Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma
Abstract
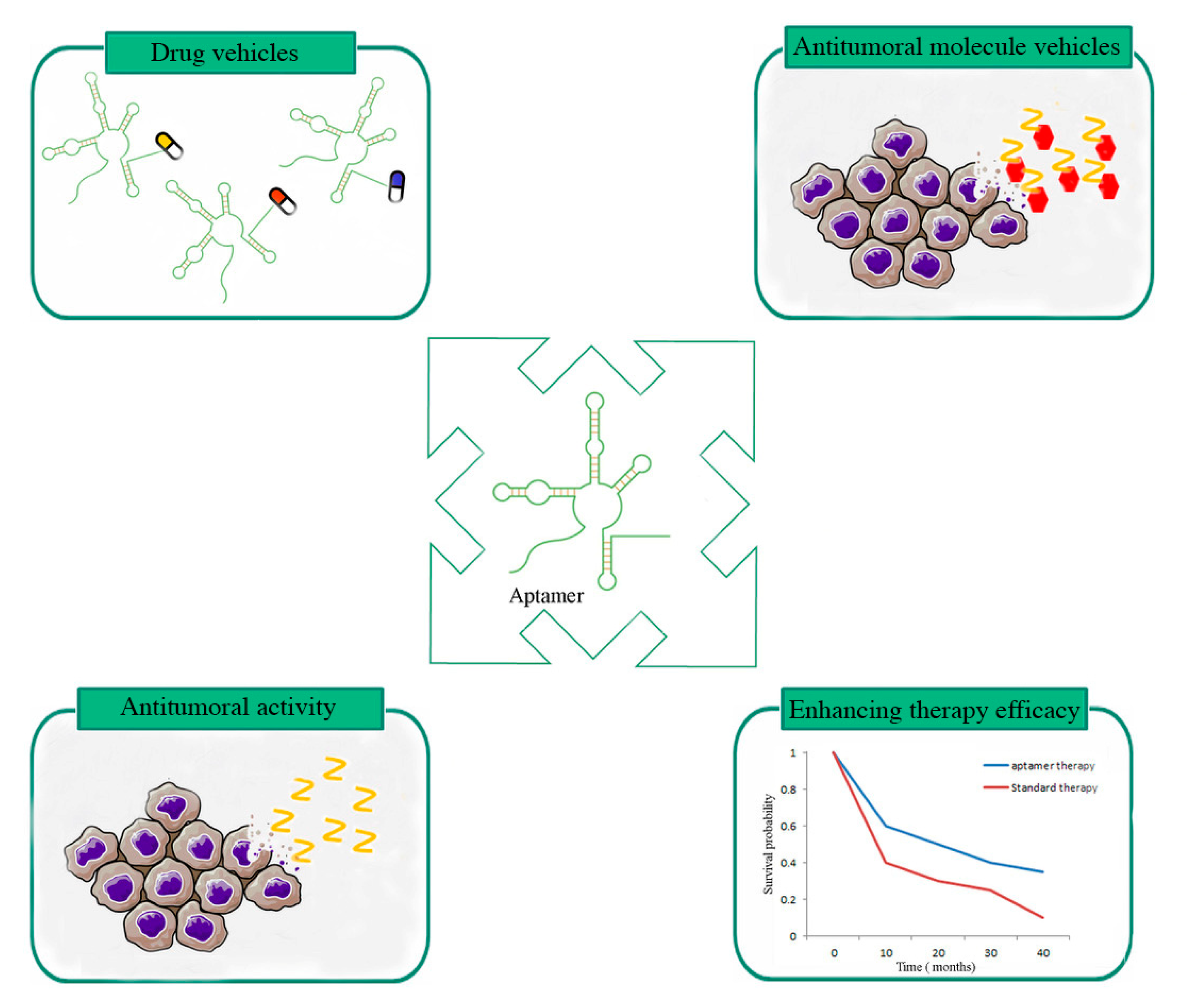
:1. Introduction
2. Aptamers Showing In Vivo Therapeutic Effects
2.1. Aptamers Used as Antitumoral Molecules
2.1.1. Gint4.T and CL4 Aptamers
2.1.2. AS1411 Aptamer
2.2. Aptamers Used as Drug Vehicles
2.2.1. AS1411 Aptamer
2.2.2. GMT8 Aptamer
2.2.3. ATP Aptamer
2.3. Aptamers Able to Enhance GBM Therapy Efficacy
2.3.1. Aptamers Enhancing Radiotherapy Sensitivity
U2 Aptamer
NOX-A12 Aptamer
AS1411 Aptamer
2.3.2. Immune-Modular Aptamers
VEGF-4-1BB Bi-specific Aptamer
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Wang, F.; Qi Xie, X. Advanced treatment in high-grade gliomas. J. BUON 2019, 24, 424–430. [Google Scholar] [PubMed]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Urak, K.T.; Shore, S.; Rockey, W.M.; Chen, S.J.; McCaffrey, A.P.; Giangrande, P.H. In vitro RNA SELEX for the generation of chemically-optimized therapeutic RNA drugs. Methods 2016, 103, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, D.; Schluesener, H.J.; Zhang, Z. Advances in SELEX and application of aptamers in the central nervous system. Biomol. Eng. 2007, 24, 583–592. [Google Scholar] [CrossRef]
- Guo, K.T.; Ziemer, G.; Paul, A.; Wendel, H.P. CELL-SELEX: Novel perspectives of aptamer-based therapeutics. Int. J. Mol. Sci. 2008, 9, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [Green Version]
- Bunka, D.H.; Stockley, P.G. Aptamers come of age—at last. Nat. Rev. Microbiol. 2006, 4, 588–596. [Google Scholar] [CrossRef]
- Kang, D.; Wang, J.; Zhang, W.; Song, Y.; Li, X.; Zou, Y.; Zhu, M.; Zhu, Z.; Chen, F.; Yang, C.J. Selection of DNA aptamers against glioblastoma cells with high affinity and specificity. PLoS ONE 2012, 7, e42731. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, Y.; Wang, H.; Wu, L.; Zhang, H.; Song, Y.; Zhu, Z.; Kang, D.; Yang, C. DNA aptamers from whole-cell SELEX as new diagnostic agents against glioblastoma multiforme cells. Analyst 2018, 143, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, C.A.; Cai, W.; Hong, H. Applications of aptamers in targeted imaging: State of the art. Curr Top. Med. Chem. 2015, 15, 1138–1152. [Google Scholar] [CrossRef]
- Hicke, B.J.; Stephens, A.W.; Gould, T.; Chang, Y.F.; Lynott, C.K.; Heil, J.; Borkowski, S.; Hilger, C.S.; Cook, G.; Warren, S.; et al. Tumor targeting by an aptamer. J. Nucl. Med. 2006, 47, 668–678. [Google Scholar] [PubMed]
- Keshtkar, M.; Shahbazi-Gahrouei, D.; Khoshfetrat, S.M.; Mehrgardi, M.A.; Aghaei, M. Aptamer-conjugated Magnetic Nanoparticles as Targeted Magnetic Resonance Imaging Contrast Agent for Breast Cancer. J. Med. Signals Sens. 2016, 6, 243–247. [Google Scholar] [PubMed]
- Rockey, W.M.; Huang, L.; Kloepping, K.C.; Baumhover, N.J.; Giangrande, P.H.; Schultz, M.K. Synthesis and radiolabeling of chelator-RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorg. Med. Chem. 2011, 19, 4080–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, P.; Rhines, L.D.; DiMeco, F.; Tyler, B.M.; Park, M.C.; Brem, H. Interstitial docetaxel (taxotere), carmustine and combined interstitial therapy: A novel treatment for experimental malignant glioma. J. Neurooncol. 2006, 80, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blank, M.; Weinschenk, T.; Priemer, M.; Schluesener, H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels. selective targeting of endothelial regulatory protein pigpen. J. Biol. Chem. 2001, 276, 16464–16468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Yang, J.; Hwang, M.; Choi, J.; Kim, H.O.; Jang, E.; Lee, J.H.; Ryu, S.H.; Suh, J.S.; Huh, Y.M.; et al. Aptamer-modified magnetic nanoprobe for molecular MR imaging of VEGFR2 on angiogenic vasculature. Nanoscale Res. Lett. 2013, 8, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Liang, H.; Tan, Y.; Yuan, C.; Li, S.; Li, X.; Li, G.; Shi, Y.; Zhang, X. Cell-SELEX aptamer for highly specific radionuclide molecular imaging of glioblastoma in vivo. PLoS ONE 2014, 9, e90752. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Abnous, K.; Ramezani, M.; Hosseinkhani, H.; Hadizadeh, F. Synthesis of AS1411-aptamer-conjugated CdTe quantum dots with high fluorescence strength for probe labeling tumor cells. J. Fluoresc. 2014, 24, 1519–1529. [Google Scholar] [CrossRef]
- Tang, J.; Huang, N.; Zhang, X.; Zhou, T.; Tan, Y.; Pi, J.; Pi, L.; Cheng, S.; Zheng, H.; Cheng, Y. Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. Int. J. Nanomed. 2017, 12, 3899–3911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fechter, P.; Cruz Da Silva, E.; Mercier, M.C.; Noulet, F.; Etienne-Seloum, N.; Guenot, D.; Lehmann, M.; Vauchelles, R.; Martin, S.; Lelong-Rebel, I.; et al. RNA Aptamers Targeting Integrin alpha5beta1 as Probes for Cyto- and Histofluorescence in Glioblastoma. Mol. Ther. Nucleic Acids 2019, 17, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Kim, Y.T.; Li, N.; Cho, S.K.; Bachoo, R.; Ellington, A.D.; Iqbal, S.M. Surface-immobilized aptamers for cancer cell isolation and microscopic cytology. Cancer Res. 2010, 70, 9371–9380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Tan, J.; Asghar, W.; Kim, Y.T.; Liu, Y.; Iqbal, S.M. Velocity effect on aptamer-based circulating tumor cell isolation in microfluidic devices. J. Phys. Chem. B 2011, 115, 13891–13896. [Google Scholar] [CrossRef]
- Wan, Y.; Mahmood, M.A.; Li, N.; Allen, P.B.; Kim, Y.T.; Bachoo, R.; Ellington, A.D.; Iqbal, S.M. Nanotextured substrates with immobilized aptamers for cancer cell isolation and cytology. Cancer 2012, 118, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, D.A.; Chen, H.; Hicke, B.J.; Swiderek, K.M.; Gold, L. A tenascin-C aptamer identified by tumor cell SELEX: Systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA 2003, 100, 15416–15421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghouts, C.; Kunz, C.; Delis, N.; Groner, B. Monomeric recombinant peptide aptamers are required for efficient intracellular uptake and target inhibition. Mol. Cancer Res. 2008, 6, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Nguyen, H.H.; Byrom, M.; Ellington, A.D. Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS ONE 2011, 6, e20299. [Google Scholar] [CrossRef]
- Borghouts, C.; Delis, N.; Brill, B.; Weiss, A.; Mack, L.; Lucks, P.; Groner, B. A membrane penetrating peptide aptamer inhibits STAT3 function and suppresses the growth of STAT3 addicted tumor cells. JAKSTAT 2012, 1, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Tamuly, D.; Allen, P.B.; Kim, Y.T.; Bachoo, R.; Ellington, A.D.; Iqbal, S.M. Proliferation and migration of tumor cells in tapered channels. Biomed. Microdevices 2013, 15, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liang, H.; Tan, Y.; Wu, X.; Li, S.; Shi, Y. A U87-EGFRvIII cell-specific aptamer mediates small interfering RNA delivery. Biomed. Rep. 2014, 2, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camorani, S.; Crescenzi, E.; Colecchia, D.; Carpentieri, A.; Amoresano, A.; Fedele, M.; Chiariello, M.; Cerchia, L. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget 2015, 6, 37570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amero, P.; Esposito, C.L.; Rienzo, A.; Moscato, F.; Catuogno, S.; de Franciscis, V. Identification of an Interfering Ligand Aptamer for EphB2/3 Receptors. Nucleic Acid Ther. 2016, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bayrac, A.T.; Akca, O.E.; Eyidogan, F.I.; Oktem, H.A. Target-specific delivery of doxorubicin to human glioblastoma cell line via ssDNA aptamer. J. Biosci. 2018, 43, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Wu, X.; Armstrong, B.; Habib, N.; Rossi, J.J. An RNA Aptamer Targeting the Receptor Tyrosine Kinase PDGFRalpha Induces Anti-tumor Effects through STAT3 and p53 in Glioblastoma. Mol. Ther. Nucleic Acids 2019, 14, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef]
- Gao, H.; Qian, J.; Yang, Z.; Pang, Z.; Xi, Z.; Cao, S.; Wang, Y.; Pan, S.; Zhang, S.; Wang, W.; et al. Whole-cell SELEX aptamer-functionalised poly(ethyleneglycol)-poly(epsilon-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. Biomaterials 2012, 33, 6264–6272. [Google Scholar] [CrossRef]
- An, S.; Lu, X.; Zhao, W.; Sun, T.; Zhang, Y.; Lu, Y.; Jiang, C. Amino Acid Metabolism Abnormity and Microenvironment Variation Mediated Targeting and Controlled Glioma Chemotherapy. Small 2016, 12, 5633–5645. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Z.; Jin, K.; Pang, Q.; Jiang, T.; Lu, H.; Liu, X.; Pang, Z.; Yu, L.; Jiang, X. Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (l-gamma-glutamylglutamine)-paclitaxel nanoconjugates. J. Colloid Interface Sci. 2017, 490, 783–796. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, H.; Liu, Y.; Wen, Y.; Wei, C.; Yu, Q.; Liu, J. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRbeta aptamer. Mol. Ther. 2014, 22, 828–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Zhao, G.; Zhang, S.; Nigim, F.; Zhou, G.; Yu, Z.; Song, Y.; Chen, Y.; Li, Y. AS1411-Induced Growth Inhibition of Glioma Cells by Up-Regulation of p53 and Down-Regulation of Bcl-2 and Akt1 via Nucleolin. PLoS ONE 2016, 11, e0167094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, C.L.; Nuzzo, S.; Catuogno, S.; Romano, S.; de Nigris, F.; de Franciscis, V. STAT3 Gene Silencing by Aptamer-siRNA Chimera as Selective Therapeutic for Glioblastoma. Mol. Ther. Nucleic Acids 2018, 10, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrand, B.; Berezhnoy, A.; Brenneman, R.; Williams, A.; Levay, A.; Kong, L.Y.; Rao, G.; Zhou, S.; Heimberger, A.B.; Gilboa, E. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol. Res. 2014, 2, 867–877. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef] [Green Version]
- Hays, E.M.; Duan, W.; Shigdar, S. Aptamers and Glioblastoma: Their Potential Use for Imaging and Therapeutic Applications. Int. J. Mol. Sci. 2017, 18, 2576. [Google Scholar] [CrossRef] [Green Version]
- Esposito, C.L.; Passaro, D.; Longobardo, I.; Condorelli, G.; Marotta, P.; Affuso, A.; de Franciscis, V.; Cerchia, L. A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death. PLoS ONE 2011, 6, e24071. [Google Scholar] [CrossRef]
- Carrasco-Garcia, E.; Saceda, M.; Grasso, S.; Rocamora-Reverte, L.; Conde, M.; Gomez-Martinez, A.; Garcia-Morales, P.; Ferragut, J.A.; Martinez-Lacaci, I. Small tyrosine kinase inhibitors interrupt EGFR signaling by interacting with erbB3 and erbB4 in glioblastoma cell lines. Exp. Cell Res. 2011, 317, 1476–1489. [Google Scholar] [CrossRef]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Li, L.; Zhu, L.J.; Smith, T.W.; Demers, A.; Ross, A.H.; Moser, R.P.; Green, M.R. A genome-wide RNA interference screen reveals an essential CREB3L2-ATF5-MCL1 survival pathway in malignant glioma with therapeutic implications. Nat. Med. 2010, 16, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Q.W.; Cheng, C.K.; Gustafson, W.C.; Charron, E.; Zipper, P.; Wong, R.A.; Chen, J.; Lau, J.; Knobbe-Thomsen, C.; Weller, M.; et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 2013, 24, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Neff, C.P.; Swiderski, P.; Li, H.; Smith, D.D.; Aboellail, T.; Remling-Mulder, L.; Akkina, R.; Rossi, J.J. Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol. Ther. 2013, 21, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, C.L.; Cerchia, L.; Catuogno, S.; De Vita, G.; Dassie, J.P.; Santamaria, G.; Swiderski, P.; Condorelli, G.; Giangrande, P.H.; de Franciscis, V. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol. Ther. 2014, 22, 1151–1163. [Google Scholar] [CrossRef] [Green Version]
- Catuogno, S.; Rienzo, A.; Di Vito, A.; Esposito, C.L.; de Franciscis, V. Selective delivery of therapeutic single strand antimiRs by aptamer-based conjugates. J. Control. Release 2015, 210, 147–159. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Kumar, S.A.; Rienzo, A.; Lawrence, C.L.; Pallini, R.; Shaw, L.; Alder, J.E.; Ricci-Vitiani, L.; Catuogno, S.; et al. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J. Control. Release 2016, 238, 43–57. [Google Scholar] [CrossRef]
- Mongelard, F.; Bouvet, P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 2010, 12, 107–114. [Google Scholar]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Girvan, A.C.; Casson, L.K.; Pierce, W.M., Jr.; Qian, M.; Thomas, S.D.; Bates, P.J. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 2007, 67, 10491–10500. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.H.; Xu, A.M.; White, F.M. Oncogenic EGFR signaling networks in glioma. Sci. Signal. 2009, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Storck, S.; Shukla, M.; Dimitrov, S.; Bouvet, P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007, 41, 125–144. [Google Scholar] [PubMed]
- Goldshmit, Y.; Trangle, S.S.; Kloog, Y.; Pinkas-Kramarski, R. Interfering with the interaction between ErbB1, nucleolin and Ras as a potential treatment for glioblastoma. Oncotarget 2014, 5, 8602–8613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuloh, G.; Pechstein, U.; Schramm, J. Motor tract monitoring during insular glioma surgery. J. Neurosurg. 2007, 106, 582–592. [Google Scholar] [CrossRef]
- Fine, H.A.; Dear, K.B.; Loeffler, J.S.; Black, P.M.; Canellos, G.P. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 1993, 71, 2585–2597. [Google Scholar] [CrossRef]
- Pope, W.B.; Lai, A.; Nghiemphu, P.; Mischel, P.; Cloughesy, T.F. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 2006, 66, 1258–1260. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Shi, X.; Hu, H.; Du, X.; Fang, Y.; Ma, Y.; Wu, H.; Yang, S. Water-soluble superparamagnetic manganese ferrite nanoparticles for magnetic resonance imaging. Biomaterials 2010, 31, 3667–3673. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, F.; Pan, Y.; Hou, C.; Ren, L.; Chen, J.; Wang, J.; Zhang, Y. Arg-Gly-Asp (RGD) peptide conjugated poly(lactic acid)-poly(ethylene oxide) micelle for targeted drug delivery. J. Biomed. Mater. Res. A 2008, 85, 797–807. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Lim, W.Q.; Luo, Z.; Phua, S.Z.F.; Huo, R.; Li, L.; Li, K.; Dai, L.; Liu, J.; et al. A Transferrin-Conjugated Hollow Nanoplatform for Redox-Controlled and Targeted Chemotherapy of Tumor with Reduced Inflammatory Reactions. Theranostics 2018, 8, 518–532. [Google Scholar] [CrossRef] [Green Version]
- Hajek, R.; Vorlicek, J.; Slavik, M. Paclitaxel (Taxol): A review of its antitumor activity in clinical studies Minireview. Neoplasma 1996, 43, 141–154. [Google Scholar]
- Nikanjam, M.; Gibbs, A.R.; Hunt, C.A.; Budinger, T.F.; Forte, T.M. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J. Control. Release 2007, 124, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Demeule, M.; Che, C.; Lavallee, I.; Poirier, J.; Gabathuler, R.; Beliveau, R.; Castaigne, J.P. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K. Clinical pharmacology of Taxol. J. Natl. Cancer Inst. Monogr. 1993, 15, 25–37. [Google Scholar]
- Son, M.J.; Song, H.S.; Kim, M.H.; Kim, J.T.; Kang, C.M.; Jeon, J.W.; Park, S.Y.; Kim, Y.J.; Groves, M.D.; Park, K.; et al. Synergistic effect and condition of pegylated interferon alpha with paclitaxel on glioblastoma. Int. J. Oncol. 2006, 28, 1385–1392. [Google Scholar] [PubMed]
- Zhan, C.; Gu, B.; Xie, C.; Li, J.; Liu, Y.; Lu, W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J. Control. Release 2010, 143, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.Y.; Zhao, B.J.; Zhao, X.; Wang, Y.; Huang, Y.; Chen, X.M.; Zhao, B.X.; Zhao, S.S.; Zhang, X.; Zhang, Q. The therapeutic efficacy of conjugated linoleic acid—paclitaxel on glioma in the rat. Biomaterials 2010, 31, 5855–5864. [Google Scholar] [CrossRef]
- Van, S.; Das, S.K.; Wang, X.; Feng, Z.; Jin, Y.; Hou, Z.; Chen, F.; Pham, A.; Jiang, N.; Howell, S.B.; et al. Synthesis, characterization, and biological evaluation of poly(L-gamma-glutamyl-glutamine)- paclitaxel nanoconjugate. Int. J. Nanomed. 2010, 5, 825–837. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Zhang, J.; Wang, L.; Chan, J.; Wang, H.; Jin, Y.; Yu, L.; Grainger, D.W.; Ying, W. A cell-based pharmacokinetics assay for evaluating tubulin-binding drugs. Int. J. Med. Sci. 2014, 11, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Yu, L.; Van, S. Clinically relevant anticancer polymer Paclitaxel therapeutics. Cancers 2010, 3, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, C.; Jiang, C.; Shen, X.; Qiao, Q.; Hu, Y. Nucleolin targeting AS1411 modified protein nanoparticle for antitumor drugs delivery. Mol. Pharm. 2013, 10, 3555–3563. [Google Scholar] [CrossRef]
- Gueritte-Voegelein, F.; Guenard, D.; Lavelle, F.; Le Goff, M.T.; Mangatal, L.; Potier, P. Relationships between the structure of taxol analogues and their antimitotic activity. J. Med. Chem. 1991, 34, 992–998. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, M.; Zhao, S.; Sun, J.; Yu, Q.; Liu, J. Pompon-like RuNPs-Based Theranostic Nanocarrier System with Stable Photoacoustic Imaging Characteristic for Accurate Tumor Detection and Efficient Phototherapy Guidance. ACS Appl. Mater. Interfaces 2017, 9, 33645–33659. [Google Scholar] [CrossRef]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Esteve, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, A.R.; Kim, S.H.; Lee, S.J.; Chung, H.; Yoon, M.Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Shen, Y.; Fu, Y.; Muroski, M.E.; Zhang, P.; Wang, Q.; Xu, C.; Lesniak, M.S.; Li, G.; Cheng, Y. Self-Assembly of Gold Nanoparticles Shows Microenvironment-Mediated Dynamic Switching and Enhanced Brain Tumor Targeting. Theranostics 2017, 7, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Liu, Z.; Dong, K.; Ren, J.; Qu, X. Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo-photothermal therapy. Biomaterials 2014, 35, 6646–6656. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, H.; Hu, J.; Han, X.; Liu, H.; Hu, Y. Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 77–84. [Google Scholar] [CrossRef]
- Luo, Z.; Cai, K.; Hu, Y.; Zhao, L.; Liu, P.; Duan, L.; Yang, W. Mesoporous silica nanoparticles end-capped with collagen: Redox-responsive nanoreservoirs for targeted drug delivery. Angew. Chem. Int. Ed. Engl. 2011, 50, 640–643. [Google Scholar] [CrossRef]
- Navath, R.S.; Kurtoglu, Y.E.; Wang, B.; Kannan, S.; Romero, R.; Kannan, R.M. Dendrimer-drug conjugates for tailored intracellular drug release based on glutathione levels. Bioconjug. Chem. 2008, 19, 2446–2455. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yu, Q.; Pan, J.; Zhou, Y.; Cao, C.; Ouyang, J.M.; Liu, J. Redox-responsive mesoporous selenium delivery of doxorubicin targets MCF-7 cells and synergistically enhances its anti-tumor activity. Acta Biomater. 2017, 54, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Tosoni, A.; Franceschi, E.; Reni, M.; Gatta, G.; Vecht, C. Glioblastoma in adults. Crit. Rev. Oncol. Hematol. 2008, 67, 139–152. [Google Scholar] [CrossRef]
- Bastien, J.I.; McNeill, K.A.; Fine, H.A. Molecular characterizations of glioblastoma, targeted therapy, and clinical results to date. Cancer 2015, 121, 502–516. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of Aptamer to U87-EGFRvIII Cells on the Proliferation, Radiosensitivity, and Radiotherapy of Glioblastoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [Green Version]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Deng, Q.; Zhou, J.; Zou, J.; Zhang, Y.; Tan, P.; Zhang, W.; Cui, H. CSN6 controls the proliferation and metastasis of glioblastoma by CHIP-mediated degradation of EGFR. Oncogene 2017, 36, 1134–1144. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Huang, Z.; Chen, Z.; Xu, R.; Wu, H.; Zang, F.; Wang, C.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.O.; Brown, J.M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008, 13, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.C.; Alomran, R.; Chernikova, S.B.; Lartey, F.; Stafford, J.; Jang, T.; Merchant, M.; Zboralski, D.; Zollner, S.; Kruschinski, A.; et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncol. 2014, 16, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Kish, P.E.; Blaivas, M.; Strawderman, M.; Muraszko, K.M.; Ross, D.A.; Ross, B.D.; McMahon, G. Magnetic resonance imaging of ethyl-nitrosourea-induced rat gliomas: A model for experimental therapeutics of low-grade gliomas. J. Neuro-Oncol. 2001, 53, 243–257. [Google Scholar] [CrossRef]
- Antosh, M.P.; Wijesinghe, D.D.; Shrestha, S.; Lanou, R.; Huang, Y.H.; Hasselbacher, T.; Fox, D.; Neretti, N.; Sun, S.; Katenka, N.; et al. Enhancement of radiation effect on cancer cells by gold-pHLIP. Proc. Natl. Acad. Sci. USA 2015, 112, 5372–5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Xu, R.; Sun, J.; Zhao, D.; Tong, J.; Sun, X. Nanoparticle surface and nanocore properties determine the effect on radiosensitivity of cancer cells upon ionizing radiation treatment. J. Nanosci. Nanotechnol. 2013, 13, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ma, J.; Sun, X.; Chen, Z.; Jiang, X.; Guo, Z.; Huang, L.; Li, Y.; Wang, M.; Wang, C.; et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res. 2009, 19, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Martinet, O.; Ermekova, V.; Qiao, J.Q.; Sauter, B.; Mandeli, J.; Chen, L.; Chen, S.H. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: Long- term remission of liver metastases in a mouse model. J. Natl. Cancer Inst. 2000, 92, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lin, G.H.; McPherson, A.J.; Watts, T.H. Immune regulation by 4-1BB and 4-1BBL: Complexities and challenges. Immunol. Rev. 2009, 229, 192–215. [Google Scholar] [CrossRef]
- Melero, I.; Shuford, W.W.; Newby, S.A.; Aruffo, A.; Ledbetter, J.A.; Hellstrom, K.E.; Mittler, R.S.; Chen, L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997, 3, 682–685. [Google Scholar] [CrossRef]
- Hirano, F.; Kaneko, K.; Tamura, H.; Dong, H.; Wang, S.; Ichikawa, M.; Rietz, C.; Flies, D.B.; Lau, J.S.; Zhu, G.; et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005, 65, 1089–1096. [Google Scholar]
- Kocak, E.; Lute, K.; Chang, X.; May, K.F., Jr.; Exten, K.R.; Zhang, H.; Abdessalam, S.F.; Lehman, A.M.; Jarjoura, D.; Zheng, P.; et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006, 66, 7276–7284. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.P.; Sauter, B.V.; Huang, T.G.; Meseck, M.; Woo, S.L.; Chen, S.H. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther. 2005, 12, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Houot, R.; Goldstein, M.J.; Kohrt, H.E.; Myklebust, J.H.; Alizadeh, A.A.; Lin, J.T.; Irish, J.M.; Torchia, J.A.; Kolstad, A.; Chen, L.; et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009, 114, 3431–3438. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Li, Q.; Shreiner, A.B.; Okuyama, R.; Jure-Kunkel, M.N.; Teitz-Tennenbaum, S.; Chang, A.E. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004, 64, 8411–8419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, L.B.; Howland, L.J.; Flynn, J.K.; West, A.C.; Devaud, C.; Duong, C.P.; Stewart, T.J.; Westwood, J.A.; Guo, Z.S.; Bartlett, D.L.; et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 2012, 72, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, R.A.; Flies, D.B.; Zhu, G.; Johnson, A.J.; Tamada, K.; Chapoval, A.I.; Strome, S.E.; Pease, L.R.; Chen, L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J. Clin. Investig. 2002, 109, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Uno, T.; Takeda, K.; Kojima, Y.; Yoshizawa, H.; Akiba, H.; Mittler, R.S.; Gejyo, F.; Okumura, K.; Yagita, H.; Smyth, M.J. Eradication of established tumors in mice by a combination antibody-based therapy. Nat. Med. 2006, 12, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Doucette, T.A.; Kong, L.Y.; Yang, Y.; Ferguson, S.D.; Yang, J.; Wei, J.; Qiao, W.; Fuller, G.N.; Bhat, K.P.; Aldape, K.; et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 2012, 14, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.H.; Boado, R.J.; Lu, J.Z.; Hui, E.K.; Pardridge, W.M. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab. Dispos. 2010, 38, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Pathmanandavel, K.; Starling, J.; Merheb, V.; Ramanathan, S.; Sinmaz, N.; Dale, R.C.; Brilot, F. Antibodies to surface dopamine-2 receptor and N-methyl-D-aspartate receptor in the first episode of acute psychosis in children. Biol. Psychiatry 2015, 77, 537–547. [Google Scholar] [CrossRef]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [Green Version]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukari, B.; Samarasinghe, R.M.; Noibanchong, J.; Shigdar, S.L. Non-Invasive Delivery of Therapeutics into the Brain: The Potential of Aptamers for Targeted Delivery. Biomedicines 2020, 8, 120. [Google Scholar] [CrossRef] [PubMed]

| Aptamer Name | Conjugate Name | Aptamer Target | Producing Method | Oligonucleotides | Modifications | References |
|---|---|---|---|---|---|---|
| Anti-tumoral activity Gint4.T CL4 AS1411 | Unconjugated | PDGFRβ EGFR Nucleolin | Cell-SELEX Cell-SELEX DNA oligonucleotides screening | RNA aptamer RNA aptamer DNA aptamer | Unmodified | [42] [42] [43] |
| Anti-tumoral molecules vehicles Gint4.T | AsiC | PDGFRβ | Cell-SELEX | RNA aptamer | STAT3 siRNA | [44] |
| Drugs vehicles AS1411 AS1411 AS1411 AS1411 GMT8 ATP aptamer | Ap-PTX-NP AS1411-PGG-PTX AsTNP RBT@MRN-SSTf/Apt ApNp 3CDIT/pOEI/DOX/ATP aptamer | Nucleolin A-172 cell line (target unknown) Intracellular ATP | DNA oligonucleotides screening Cell-SELEX Conventional SELEX | DNA aptamer DNA aptamer DNA aptamer | PEG-PLGA NPs loaded with PTX PEG-PGG Nps loaded with PTX TGN + PEG-PCL Nps loaded with DTX Tf + SS + MRN NPs loaded with RBT PEG-PCL Nps loaded with DTX 3CDIT + pOEI + DOX | [36] [40] [37] [41] [38] [39] |
| Enhancing therapy efficacy U2 NOX-A12 (Olaptesed pegol) AS1411 VEGF aptamer + 4-1BB | 188Re-U2 NOX-A12 (Olaptesed pegol) Cy5-AsNPs VEGF-4-1BB | EGFRvIII SDF-1 Nucleolin VEGF + CD8+ cells | Cell-SELEX Conventional SELEX DNA oligonucleotides screening Conventional SELEX | DNA aptamer RNA aptamer DNA aptamer RNA aptamer | 188Re PEG Cy5-AgNPS-PEG Unmodified | [102] [109] [46] [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. https://doi.org/10.3390/molecules25184267
Cesarini V, Scopa C, Silvestris DA, Scafidi A, Petrera V, Del Baldo G, Gallo A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules. 2020; 25(18):4267. https://doi.org/10.3390/molecules25184267
Chicago/Turabian StyleCesarini, Valeriana, Chiara Scopa, Domenico Alessandro Silvestris, Andrea Scafidi, Valerio Petrera, Giada Del Baldo, and Angela Gallo. 2020. "Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma" Molecules 25, no. 18: 4267. https://doi.org/10.3390/molecules25184267
APA StyleCesarini, V., Scopa, C., Silvestris, D. A., Scafidi, A., Petrera, V., Del Baldo, G., & Gallo, A. (2020). Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules, 25(18), 4267. https://doi.org/10.3390/molecules25184267






