Recent Advances in Catalytic Synthesis of Benzosultams
Abstract
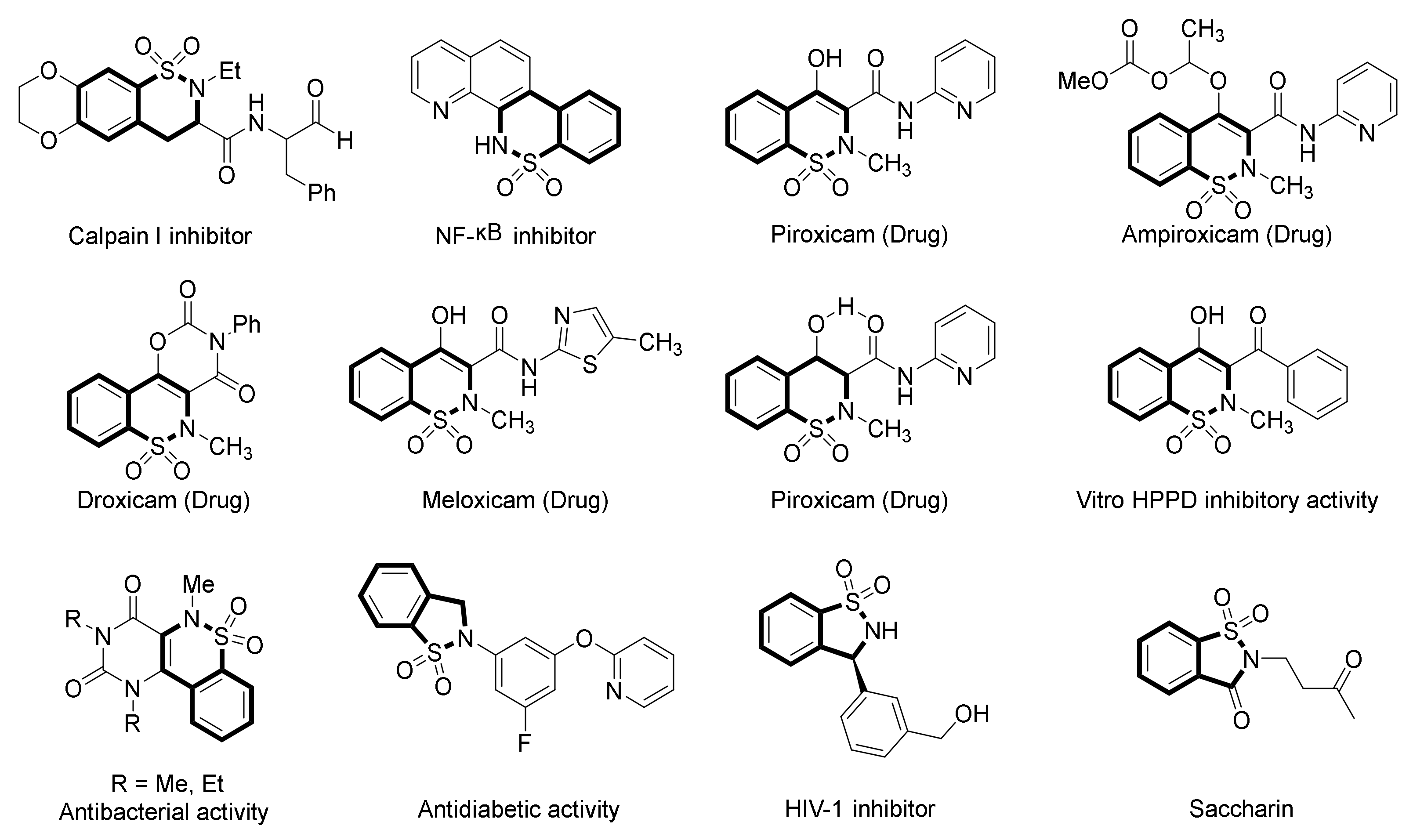
1. Introduction
2. Transition Metal-Catalyzed Benzosultam Synthesis
2.1. Synthesis of Benzosultams via C(sp2)–H Functionalization
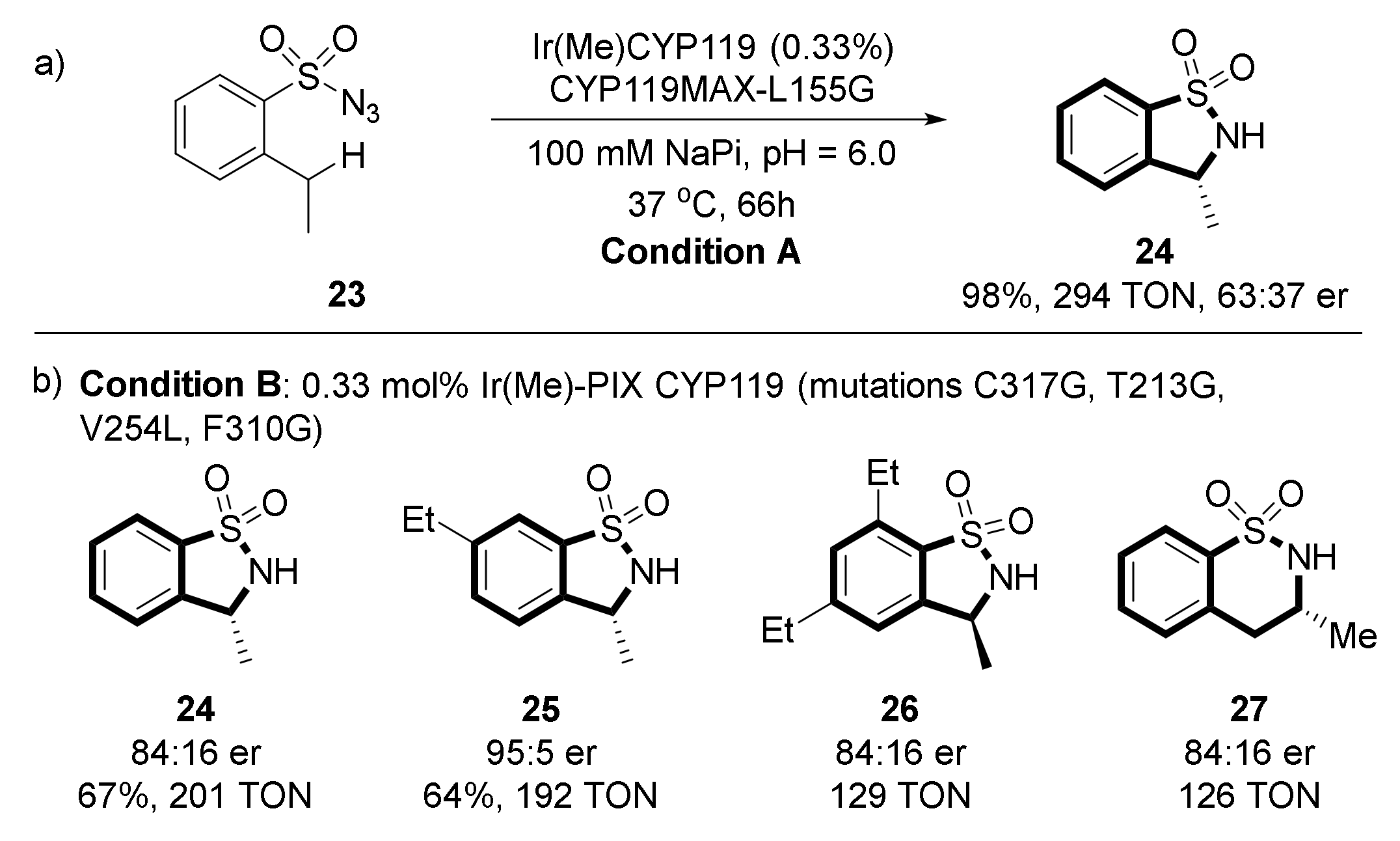
2.2. Synthesis of Benzosultams via C(sp3)–H Amination
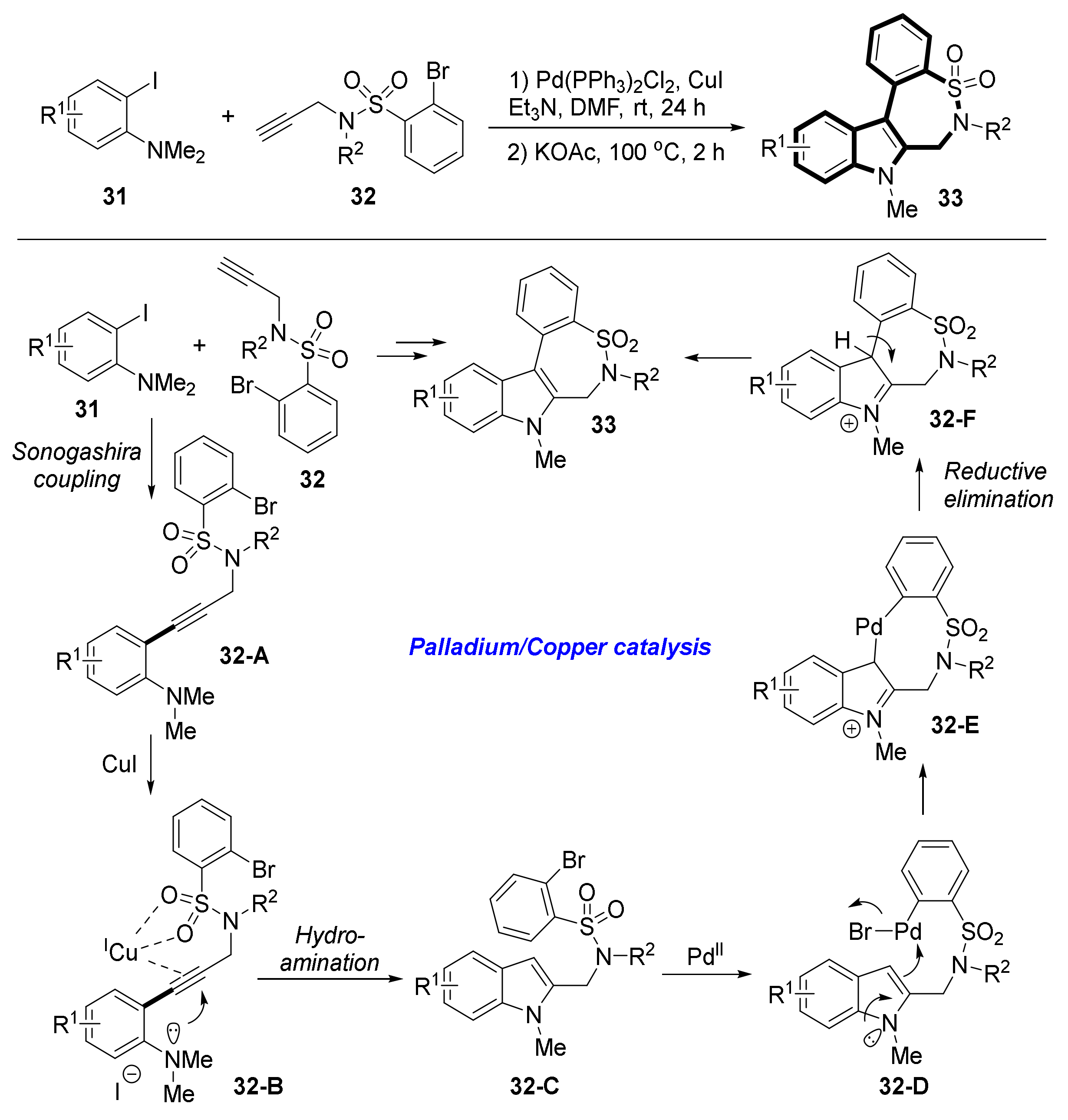
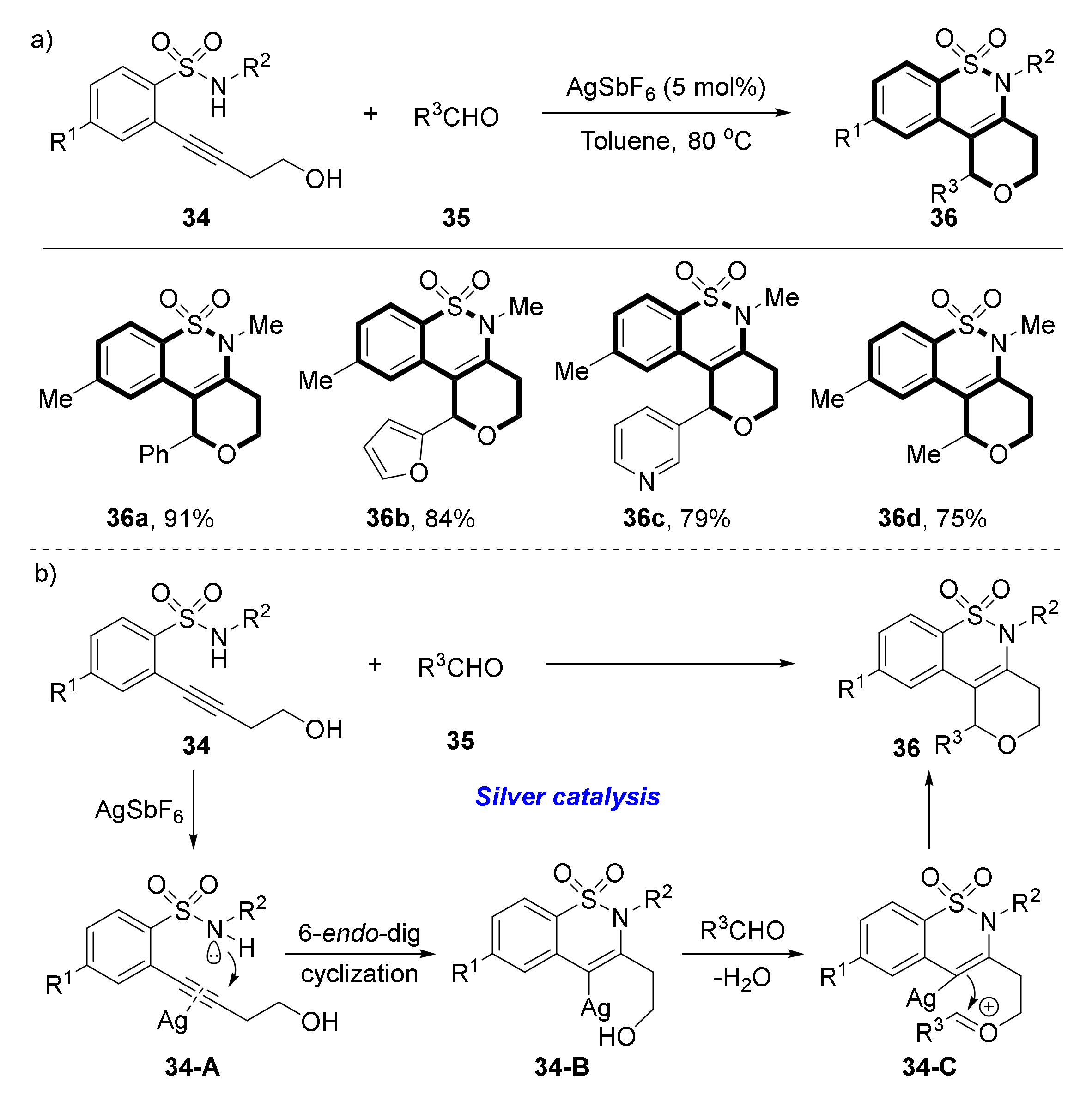
2.3. Synthesis of Benzosultams via Hydroamination of Alkyne
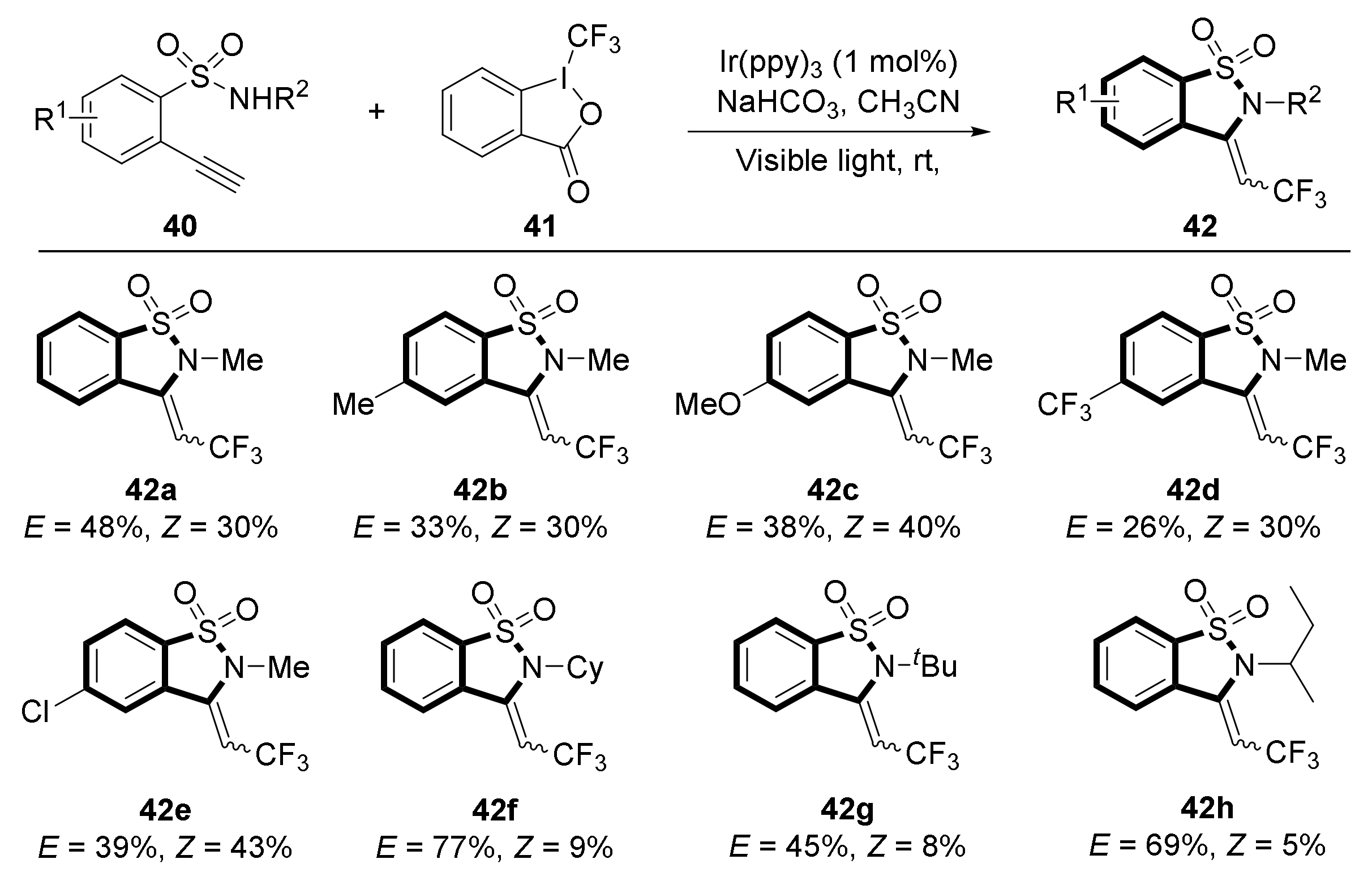
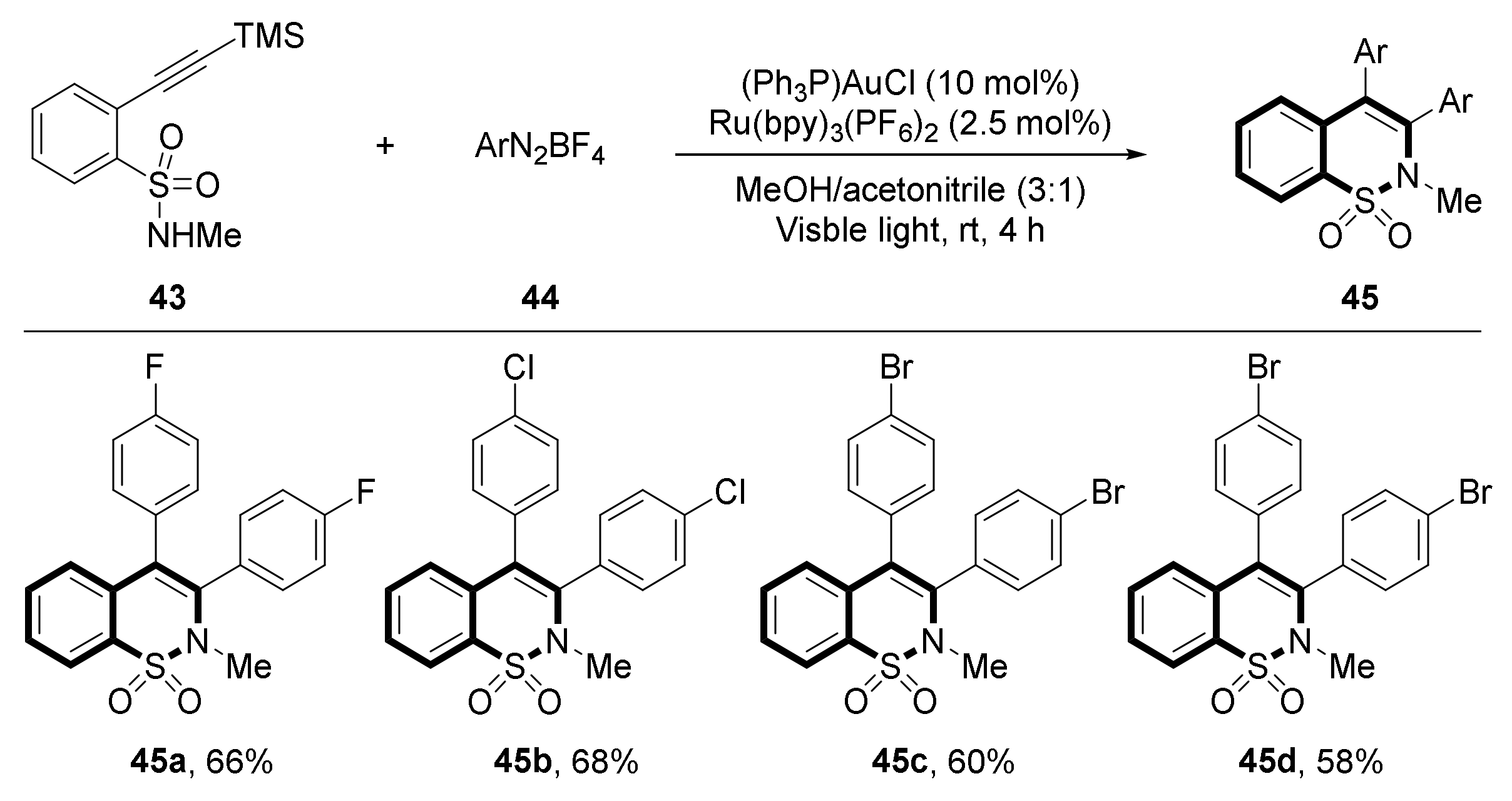
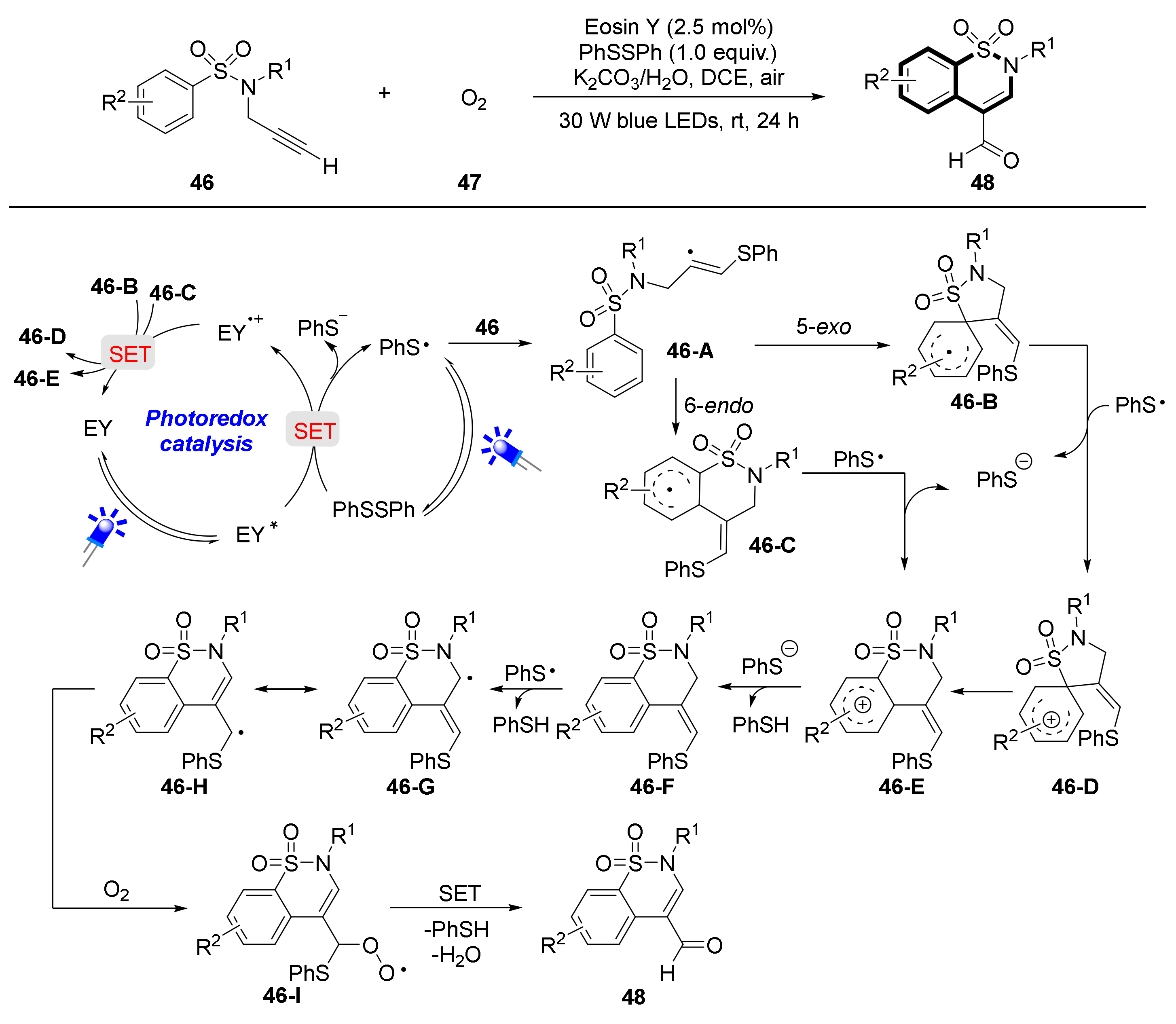
3. Visible Light Photocatalytic Synthesis of Benzosultams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Domagk, G. Ein Beitrag zur Chemotherapie der bakteriellen Infektionen. Dtsch. Med. Wochenschr. 1935, 61, 250–253. [Google Scholar] [CrossRef]
- Hanessian, S.; Sailes, H.; Therrien, E. Synthesis of Functionally Diverse Bicyclic Sulfonamides as Constrained Proline Analogues and Application to the Design of Potential Thrombin Inhibitors. Tetrahedron 2003, 59, 7047–7056. [Google Scholar] [CrossRef]
- Tanimukai, H.; Inui, M.; Harigushi, S.; Kaneko, Z. Antiepileptic Property of Inhibitors of Carbonic Anhydrase. J. Biochem. Pharm. 1965, 14, 961–970. [Google Scholar]
- Wells, G.J.; Tao, M.; Josef, K.A.; Bihovsky, R. 1,2-Benzothiazine 1,1-Dioxide P2-P3 Peptide Mimetic Aldehyde Calpain I Inhibitors. J. Med. Chem. 2001, 44, 3488–3503. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Saczewski, F.; Neamati, N. Bioorg. Synthesis and Anti-HIV-1 Activity of a Novel Series of 1,4,2-benzodithiazine-dioxides. Med. Chem. Lett. 2006, 16, 5298–5302. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gong, G.; Liu, Y.; Deng, S.; Rinderspacher, A.; Branden, L.; Landry, D.W. Convenient Preparation of N-8-quinolinyl Benzenesultams as Novel NF-κB Inhibitors. Tetrahedron Lett. 2008, 49, 2320–2323. [Google Scholar] [CrossRef]
- Ahmad, N.; Zia-ur-Rehman, M.; Siddiqui, H.L.; Ullah, M.F.; Parvez, M. Microwave Assisted Synthesis and Structure-Activity Relationship of 4-Hydroxy-N’-[1-phenylethylidene]-2H/2-methyl-1,2-benzo- thiazine-3-carbohydrazide 1,1-dioxides as Anti-Microbial Agents. Eur. J. Org. Chem. 2011, 46, 2368–2377. [Google Scholar]
- Ivanova, D.; Deneva, V.; Nedeltcheva, D.; Kamounah, F.S.; Gergov, G.; Hansen, P.E.; Kawauchi, S.; Antonov, L. Tautomeric Transformations of Piroxicam in Solution: A Combined Experimental and Theoretical Study. RSC Adv. 2015, 5, 31852–31860. [Google Scholar] [CrossRef]
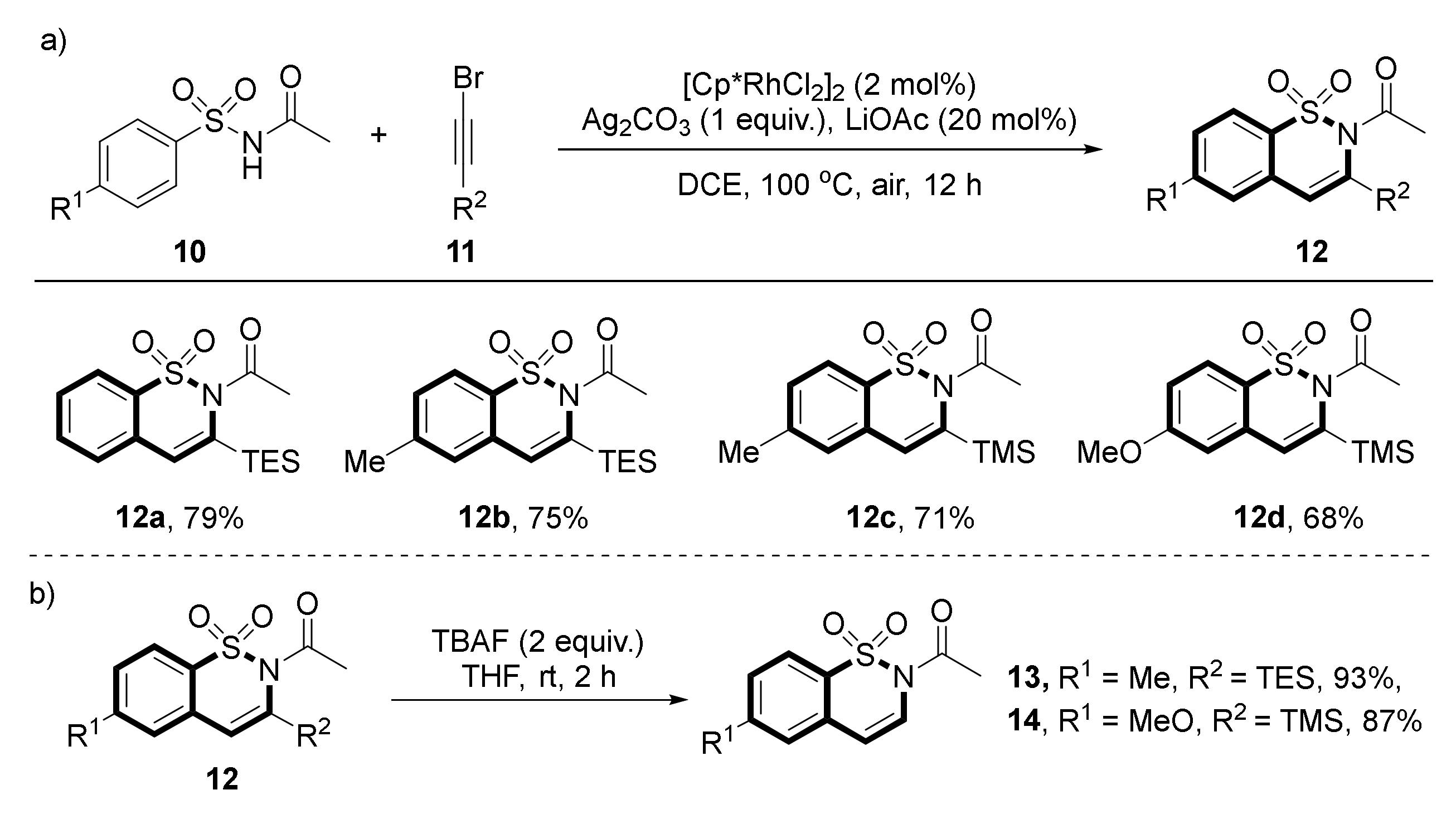
- Li, X.; Dong, Y.; Qu, F.; Liu, G. Synthesis of Benzofused Five-Ring Sultams via Rh-Catalyzed C–H Olefination Directed by an N-Ac-Substituted Sulfonamide Group. J. Org. Chem. 2015, 80, 790–798. [Google Scholar] [CrossRef]
- Azevedo, C.M.; Watterson, K.R.; Wargent, E.T.; Hansen, S.V.; Hudson, B.D.; Kepczynska, M.A.; Dunlop, J.; Shimpukade, B.; Christiansen, E.; Milligan, G.; et al. Non-Acidic Free Fatty Acid Receptor 4 Agonists with Antidiabetic Activity. J. Med. Chem. 2016, 59, 8868–8878. [Google Scholar] [CrossRef]
- Lei, K.; Hua, X.W.; Tao, Y.Y.; Liu, Y.; Liu, N.; Ma, Y.; Li, Y.H.; Xu, X.H.; Kong, C.H. Discovery of (2-Benzoylethen-1-ol)-Containing 1,2-Benzothiazine Derivatives as Novel 4-Hydroxyphenylpyruvate Dioxygenase (HPPD) Inhibiting-Based Herbicide Lead Compounds. Bioorg. Med. Chem. 2016, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mukherjee, S.; Malakar, S.; Debnath, S.; Roy, P.; Sinha Babu, S.P. Studying the Biological Activities and Molecular Docking of Some Novel Benzosultams and Benzosultones. Curr. Bioact. Comp. 2017, 13, 347–355. [Google Scholar] [CrossRef]
- Ahn, K.H.; Kim, S.K.; Ham, C. The Evaluation of Chiral Benzosultams as Auxiliaries in Asymmetric Azidation Reaction. Tetrahedron Lett. 1998, 39, 6321–6322. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Chen, X.-Q.; Pang, H.-L.; Tian, T.; Li, B.; Song, X.-Q.; Meng, W.; Yang, Z.; Zhao, Y.-D.; Liu, Y.-Y. Diastereoselective Synthesis of Highly Functionalized Polycyclic Benzosultams via Tandem Cyclisations of Cyclic N-Sulfonylimines with in Situ Generated Huisgen 1,4-Dipoles. RSC Adv. 2016, 6, 61732–61739. [Google Scholar] [CrossRef]
- Laha, J.K.; Jethava, K.P. Access to Imidazolidine-Fused Sulfamidates and Sulfamides Bearing a Quaternary Center via 1,3-Dipolar Cycloaddition of Nonstabilized Azomethine Ylides. J. Org. Chem. 2017, 82, 3597–3604. [Google Scholar] [CrossRef]
- Nishimura, T.; Noishiki, A.; Tsui, G.C.; Hayashi, T. Asymmetric Synthesis of (Triaryl)methylamines by Rhodium-Catalyzed Addition of Arylboroxines to Cyclic N-Sulfonyl Ketimines. J. Am. Chem. Soc. 2012, 134, 5056–5059. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Xu, M.H. Simple Branched Sulfur-Olefins as Chiral Ligands for Rh-Catalyzed Asymmetric Arylation of Cyclic Ketimines: Highly Enantioselective Construction of Tetrasubstituted Carbon Stereocenters. J. Am. Chem. Soc. 2013, 135, 971–974. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Xu, M.H. Rhodium-Catalyzed Asymmetric Arylation of Cyclic N-Sulfonyl Aryl Alkyl Ketimines: Efficient Access to Highly Enantioenriched Alpha-Tertiary Amines. Org. Lett. 2015, 17, 528–531. [Google Scholar] [CrossRef]
- Qiao, B.; Huang, Y.J.; Nie, J.; Ma, J.A. Highly Regio-, Diastereo-, and Enantioselective Mannich Reaction of Allylic Ketones and Cyclic Ketimines: Access to Chiral Benzosultam. Org. Lett. 2015, 17, 4608–4611. [Google Scholar] [CrossRef]
- Yu, J.-S.; Zhou, J. Organocatalytic Enantioselective Mukaiyama–Mannich Reaction of Fluorinated Enol Silyl Ethers and Cyclic N-Sulfonyl Ketimines. Org. Chem. Front. 2016, 3, 298–303. [Google Scholar] [CrossRef]
- Hou, L.; Kang, T.; Yang, L.; Cao, W.; Feng, X. Enantioselective Imino-Ene Reaction of N-Sulfonyl Ketimines with Silyl Enol Ethers: Access to Chiral Benzosultams. Org. Lett. 2020, 22, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Xiong, M.; Jiang, J.; Wang, J. Cp*CoIII-Catalyzed C–H Alkenylation/Annulation to Afford Spiro Indenyl Benzosultam. J. Org. Chem. 2016, 81, 6093–6099. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.N.; Rao, M.V.K.; Sridhar, B.; Reddy, B.V.S. PdII-Catalyzed Spiroannulation of Cyclic N-Sulfonyl Ketimines with Aryl Iodides through C–H Bond Activation. Eur. J. Org. Chem. 2017, 2017, 4085–4090. [Google Scholar] [CrossRef]
- Nagarjuna, R.K.; Subhadra, U.; Sridhar, B.; Subba, R.B.V. Ru(II)-Catalyzed Spirocyclization of Aryl N-Sulfonyl Ketimines with Aryl Isocyanates Through an Aromatic C–H bond activation. Org. Biomol. Chem. 2018, 16, 2522–2526. [Google Scholar]
- Li, Q.; Yuan, X.; Li, B.; Wang, B. The Regioselective Annulation of Alkylidenecyclopropanes by Rh(III)-Catalyzed C–H/C–C Activation to Access Spirocyclic Benzosultams. Chem. Commun. 2020, 56, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Seppelt, M. Catalytic Enantioselective Synthesis of 3-Substituted Benzosultams via Corey-Bakshi-Shibata Reduction of Cyclic N-Sulfonylimines. Synlett 2011, 2011, 402–404. [Google Scholar] [CrossRef]
- Pan, J.; Wu, J.H.; Zhang, H.; Ren, X.; Tan, J.P.; Zhu, L.; Zhang, H.S.; Jiang, C.; Wang, T. Highly Enantioselective Synthesis of Fused Tri- and Tetrasubstituted Aziridines: Aza-Darzens Reaction of Cyclic Imines with α-Halogenated Ketones Catalyzed by Bifunctional Phosphonium Salt. Angew. Chem. Int. Ed. 2019, 58, 7425–7430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Q.; Qiu, G.; Wu, J. Synthesis of Benzosultams via an Intramolecular Sp2 C–H bond Amination Reaction of o-Arylbenzenesulfonamides under Metal-Free Conditions. Org. Biomol. Chem. 2014, 12, 149–155. [Google Scholar] [CrossRef]
- Ghandi, M.; Kia, M.A.; Sadeghzadegh, M.; Bozcheloei, A.H.; Kubicki, M. Diastereoselective Synthesis of Novel Pyrrolidine or Pyrrolizine-Fused Benzo-δ-Sultams via 1,3-Dipolar Cycloadditions. J. Heterocycl. Chem. 2015, 52, 1646–1653. [Google Scholar] [CrossRef]
- Grosheva, D.S.; Rassadin, V.A.; Sokolov, V.V. A Route to Benzo-Annelated δ-Sultams through Michael Cyclization. Eur. J. Org. Chem. 2015, 2015, 1355–1363. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Makosza, M. Synthesis of Benzosultams via Intramolecular Vicarious Nucleophilic Substitution of Hydrogen. Synthesis 1992, 1992, 571–576. [Google Scholar] [CrossRef]
- Wu, C. Novel Synthesis of N-Methyl-1,2-benzosultams, an Unsuspected Demethylative Intramolecular Cyclization Reaction. J. Org. Chem. 1998, 63, 2348–2350. [Google Scholar] [CrossRef]
- Ahn, K.H.; Baek, H.H.; Lee, S.J.; Cho, C.W. Synthesis of Chiral Benzosultams: 3-Functionalized 1,2-Benzisothiazoline 1,1-dioxides. J. Org. Chem. 2000, 65, 7690–7696. [Google Scholar] [CrossRef]
- Philippe, D.; Dodd, R.H. Intramolecular Bromine-Catalyzed Aziridination: A New Direct Access to Cyclic Sulfonamides. Tetrahedron Lett. 2001, 42, 1037–1040. [Google Scholar]
- Liu, Z.; Shibata, N.; Takeuchi, Y. Facile Synthesis of Disubstituted and Spiro Five-Membered Benzosultams Mediated by TMSCl–NaI–MeCN Reagent. J. Chem. SocPerkin Trans. 2002, 1, 302–303. [Google Scholar] [CrossRef]
- Fang, F.; Wang, R.; Liu, Z.-P.; Ji, A.-G. Novel Method for the Synthesis of 3-Monosubstituted Six-Membered Benzosultams. Heterocycles 2007, 71, 2377–2388. [Google Scholar]
- Sun, H.M.; Liu, Z.P.; Tang, L.Q. Synthesis of Novel Chiral Fluorinating Agents, (+)- and (−)-N-Fluoro-3-Methyl-3-(4-Methylphenyl)-2H-Benzo[e][1,2]Thiazine-1,1,4-Triones. Chin. Chem. Lett. 2008, 19, 907–910. [Google Scholar] [CrossRef]
- Liu, Z.P.; Dong, Y.H.; Ni, Q.W.; Ma, S.T. Iodotrimethylsilane and Catalytic Iodine Promoted Cyclization for the Facile Synthesis of 3-Monoarylated Five-Membered Benzosultams. Heterocycles 2010, 81, 637–648. [Google Scholar] [CrossRef]
- Lorion, M.; Agouridas, V.; Couture, A.; Deniau, E.; Grandclaudon, P. Cyclic Sulfamidates as Vehicles for the Synthesis of Poly- and Diversely Substituted Benzosultams via Unusual S(O)2-O Bond Cleavage. Org. Lett. 2010, 12, 1356–1359. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S. Recent Developments in the Synthesis of Fused Sultams. Chem. Rev. 2011, 111, 7749–7773. [Google Scholar] [CrossRef]
- Debnath, S.; Mondal, S. Sultams: Recent Syntheses and Applications. Eur. J. Org. Chem. 2018, 2018, 933–956. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Vashchenko, B.V.; Blahun, O.P.; Zhersh, S. Saturated Bicyclic Sultams. Eur. J. Org. Chem. 2020. [Google Scholar] [CrossRef]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C–C Bond Formation via Heteroatom-Directed C–H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, W.J.; Jun, C.H. Metal-Organic Cooperative Catalysis in C–H and C–C Bond Activation. Chem. Rev. 2017, 117, 8977–9015. [Google Scholar] [CrossRef]
- Labinger, J.A. Platinum-Catalyzed C–H Functionalization. Chem. Rev. 2017, 117, 8483–8496. [Google Scholar] [CrossRef]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2018, 119, 2192–2452. [Google Scholar] [CrossRef]
- Yamagishi, M.; Nishigai, K.; Ishii, A.; Hata, T.; Urabe, H. Facile Preparation of Indoles and 1,2-Benzothiazine 1,1-dioxides: Nucleophilic Addition of Sulfonamides to Bromoacetylenes and Subsequent Palladium-Catalyzed Cyclization. Angew. Chem. Int. Ed. 2012, 51, 6471–6474. [Google Scholar] [CrossRef]
- Mondal, S.; Debnath, S.; Pal, S.; Das, A. Synthesis of Uracil-, Coumarin- and Quinolone-Fused Benzosultams and Benzosultones. Synthesis 2015, 47, 3423–3433. [Google Scholar] [CrossRef]
- Laha, J.K.; Jethava, K.P.; Dayal, N. Palladium-Catalyzed Intramolecular Oxidative Coupling Involving Double C(sp2)-H Bonds for the Synthesis of Annulated Biaryl Sultams. J. Org. Chem. 2014, 79, 8010–8019. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Dayal, N.; Jethava, K.P.; Prajapati, D.V. Access to Biaryl Sulfonamides by Palladium-Catalyzed Intramolecular Oxidative Coupling and Subsequent Nucleophilic Ring Opening of Heterobiaryl Sultams with Amines. Org. Lett. 2015, 17, 1296–1299. [Google Scholar] [CrossRef]
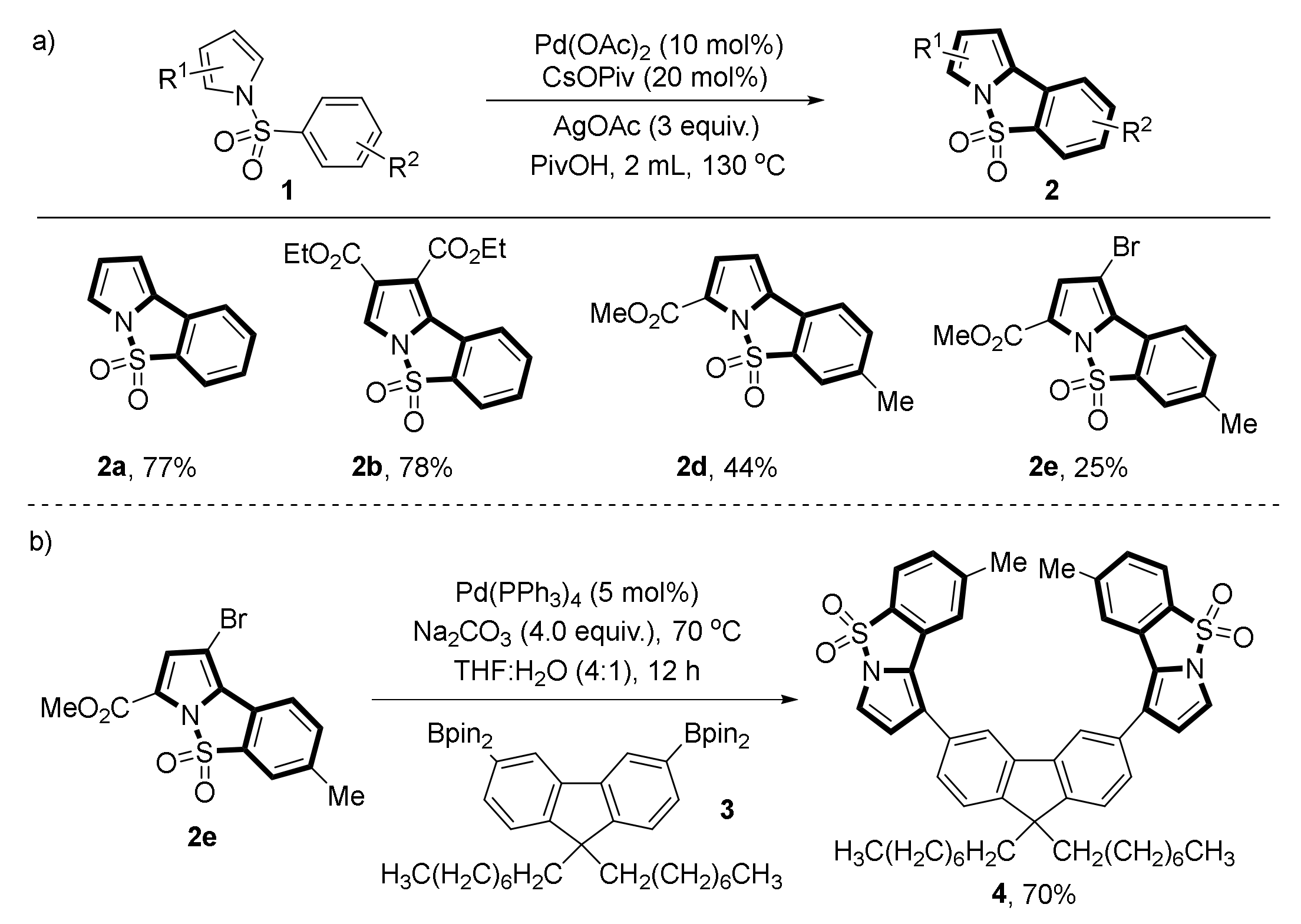
- Laha, J.K.; Bhimpuria, R.A.; Kumar, A.M. Post-Synthetic Diversification of Pyrrole-Fused Benzosultams via Trans-Sulfonylations and Reactions on the Periphery of Pyrrole. Org. Chem. Front. 2017, 4, 2170–2174. [Google Scholar] [CrossRef]
- Laha, J.K.; Bhimpuria, R.A.; Hunjan, M.K. Intramolecular Oxidative Arylations in 7-Azaindoles and Pyrroles: Revamping the Synthesis of Fused N-Heterocycle Tethered Fluorenes. Eur. Chem. J. 2017, 23, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
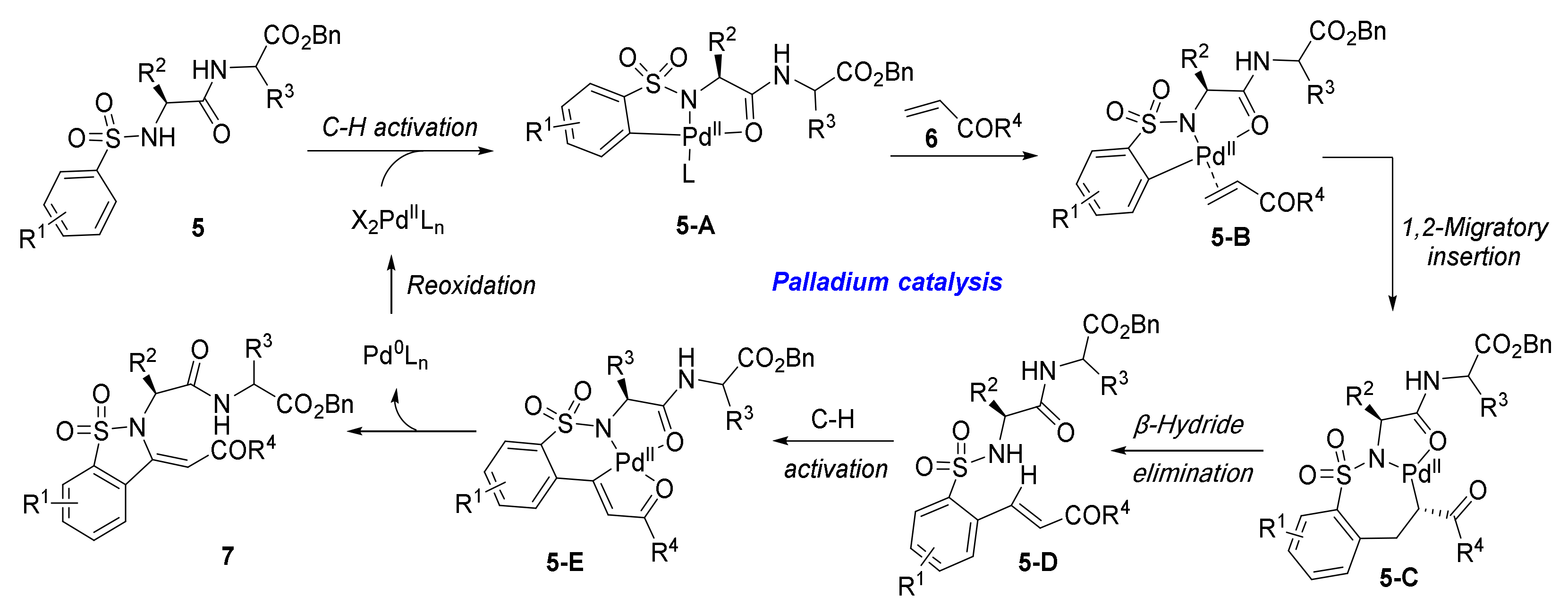
- Tang, J.; Chen, H.; He, Y.; Sheng, W.; Bai, Q.; Wang, H. Peptide-Guided Functionalization and Macrocyclization of Bioactive Peptidosulfonamides by Pd(II)-Catalyzed Late-Stage C–H Activation. Nat. Commun. 2018, 9, 3383. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Rechenmacher, F.; Kessler, H. Cilengitide: The First Anti-angiogenic Small Molecule Drug Candidate Design, Synthesis and Clinical Evaluation. Anticancer Agents Med. Chem. 2010, 10, 753–768. [Google Scholar] [CrossRef]
- Pham, M.V.; Ye, B.; Cramer, N. Access to Sultams by Rhodium(III)-Catalyzed Directed C–H Activation. Angew. Chem. Int. Ed. 2012, 51, 10610–10614. [Google Scholar] [CrossRef]
- Xie, W.; Yang, J.; Wang, B.; Li, B. Regioselective Ortho Olefination of Aryl Sulfonamide via Rhodium-Catalyzed Direct C–H Bond Activation. J. Org. Chem. 2014, 79, 8278–8287. [Google Scholar] [CrossRef]
- Ran, Y.; Yang, Y.; You, H.; You, J. RhCl3-Catalyzed Oxidative C–H/C–H Cross-Coupling of (Hetero)aromatic Sulfonamides with (Hetero)arenes. ACS Catal. 2018, 8, 1796–1801. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Pu, S.; Chen, J. Rhodium-Catalyzed Direct C–H Bond Alkynylation of Aryl Sulfonamides with Bromoalkynes. Org. Biomol. Chem. 2019, 17, 2948–2953. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, H.; Sun, M.; Zhou, Z.; Yi, W. Experimental and Computational Studies on Cp*(Cy)Rh(III)/KOPiv-Catalyzed Intramolecular Dehydrogenative Cross-Couplings for Building Eight-Membered Sultam/Lactam Frameworks. Org. Lett. 2020, 22, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, D.; Sundararaju, B. Cobalt Catalyzed C–H and N-H Bond Annulation of Sulfonamide with Terminal and Internal Alkynes. Org. Lett. 2015, 17, 6118–6121. [Google Scholar] [CrossRef] [PubMed]
- Thrimurtulu, N.; Nallagonda, R.; Volla, C.M. Cobalt-Catalyzed Aryl C–H Activation and Highly Regioselective Intermolecular Annulation of Sulfonamides with Allenes. Chem. Commun. 2017, 53, 1872–1875. [Google Scholar] [CrossRef]
- Chrostowska, A.; Gracian, F.; Sotiropoulos, J.-M.; Pfister-Guillouzo, G.; Wojciechowski, K. UV Photoelectron Spectroscopy Studies of the Products of Thermal Extrusion of Sulfur Dioxide from Benzosultams. Eur. J. Org. Chem. 2000, 2000, 313–318. [Google Scholar] [CrossRef]
- Bunnett, J.F.; Kato, T.; Flynn, R.R.; Skorcz, J.A. Studies of Ring Closure via Aryne Intermediates. J. Org. Chem. 1963, 28, 1–6. [Google Scholar] [CrossRef]
- Wojciechowski, K. Synthesis of Nitrobenzophenones from Nitro-α-Sulfonyldiphenylmethane Derivatives. Synth. Commun. 1997, 27, 135–144. [Google Scholar] [CrossRef]
- Moutrille, C.; Zard, S.Z. A New Approach for the ortho-Substitution of Anilines and for the Synthesis of Indolines. Tetrahedron Lett. 2004, 45, 4631–4634. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J. Synthesis of Benzo-Gamma-Sultams via the Rh-Catalyzed Aromatic C–H Functionalization of Diazosulfonamides. Chem. Commun. 2014, 50, 3616–3618. [Google Scholar] [CrossRef]
- Huang, P.; Yang, Z.; Xu, J. Specific Intramolecular Aromatic C–H Insertion of Diazosulfonamides. Tetrahedron 2017, 73, 3255–3265. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef]
- Ruppel, J.V.; Kamble, R.M.; Zhang, X.P. Cobalt-Catalyzed Intramolecular C−H Amination with Arylsulfonyl Azides. Org. Lett. 2007, 9, 4889–4892. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, H.; Wojtas, L.; Zhang, X.P. Selective Intramolecular C–H Amination through the Metalloradical Activation of Azides: Synthesis of 1,3-Diamines under Neutral and Nonoxidative Conditions. Angew. Chem. Int. Ed. 2010, 49, 10192–10196. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lang, K.; Li, C.; Gill, J.B.; Kim, I.; Lu, H.; Fields, K.B.; Marshall, M.; Cheng, Q.; Cui, X.; et al. Enantioselective Radical Construction of 5-Membered Cyclic Sulfonamides by Metalloradical C–H Amination. J. Am. Chem. Soc. 2019, 141, 18160–18169. [Google Scholar] [CrossRef] [PubMed]
- Podust, L.M.; Sherman, D.H. Diversity of P450 Enzymes in the Biosynthesis of Natural Products. Nat. Prod. Rep. 2012, 29, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.J.; Bell, S.G.; Wong, L.L. P450BM3 (CYP102A1): Connecting the Dots. Chem. Soc. Rev. 2012, 41, 1218–1260. [Google Scholar] [CrossRef]
- Bordeaux, M.; Galarneau, A.; Fajula, F.; Drone, J. A regioselective biocatalyst for alkane activation under mild conditions. Angew. Chem. Int. Ed. 2011, 50, 2075–2079. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Coelho, P.S.; Farwell, C.C.; Wang, Z.J.; Lewis, J.C.; Brown, T.R.; Arnold, F.H. Enantioselective Intramolecular C–H Amination Catalyzed by Engineered Cytochrome P450 Enzymes in Vitro and in Vivo. Angew. Chem. Int. Ed. 2013, 52, 9309. [Google Scholar] [CrossRef]
- Hyster, T.K.; Farwell, C.C.; Buller, A.R.; McIntosh, J.A.; Arnold, F.H. Enzyme-Controlled Nitrogen-Atom Transfer Enables Regiodivergent C–H Amination. J. Am. Chem. Soc. 2014, 136, 15505–15508. [Google Scholar] [CrossRef]
- Bordeaux, M.; Singh, R.; Fasan, R. Intramolecular C(sp3)-H Amination of Arylsulfonyl Azides with Engineered and Artificial Myoglobin-Based Catalysts. Bioorg. Med. Chem. 2014, 22, 5697–5704. [Google Scholar] [CrossRef]
- Singh, R.; Bordeaux, M.; Fasan, R. P450-Catalyzed Intramolecular sp3 C–H Amination with Arylsulfonyl Azide Substrates. ACS Catal. 2014, 4, 546–552. [Google Scholar] [CrossRef]
- Hyster, T.K.; Ward, T.R. Genetic Optimization of Metalloenzymes: Enhancing Enzymes for Non-Natural Reactions. Angew. Chem. Int. Ed. 2016, 55, 7344–7357. [Google Scholar] [CrossRef] [PubMed]
- Dydio, P.; Key, H.M.; Hayashi, H.; Clark, D.S.; Hartwig, J.F. Chemoselective, Enzymatic C–H Bond Amination Catalyzed by a Cytochrome P450 Containing an Ir(Me)-PIX Cofactor. J. Am. Chem. Soc. 2017, 139, 1750–1753. [Google Scholar] [CrossRef]
- Scamp, R.J.; Scheffer, B.; Schomaker, J.M. Regioselective Differentiation of Vicinal Methylene C–H bonds Enabled by Silver-Catalysed Nitrene Transfer. Chem. Commun. 2019, 55, 7362–7365. [Google Scholar] [CrossRef] [PubMed]
- Barange, D.K.; Nishad, T.C.; Swamy, N.K.; Bandameedi, V.; Kumar, D.; Sreekanth, B.R.; Vyas, K.; Pal, M. A Remarkable Accelerating Effect of Ag-Salt on Intramolecular Cyclization of o-(1-Alkynyl)Benzenesulfonamides. J. Org. Chem. 2007, 72, 8547–8550. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, D.; Murthy, P.V.N.S.; Prasad, K.R.S.; Kandale, A.; Deora, G.S.; Basaveswara Rao, M.V.; Pal, M. AgNO3 Mediated C–N Bond Forming Reaction: Synthesis of 3-Substituted Benzothiazines as Potential COX Inhibitors. Tetrahedron Lett. 2012, 53, 6577–6583. [Google Scholar] [CrossRef]
- Debnath, S.; Mondal, S. One-pot Sonogashira Coupling-Cyclization toward Regioselective Synthesis of Benzosultams. J. Org. Chem. 2015, 80, 3940–3948. [Google Scholar] [CrossRef]
- Ha, T.M.; Yao, B.; Wang, Q.; Zhu, J. Sulfonamide and Tertiary Amine as Nucleophiles in Pd(II)-Catalyzed Diamination of Alkynes: Synthesis of Tetracyclic Indolobenzothiazine S,S-Dioxides. Org. Lett. 2015, 17, 5256–5259. [Google Scholar] [CrossRef]
- Debnath, S.; Mondal, S. One-Pot Sonogashira Coupling, Hydroamination of Alkyne and Intramolecular C H Arylation Reactions toward the Synthesis of Indole-fused Benzosultams. Tetrahedron Lett. 2018, 59, 2260–2263. [Google Scholar] [CrossRef]
- Maheshwar Rao, B.; Yadav, J.S.; Sridhar, B.; Subba Reddy, B.V. Silver(I)-Catalyzed Sequential Hydroamination and Prins Type Cyclization for the Synthesis of Fused Benzo-δ-sultams. Org. Biomol. Chem. 2018, 16, 5163–5166. [Google Scholar] [CrossRef]
- Pertschi, R.; Weibel, J.M.; Pale, P.; Blanc, A. Benzosultam Synthesis by Gold(I)-Catalyzed Ammonium Formation/Nucleophilic Substitution. Org. Lett. 2019, 21, 5616–5620. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, K. Photoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2009, 48, 9785–9789. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible Light Photocatalysis as a Greener Approach to Photochemical Synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.R.J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar]
- Xuan, J.; Xiao, W.J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Albini, A. Photoorganocatalysis. What for? Chem. Soc. Rev. 2013, 42, 97–113. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Chen, J.R.; Xiao, W.J. Homogeneous Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2013, 52, 11701–11703. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Sahoo, B.; Li, J.L.; Glorius, F. Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Eur. Chem. J. 2014, 20, 3874–3886. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 985. [Google Scholar] [CrossRef]
- Chen, J.R.; Hu, X.Q.; Lu, L.Q.; Xiao, W.J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A. Photoinduced Multicomponent Reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; Konig, B. Visible-Light Photocatalysis: Does It Make Difference in Organic Synthesis ? Anfgew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Strieth-Kalthoff, F.; James, M.J.; Teders, M.; Pitzer, L.; Glorius, F. Energy Transfer Catalysis Mediated by Visible Light: Principles, Applications, Directions. Chem. Soc. Rev. 2018, 47, 7190–7202. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Zou, Y.Q.; Lu, L.Q.; Xiao, W.J. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem. Int. Ed. 2019, 58, 1586–1604. [Google Scholar] [CrossRef]
- Xiang, Y.; Kuang, Y.; Wu, J. Generation of Benzosultams via Trifluoromethylation of 2-Ethynylbenzenesulfonamide under Visible Light. Org. Chem. Front. 2016, 3, 901–905. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Busto, E.; Herrera, F.; Lázaro-Milla, C.; Luna, A. Photopromoted Entry to Benzothiophenes, Benzoselenophenes, 3H-Indoles, Isocoumarins, Benzosultams, and (Thio)flavones by Gold-Catalyzed Arylative Heterocyclization of Alkynes. Adv. Synth. Catal. 2017, 359, 2640–2652. [Google Scholar] [CrossRef]
- Chen, F.; Yang, C.; Hu, X.; Zhang, X.; Xie, H.; Jiang, H.; Jiang, F.; Zeng, W. Photocatalyzed Formal Carbooxygenation of Terminal Alkynes. Org. Chem. Front. 2020, 7, 1600–1605. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Hu, X.Q.; Yang, M.N.; Chen, J.R.; Xiao, W.J. A Visible-Light Photocatalytic N-Radical Cascade of Hydrazones for the Synthesis of Dihydropyrazole-Fused Benzosultams. Chem. Commun. 2016, 52, 12749–12752. [Google Scholar] [CrossRef] [PubMed]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.-Q.; Hu, X.-Q. Recent Advances in Catalytic Synthesis of Benzosultams. Molecules 2020, 25, 4367. https://doi.org/10.3390/molecules25194367
Zhao Q-Q, Hu X-Q. Recent Advances in Catalytic Synthesis of Benzosultams. Molecules. 2020; 25(19):4367. https://doi.org/10.3390/molecules25194367
Chicago/Turabian StyleZhao, Quan-Qing, and Xiao-Qiang Hu. 2020. "Recent Advances in Catalytic Synthesis of Benzosultams" Molecules 25, no. 19: 4367. https://doi.org/10.3390/molecules25194367
APA StyleZhao, Q.-Q., & Hu, X.-Q. (2020). Recent Advances in Catalytic Synthesis of Benzosultams. Molecules, 25(19), 4367. https://doi.org/10.3390/molecules25194367






