874.32 * | 107.74 |
| 0-30 °C Control | 500 | 11.46 | 0.16 | 62.28 | 0.61 | 4383.77 | 66.06 |
| 0-30 °C Trial | 500 | 4.76 * | 0.14 | 56.16 * | 0.69 | 1776.63 * | 51.84 |
| 0%-30 °C Control | 1000 | 14.91 | 0.20 | 60.90 | 0.44 | 5670.07 | 84.65 |
| 0%-30 °C Trial | 1000 | 7.10 * | 0.20 | 56.07 * | 0.55 | 2646.63 * | 76.83 |
| 0%-30 °C Control | 1500 | 18.67 | 0.80 | 61.22 | 0.37 | 7109.64 | 313.8 |
| 0%-30 °C Trial | 1500 | 9.39 * | 0.30 | 56.15 * | 0.66 | 3503.16 * | 120.11 |
| 7.5%-22.5 °C Control | 500 | 9.83 | 0.46 | 63.78 | 1.95 | 3782.41 | 161.68 |
| 7.5%-22.5 °C Trial | 500 | 4.93 * | 0.10 | 59.81 * | 0.85 | 1869.43 * | 41.9 |
| 7.5%-22.5 °C Control | 1000 | 14.76 | 0.47 | 61.12 | 0.79 | 5617.58 | 195.18 |
| 7.5%-22.5 °C Trial | 1000 | 8.26 * | 0.32 | 59.18 | 0.57 | 3119.47 * | 128.02 |
| 7.5%-22.5 °C Control | 1500 | 17.06 | 0.61 | 61.76 | 0.39 | 6511.12 | 239.88 |
| 7.5%-22.5 °C Trial | 1500 | 9.88 * | 0.30 | 59.56 | 0.43 | 3736.88 * | 118.75 |
| 15%-15 °C Control | 500 | 10.68 | 0.18 | 61.97 | 0.19 | 4081.13 | 70.95 |
| 15%-15 °C Trial | 500 | 5.35 * | 0.09 | 60.12 * | 0.43 | 2030.54 * | 38.17 |
| 15%-15 °C Control | 1000 | 14.72 | 0.54 | 61.74 | 0.53 | 5616.28 | 217.58 |
| 15%-15 °C Trial | 1000 | 8.01 * | 0.14 | 59.89* | 0.30 | 3032.60 * | 54.27 |
| 15%-15 °C Control | 1500 | 18.05 | 0.83 | 62.22 | 0.34 | 6900.68 | 325.69 |
| 15%-15 °C Trial | 1500 | 10.70 * | 0.38 | 60.64 * | 0.42 | 4065.38 * | 151.15 |
| 15%-30 °C Control | 500 | 11.24 | 0.26 | 63.70 | 0.77 | 4324.30 | 101.11 |
| 15%-30 °C Trial | 500 | 6.75 * | 0.17 | 63.88 | 0.35 | 2599.33 * | 69.52 |
| 15%-30 °C Control | 1000 | 16.90 | 0.39 | 62.04 | 0.20 | 6456.06 | 150.41 |
| 15%-30 °C Trial | 1000 | 11.34 * | 0.83 | 63.68 | 1.77 | 4360.63 * | 305.77 |
| 15%-30 °C Control | 1500 | 18.56 | 0.57 | 62.80 | 0.21 | 7111.70 | 220.79 |
| 15%-30 °C Trial | 1500 | 13.07 * | 0.29 | 62.90 | 0.46 | 5008.61 * | 119.10 |
| Condition | 500 mg/L | StDev | 1000 mg/L | StDev | 1500 mg/L | StDev |
|---|---|---|---|---|---|---|
| 0%EtOH-15 °C | 0.1424 | 0.0107 | 0.2913 | 0.0169 | 0.4733 | 0.0103 |
| 0%EtOH-30 °C | 0.1101 | 0.0085 | 0.2489 | 0.0102 | 0.3719 | 0.0067 |
| 7.5%EtOH-22.5°C | 0.0964 | 0.0061 | 0.2206 | 0.0042 | 0.3135 | 0.0021 |
| 15%EtOH-15 °C | 0.1086 | 0.0115 | 0.2359 | 0.0069 | 0.3492 | 0.0135 |
| 15%EtOH-30 °C | 0.0840 | 0.0032 | 0.1879 | 0.0037 | 0.2272 | 0.0078 |
| Condition | 500 mg/L | StDev | 1000 mg/L | StDev | 1500 mg/L | StDev |
|---|---|---|---|---|---|---|
| 0%EtOH-15 °C | 0.1136 | 0.0024 | 0.2000 | 0.0084 | 0.2641 | 0.0081 |
| 0%EtOH-30 °C | 0.1007 | 0.0036 | 0.1910 | 0.0046 | 0.2381 | 0.0093 |
| 7.5%EtOH-22.5 °C | 0.1027 | 0.0027 | 0.1707 | 0.0026 | 0.2123 | 0.0114 |
| 15%EtOH-15 °C | 0.0748 | 0.0085 | 0.1119 | 0.0132 | 0.1174 | 0.0091 |
| 15%EtOH-30 °C | 0.0616 | 0.0103 | 0.0910 | 0.0175 | 0.1051 | 0.0075 |
| Condition | Average MM Reduction (With A+) | StDev | Average MM Reduction (Without A+) | StDev |
|---|---|---|---|---|
| 0%-15 °C | 4622.81 | 186.01 | 2688.07 | 90.33 |
| 0%-30 °C | 4040.90 | 224.11 | 1713.57 | 231.24 |
| 7.5%-22.5 °C | 3775.43 | 508.73 | 1444.71 | 362.84 |
| 15%-15 °C | 4049.18 | 296.20 | 1659.02 | 247.51 |
| 15%-30 °C | 2872.66 | 133.12 | 1718.05 | 357.99 |
References
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology (Vol. 2): The Chemistry of Wine Stabilization and Treatments; John Wiley and Sons Incorporated: Hoboken, NJ, USA, 2001; pp. 172–173. [Google Scholar]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between grape-derived proanthocyanidins and cell wall material. 2. implications for vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 1. Effect on Proanthocyanidin Composition and Molecular Mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochim. Biophys. Acta-Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.W.; Medina-Plaza, C.; Miller, K.V.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. Effects of temperature and ethanol on the kinetics of proanthocyanidin adsorption in model wine systems. J. Agric. Food Chem. 2020, 68, 2891–2899. [Google Scholar] [CrossRef]
- Miller, K.V.; Noguera, R.; Beaver, J.; Medina-plaza, C.; Oberholster, A.; Block, D.E. A Mechanistic Model for the Extraction of Phenolics from Grapes During Red Wine Fermentation. Molecules 2019, 24, 1275. [Google Scholar] [CrossRef]
- Beaver, J.W.; Medina-Plaza, C.; Miller, K.V.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. Heat-dependent desorption of proanthocyanidins from grape-derived cell wall material under variable ethanol concentrations in model wine systems. Molecules 2019, 24, 3561. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Cano-Lechuga, M.; Ruiz-García, Y.; Gómez-Plaza, E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chem. 2014, 152, 558–565. [Google Scholar] [CrossRef]
- Singleton, V.L.; Trousdale, E.K. Anthocyanin-Tannin Interactions Explaining differences in polymeric phenols between white and red wines. Am. J. Enol. Vitic. 1992, 43, 63–70. [Google Scholar]
- Bautista-Ortín, A.B.; Martínez-Hernández, A.; Ruiz-García, Y.; Gil-Muñoz, R.; Gómez-Plaza, E. Anthocyanins influence tannin-cell wall interactions. Food Chem. 2016, 206, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules 2019, 24, 3350. [Google Scholar] [CrossRef]
- Singleton, V.L.; Draper, D.E. The Transfer of Polyphenolic Compounds from Grape Seeds into Wines. Am. J. Enol. Vitic. 1964, 15, 34–40. [Google Scholar]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction. Am. J. Enol. Vitic. 2005, 48, 197–206. [Google Scholar]
- Zanchi, D.; Vernhet, A.; Poncet-Legrand, C.; Cartalade, D.; Tribet, C.; Schweins, C.; Cabane, B. Colloidal Dispersions of Tannins in Water−Ethanol. Langmuir 2007, 23, 9949–9959. [Google Scholar] [CrossRef]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-specific and developmental modifications of grape cell walls influence the adsorption of proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef]
- Allegrom, G.; Bautista-Ortin, A.B.; Gomez-Plaza, E.; Pasore, C.; Valentini, G.; Filipetti, I. Impact of flavaonoid and cell wall material changes on phenolic maurity in cv. merlot (Vitis vinifera L.). Am. J. Enol. Vitic. 2018, 69, 417–421. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Molero, N.; Marín, F.; Ruiz-García, Y.; Gómez-Plaza, E. Reactivity of pure and commercial grape skin tannins with cell wall material. Eur. Food Res. Technol. 2014, 240, 645–654. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley Publishing: Hoboken, NJ, USA, 2016; pp. 118–126. [Google Scholar]
- Soderberg, T. Organic Chemistry with a Biological Emphasis. Chemistry Publications. 2016, Volume I. Available online: https://digitalcommons.morris.umn.edu/chem_facpubs/1 (accessed on 8 August 2020).
- Teng, B.; Hayasaka, Y.; Smith, P.A.; Bindon, K.A. Effect of Grape Seed and Skin Tannin Molecular Mass and Composition on the Rate of Reaction with Anthocyanin and Subsequent Formation of Polymeric Pigments in the Presence of Acetaldehyde. J. Agric. Food Chem. 2019, 67, 8938–8949. [Google Scholar] [CrossRef]
- Dallas, C.; Ricardo da Silva, J.M.; Laureano, O. Interactions of Oligomeric Procyanidins in Model Wine Solutions Containing Malvidin-3-Glucoside and Acetaldehyde. J. Sci. Food Agric. 1996, 70, 493–500. [Google Scholar] [CrossRef]
- Thorngate, J.H.; Singleton, V.L. Reactions of Monomeric and Polymeric Flavan-3-Ols with Monomeric Pigment in Model Wine Solutions. Am. J. Enol. Vitic. 1994, 45, 349–352. [Google Scholar]
- Timberlake, C.F.; Bridle, P. Interactions Between Anthocyanins, Phenolic Compounds, and Acetaldehyde and Their Significance in Red Wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- Vidal, S.; Williams, P.; Neill, M.A.O.; Pellerin, P. Polysaccharides from grape berry cell walls Part I: Tissue distribution and structural characterization of the pectic polysaccharides. Carbohydr. Polym. 2001, 45, 315–323. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P.; Pa, P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Oberholster, A.; Peng, Z.; Sefton, M.A.; Iland, P.G.; Waters, E.J. Analysis of pigmented polymers in red wine by reverse phase HPLC. Aust. J. Grape Wine R. 2008, 8, 70–75. [Google Scholar]
- Ruiz-Garcia, Y.; Smith, P.A.; Bindon, K.A. Selective extraction of polysaccharide affects the adsorption of proanthocyanidin by grape cell walls. Carbohydr. Polym. 2014, 114, 102–114. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ortega-Regules, A.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Differences in anthocyanin extractability from grapes to wines according to variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar]
- Nagel, C.W.; Wulf, L.W. Changes in the anthocyanins, flavonoids and hydroxycinamic acid esters during fermentation and aging of Merlot and Cabernet Sauvignon. Am. J. Enol. Vitic. 1979, 30, 111–116. [Google Scholar]
- Petrie, P.R.; Clingeleffer, P.R. Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: Outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate. Aust. J. Grape Wine Res. 2006, 12, 21–29. [Google Scholar] [CrossRef]
- Boulton, R.; Skogerson, K.; Downey, M.; Mazza, M. Rapid determination of phenolic components in red wines from UV-visible spectra and the method of partial least squares. Am. J. Enol. Vitic. 2007, 58, 318–325. [Google Scholar]
- McLeod, S.; Colantuoni, G. Wine X-ray: Total Iron-reactive phenolics. Available online: https://www.winexray.com/how-we-do-it (accessed on 8 August 2020).
- Kennedy, J.A.; Taylor, A. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Skogerson, K.; Downey, M.; Boulton, R. Modified Adams’ assay for phenolics in wine: Total iron reactive phenolics. Available online: http://boulton.ucdavis.edu/uv-vis/Model.htm (accessed on 8 August 2020).
Sample Availability: Samples from the described study are not available from the authors. |
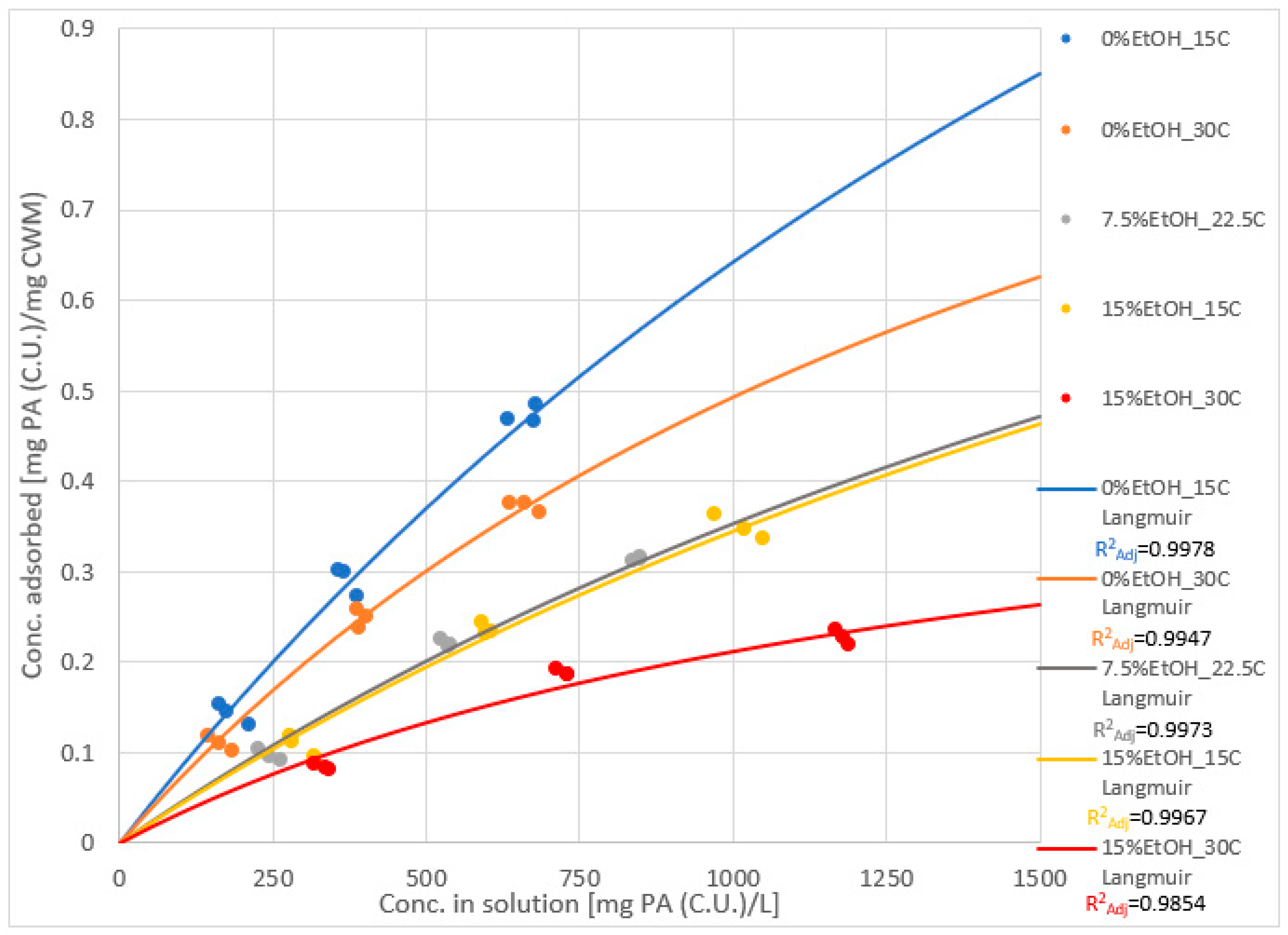

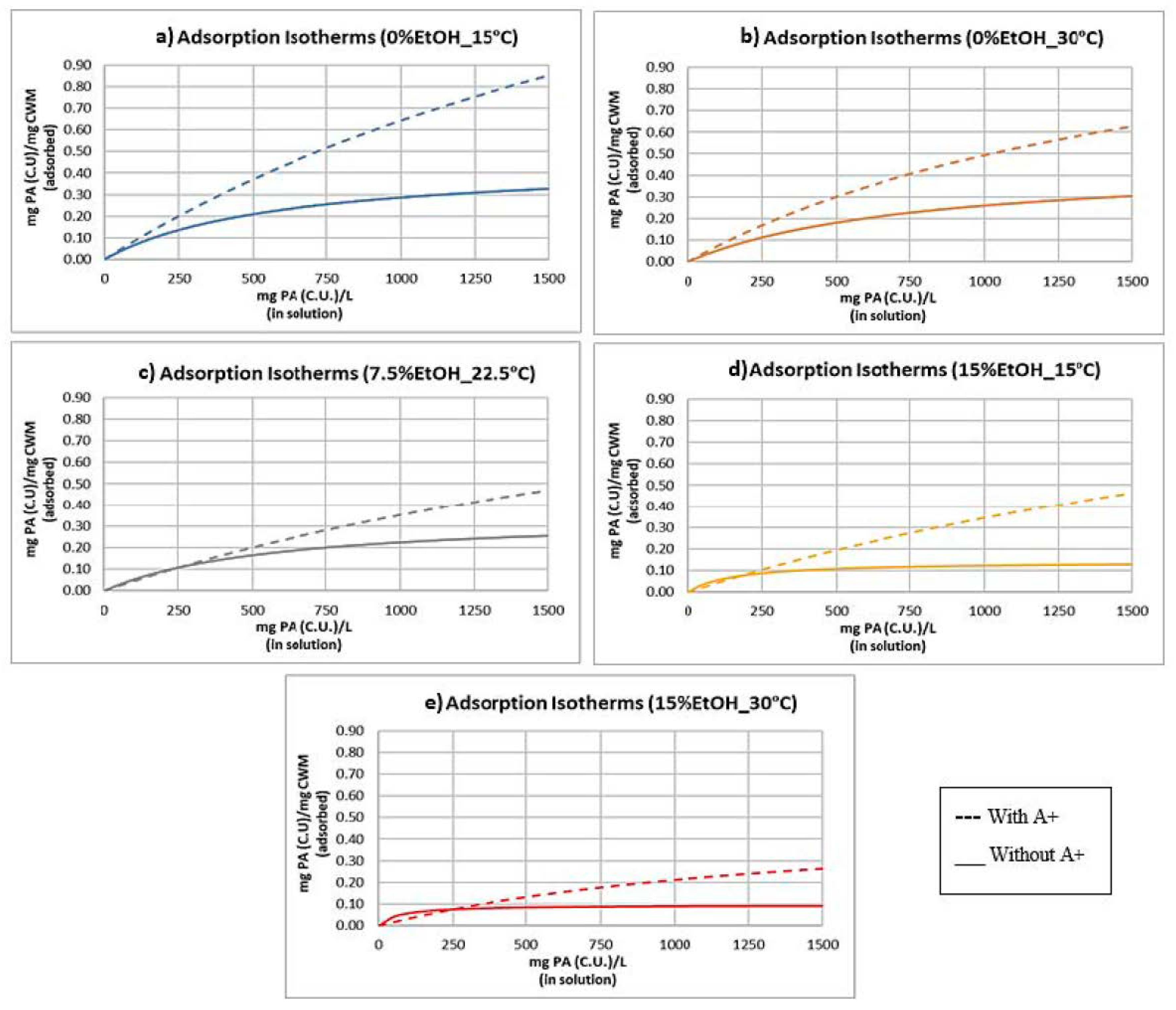


| Experimental Condition | Keq | SCWM |
|---|---|---|
| 0%EtOH-15 °C | 3.63 × 10−4 | 2.41 |
| 0%EtOH-30 °C | 5.70 × 10−4 | 1.36 |
| 7.5%EtOH-22.5 °C | 3.28 × 10−4 | 1.43 |
| 15%EtOH-15 °C | 3.02 × 10−4 | 1.48 |
| 15%EtOH-30 °C | 6.98 × 10−4 | 5.14 × 10−1 |
| Starting Concentration of PA Solution (mg/L) | Incubation Temperature (°C) | Ethanol Percentage of Model Wine (v/v) |
|---|---|---|
| 500 | 15 | 0% |
| 1000 | 15 | 0% |
| 1500 | 15 | 0% |
| 500 | 15 | 15% |
| 1000 | 15 | 15% |
| 1500 | 15 | 15% |
| 500 | 22.5 | 7.5% |
| 1000 | 22.5 | 7.5% |
| 1500 | 22.5 | 7.5% |
| 500 | 30 | 0% |
| 1000 | 30 | 0% |
| 1500 | 30 | 0% |
| 500 | 30 | 15% |
| 1000 | 30 | 15% |
| 1500 | 30 | 15% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaver, J.W.; Miller, K.V.; Medina-Plaza, C.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. The Effects of Temperature and Ethanol on Proanthocyanidin Adsorption to Grape Cell Wall Material in the Presence of Anthocyanins. Molecules 2020, 25, 4139. https://doi.org/10.3390/molecules25184139
Beaver JW, Miller KV, Medina-Plaza C, Dokoozlian N, Ponangi R, Blair T, Block D, Oberholster A. The Effects of Temperature and Ethanol on Proanthocyanidin Adsorption to Grape Cell Wall Material in the Presence of Anthocyanins. Molecules. 2020; 25(18):4139. https://doi.org/10.3390/molecules25184139
Chicago/Turabian StyleBeaver, Jordan W., Konrad V. Miller, Cristina Medina-Plaza, Nick Dokoozlian, Ravi Ponangi, Thomas Blair, David Block, and Anita Oberholster. 2020. "The Effects of Temperature and Ethanol on Proanthocyanidin Adsorption to Grape Cell Wall Material in the Presence of Anthocyanins" Molecules 25, no. 18: 4139. https://doi.org/10.3390/molecules25184139
APA StyleBeaver, J. W., Miller, K. V., Medina-Plaza, C., Dokoozlian, N., Ponangi, R., Blair, T., Block, D., & Oberholster, A. (2020). The Effects of Temperature and Ethanol on Proanthocyanidin Adsorption to Grape Cell Wall Material in the Presence of Anthocyanins. Molecules, 25(18), 4139. https://doi.org/10.3390/molecules25184139








