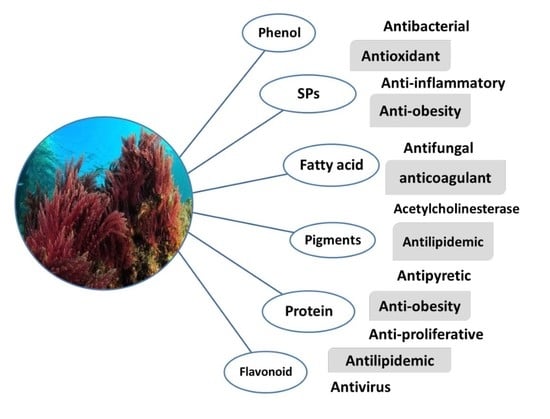
Therapeutic Uses of Red Macroalgae
Abstract
:1. Introduction
2. Antimicrobial Activity
3. Antiviral Activity
4. Antioxidant Activity
5. Anticancer and Antiproliferative Activities
6. Anti-Inflammatory Activity
7. Analgesic and Antipyretic Activities
8. Anticoagulant and Antithrombotic Activities
9. Antidiabetic Activity
10. Anti-Obesity Activity
11. Antihypertensive Activity
12. Acetylcholinesterase Inhibitory ‘’Alzheimer’s Disease”
13. Macroalgae for Skincare
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Ismail, M.M.; Gheda, S.F.; Pereira, L. Variation in bioactive compounds in some seaweed from Abo Qir bay, Alexandria, Egypt. Rend Lincei-Sci. Fis. 2016, 27, 269–279. [Google Scholar]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Carvalho, A.P.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material, total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [PubMed] [Green Version]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, antioxidant, and anti-Tumor activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2020. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El Sabbagh, S.; Abd El Samea, B. Control of some microbial skin diseases by some marine algal extract. J. Agric. Chem. Biotechnol. 2016, 7, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and antibacterial activity of red seaweed; Kappaphycus alvarezii against pathogenic bacteria. Global J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar]
- Ratnawati, R.; Prasetyaningrum, A.; Wardhani, D.H. Kinetics and thermodynamics of ultrasound-assisted depolymerization of ҡ-carrageenan. Bull. Chem. React. Eng. Catal. 2016, 11, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Chen, Y.; Yao, F.; Chen, W.; Shi, G. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar. Drugs 2012, 10, 2634–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Ioniols I and II, tetracyclic diterpenes with antibacterial activity, from Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Cueto, M.; Díaz-Marrero, A.R.; Darias, J.; San Martín, A. Oxachamigrenes, New Halogenated Sesquiterpenes from Laurencia obtusa. J. Nat. Prod. 2002, 65, 946–948. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2003, 20, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, K.; Ayyakkannu, K. Seasonal variation of antibacterial and antifungal activities of the extract of marine algae from southern coasts of India. Bot. Mar. 1997, 40, 507–515. [Google Scholar] [CrossRef]
- Saleh, B.; Al-Mariri, A. Antifungal activity of crude seaweed extracts collected from Lattakia Coast, Syria. J. Fish. Aquat. Sci. 2018, 13, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, G.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and antimicrobial potential of bromoditerpenes isolated from the Red Alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Pandian, P.; Selvamuthukumar, S.; Manavalan, R.; Parthasarathy, V. Screening of antibacterial and antifungal activities of red marine algae Acanthaphora spicifera (Rhodophyceae). J. Biomed. Sci. Res. 2011, 3, 444–448. [Google Scholar]
- Mendis, E.; Kim, S.K. Present and future prospects of seaweed in developing functional foods. Adv. Food Nutr. Res. 2011, 64, 1–15. [Google Scholar]
- Kwon, H.J.; Ryu, Y.B.; Kim, Y.M.; Song, N.; Kim, C.Y.; Rho, M.-C.; Jeong, J.-H.; Cho, K.-O.; Lee, W.S.; Park, S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg. Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweed. Carbohydr. Res. 2017, 453–454. [Google Scholar]
- Gheda, S.F.; El-Adawib, H.L.; EL-Deebc, N.M. Antiviral profile of brown and red seaweed polysaccharides against hepatitis C virus. Iran. J. Pharmaceut. Res. 2016, 15, 483–491. [Google Scholar]
- Diogo, J.V.; Novo, S.G.; Gonzalez, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Veter. Sci. 2015, 98, 142–144. [Google Scholar]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar]
- Morán-Santibañez, K.; Peña-Hernández, M.A.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Skouta, R.; Vasquez, A.H.; Rodríguez-Padilla, C.; Trejo-Avila, L. Virucidal and synergistic activity of polyphenol-rich extracts of seaweeds against measles virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweed collected from the Qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial, the potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Vizetto-Duarte, C.; Cust-odio, L.; Acosta, G.; Lago, J.H.G.; Morais, T.R.; de Sousa, C.B.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Lima, R.T.; et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. Peer J. 2016, 4, 1704. [Google Scholar]
- Ina, A.; Kamei, Y. Vitamin B (12), a chlorophyll-related analog to pheophytin a from marine brown algae, promotes neurite outgrowth and stimulates differentiation in PC12 cells. Cytotechnol 2006, 52, 181–187. [Google Scholar]
- Manlusoc, J.K.T.; Hsieh, C.-L.; Hsieh, C.-Y.; Salac, E.S.; Lee, Y.-T.; Tsai, P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Pangestuti, R.; Kim, S. Biological activities and potential health benefits of sulphate polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Benattouche, Z.; Raho, G.B.; Sahnouni, F.; Hariri, A.; Bouhadi, G.; Benchohra, M. Anioxidant activities of sulfated polysaccharide obtained from red algae Corallina officinalis. IJP 2017, 4, 88–91. [Google Scholar]
- Punampalam, R.; Khoo, K.S.; Sit, N.M. Evaluation of antioxidant properties of phycobiliproteins and phenolic compounds extracted from Bangia atropurpurea. Malays. J. Fundam. Appl Sci. 2018, 14, 289–297. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin, Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; El Zokm, G.M.; El-Sayed, A.M. Variation in biochemical constituents and master elements in common seaweed from Alexandria Coast, Egypt, with special reference to their antioxidant activity and potential food uses, prospective equations. Environ. Monit Assessm. 2017, 189, 648. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics, From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweed Ulva armoricana, and Solieria chordalis from Brittany (France), An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed]
- De alencar, D.; Diniz, J.; Rocha, S.; Pires-cavalcante, K.; De Lima, R.L.; De Sousa, K.C.; Freitas, J.O.; Bezerra, R.M.; Baracho, B.M.; Sampaio, A.H.; et al. Fatty acid composition from the marine red algae Pterocladiella capillacea (S. G. Gmelin) Santelices & Hommersand 1997 and Osmundaria obtusiloba (C.; Agardh) R. E. Norris 1991 and its antioxidant activity. An. Acad Bras. Cienc. 2018, 90, 449–459. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jonsson, J.O.; Thorkelsson, G.; Olafasdottir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci. Tech. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Gheda, S.; El-Sheekh, M.; Abou-Zeid, A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pacif. J. Trop. Med. 2018, 11, 583–589. [Google Scholar]
- Erfani, N.; Nazemosadat, Z.; Moein, M. Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D. Pharm. Res. 2015, 7, 133–137. [Google Scholar]
- Raman, M.; Doble, M. ҡ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Foods. 2015, 15, 354–364. [Google Scholar] [CrossRef]
- Luo, M.; Shao, B.; Nie, W.X.; Wei, X.; Li, Y.-L.; Wang, B.-L.; He, Z.-Y.; Liang, Z.; Ye, T.-H.; Wei, Y.-Q. Antitumor and adjuvant activity of lamb-dacarrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 12. [Google Scholar]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264 7 mcrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; De Araújo, I.W.F.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food. 2012, 15, 1091–1095. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin, A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [Green Version]
- Ravi, M.; Tentu, S.; Baskar, G.; Prasad, S.R.; Raghavan, S.; Jayaprakash, P.; Jeyakanthan, J.; Rayala, S.K.; Venkatraman, G. Molecular mechanism of anti-cancer activity of phycocyanin in triple-negative breast cancer cells. BMC Cancer 2015, 15, 768. [Google Scholar] [CrossRef] [Green Version]
- Dellai, A.; Laajili, S.; Le Morvanb, V.; Robert, J.; Bouraoui, A. Antiproliferative activity and phenolics of the Mediterranean seaweed Laurencia obusta. Ind. Crop. Prod. 2013, 47, 252–255. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three Mediterranean brown seaweeds of the genus Cystoseira. Iran. J. Pharm Res. 2014, 13, 207–220. [Google Scholar] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Hassing, M.R.; Lewandowski, P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer 2013, 108, 486–492. [Google Scholar]
- Gunerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefneries. Biotechnol Adv. 2015, 33, 243–260. [Google Scholar]
- Morris, C.E. How does fertility of the substrate affect intra-specific competition? Evidence and synthesis from self-thinning. Ecol. Res. 2003, 18, 287–305. [Google Scholar] [CrossRef]
- Mittal, R.; Raghavarao, K.S.M.S. Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res. 2018, 34, 1–11. [Google Scholar]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse Palmaria palmata resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar]
- Van Ginneken, C.; Schafer, K.H.; Van Dam, D.; Huygelen, V.; De Deyn, P.P. Morphological changes in the enteric nervous system of aging and APP23 transgenic mice. Brain Res. 2011, 1378, 43–53. [Google Scholar] [CrossRef]
- Hong, D.D.; Hien, H.M.; Anh, H.T. Studies on the analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulata Forsskal (Chlorophyta) in experiment animal models. Afr. J. Biotechnol. 2011, 10, 2308–2314. [Google Scholar]
- Vázquez, A.I.; Sánchez, C.M.D.; Delgado, N.G.; Alfonso, A.M.S.; Ortega, Y.S.; Sánchez, H.C. Anti-inflammatory and analgesic activities of red seaweed Dichotomaria obtusata. Braz J. Pharmaceut Scis. 2011, 47, 111–118. [Google Scholar]
- Tan, L.T.; Williamson, R.T.; Gerwick, W.H.; Watts, K.H.; Mcgough, K.; Jacobs, R. Cis and trans, transceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000, 65, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Germain, S.; Bum, E.N.; Talla, E.; Dimo, T.; Weiss, N.; Sidiki, N.; Dawe, A.; Moto, F.C.O.; Dzefiet, P.D.; Waard, M. Antipyretic and antinociceptive effects of Nauclealatifolia roots decoction and possible mechanisms of action. Pharm. Biol. 2011, 49, 15–25. [Google Scholar]
- Chandrasekharan, N.V.; Simmons, D.L. The cycloox-ygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, J.P.; Devi, S.D.K. Evaluation of antipyretic activity of methanol extract of Hypnea musciformis (wulf) Lamouroux (red seaweed) in Manapad coast, Tamil Nadu. Ind. Int. J.; Med. Chem. Anal. 2015, 5, 74–78. [Google Scholar]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.I.; Pinto, M.M.; Sousa, A.; Correia-da-Silva, M. Antithrombotics from the Sea, Polysaccharides and Beyond. Mar. Drugs 2019, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 14, 17. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanas esfocus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef]
- Güven, K.C.; Coban, B.; Sezik, E. Anticoagulant and antilipaemic activities of polysaccharides from marine algae. J. Black Sea Med. Env. 2019, 25, 22–257. [Google Scholar]
- Necas, J.; Bartosikova, L. Carrageenan, A Review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Venkatraman, A.; Yahoob, S.A.M.; Nagarajan, Y.; Harikrishnan, S.; Vasudevan, S.; Murugasamy, T. Pharmacological activity of biosynthesized gold nanoparticles from brown algae seaweed Turbinaria conoide. Nanoworld J. 2018, 4, 17–22. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Antidiabetic and anti-inflammatory potential of Sulphated polygalactans from red seaweed Kappaphycus alvarezii and Gracilaria opuntia. Int. J. Food Prop. 2017, 20, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Nguyen, T.H.; Nguyen, V.M.; Nguyen, T.L.P.; Tran, T.V.A.; Do, A.D.; Kim, S.M. Antidiabetic and antioxidant activities of red seaweed Laurencia dendroidea. Asian Pac. J. Trop. Biomed. 2019, 9, 501–509. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, L.; Yan Agaro, X.J. Bioactivity research of oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Histopathological studies on liver, kidney and heart of normal and dietary induced hyperlipidaemic rats fed with tropical red seaweed Gracilaria changii. J. Funct. Foods 2015, 17, 202–213. [Google Scholar] [CrossRef]
- Seung-Hong, L.; You-Jin, J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar]
- Kang, M.-C.; Ko, N.K.; Kim, Y.-B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef]
- Lee, H.-G.; Lu, Y.A.; Li, X.; Hyun, J.-M.; Kim, H.-S.; Lee, J.J.; Kim, T.H.; Kim, H.M.; Kang, M.-C. Anti-Obesity effects of Grateloupia elliptica, a red seaweed, in mice with high-fat diet-induced obesity via suppression of adipogenic factors in white adipose tissue and increased thermogenic factors in brown adipose tissue. Nutrients 2020, 12, 308. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.-M.; Kim, H.-S.; Kim, T.H.; Kim, H.-M.; Lee, J.J.; Kang, M.-C.; Jeon, Y.J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. 2020. [Google Scholar] [CrossRef]
- Seo, M.-J.; Lee, O.-H.; Choi, H.-S.; Lee, B.-Y. Extract from edible red seaweed (Gelidium amansii) inhibits lipid accumulation and ros production during differentiation in 3T3-L1 cells. Prev. Nutr. Food Sci. 2012, 17, 129–135. [Google Scholar] [PubMed] [Green Version]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products, Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar]
- Kim, K.-J.; Lee, O.-H.; Le, B.-Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [PubMed]
- Seca, A.M.L.; Diana, C.G.A.; Pinto, D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart health peptides from macroalgae and their potential use in functional foods. J. Agricul. Food Chem. 2011, 59, 6829–6836. [Google Scholar]
- Saito, M.; Kawai, M.; Hagino, H.; Okada, J.; Yamamoto, K.; Hayashida, M.; Ikeda, T.P. Antihypertensive effect of Nori-peptides derived from red alga Porphyra yezoensis in hypertensive patients. Amer. J. Hypertension. 2002, 15, 210A. [Google Scholar]
- McNamara, Y. Alzheimer’s association international conference (AAIC) copenhagen, denmark-July 12–17, 2014. Drugs Future 2014, 39, 651–656. [Google Scholar]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.L.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacogn. 2015, 25, 657–662. [Google Scholar]
- Ghannadi, A.; Plubrukarn, A.; Zandi, K.; Sartavi, K.; Yegdaneh, A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res. Pharm. Sci. 2013, 8, 113–118. [Google Scholar]
- Olasehindea, T.A.; Mabinyaa, L.V.; Olaniranc, A.O.; Okoha, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr Diet. Fibre 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of Tramiprosate (homotaurine) against Alzheimer’s disease, A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.N.; Poon, B.P.; Salvi, J.; Olsen, J.B.; Emili, A.; Mikhail, A. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev. Cell 2011, 20, 867–879. [Google Scholar] [CrossRef]
- Leandro, A.; Leonel Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different levels of skin whitening activity among 3,6-Anhydro-L-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Senphan, T. Antioxidant activity of the extracts from freshwater macroalgae (Cladophora glomerata) grown in northern Thailand and its preventive effect against lipid oxidation of refrigerated eastern little tuna slice. Turk. J. Fish. Aquat Sci. 2018, 19, 209–219. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. https://doi.org/10.3390/molecules25194411
Ismail MM, Alotaibi BS, EL-Sheekh MM. Therapeutic Uses of Red Macroalgae. Molecules. 2020; 25(19):4411. https://doi.org/10.3390/molecules25194411
Chicago/Turabian StyleIsmail, Mona M., Badriyah S. Alotaibi, and Mostafa M. EL-Sheekh. 2020. "Therapeutic Uses of Red Macroalgae" Molecules 25, no. 19: 4411. https://doi.org/10.3390/molecules25194411
APA StyleIsmail, M. M., Alotaibi, B. S., & EL-Sheekh, M. M. (2020). Therapeutic Uses of Red Macroalgae. Molecules, 25(19), 4411. https://doi.org/10.3390/molecules25194411