Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots
Abstract
1. Introduction
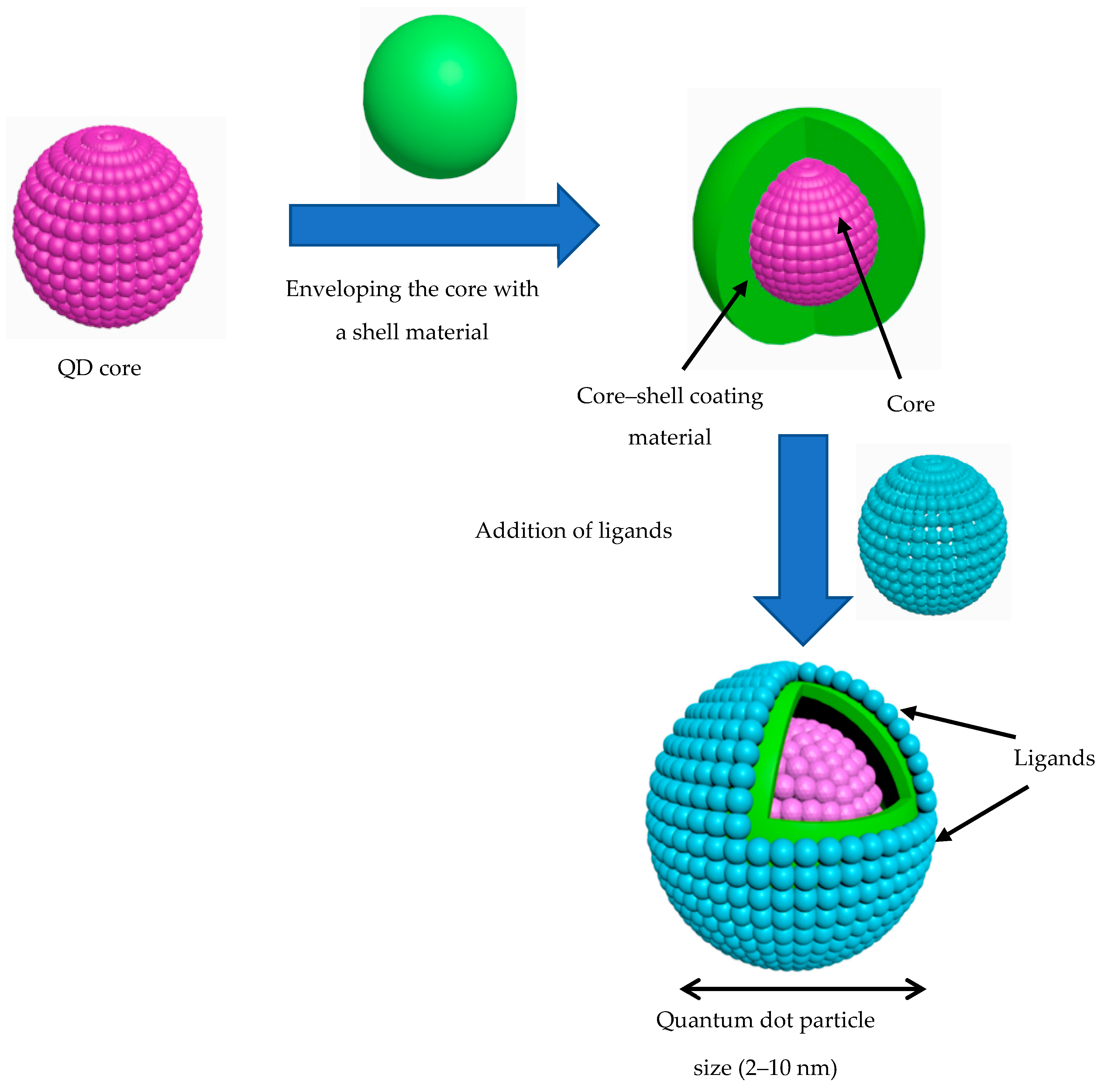
2. Structural Composition of QDs
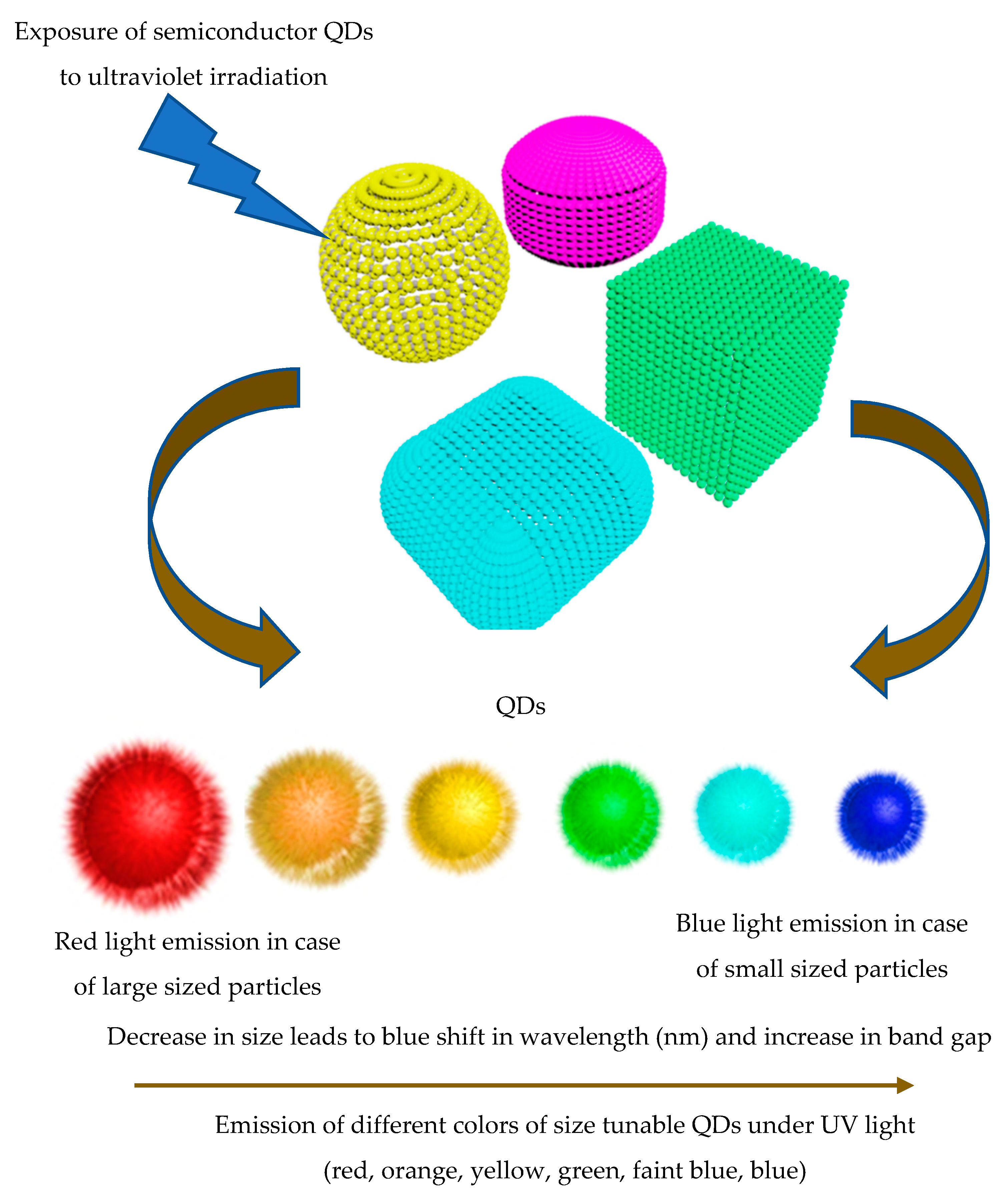
3. Physicochemical Properties of QDs
3.1. Blinking
3.2. Stokes Shift
4. Microbial Synthesis of QDs
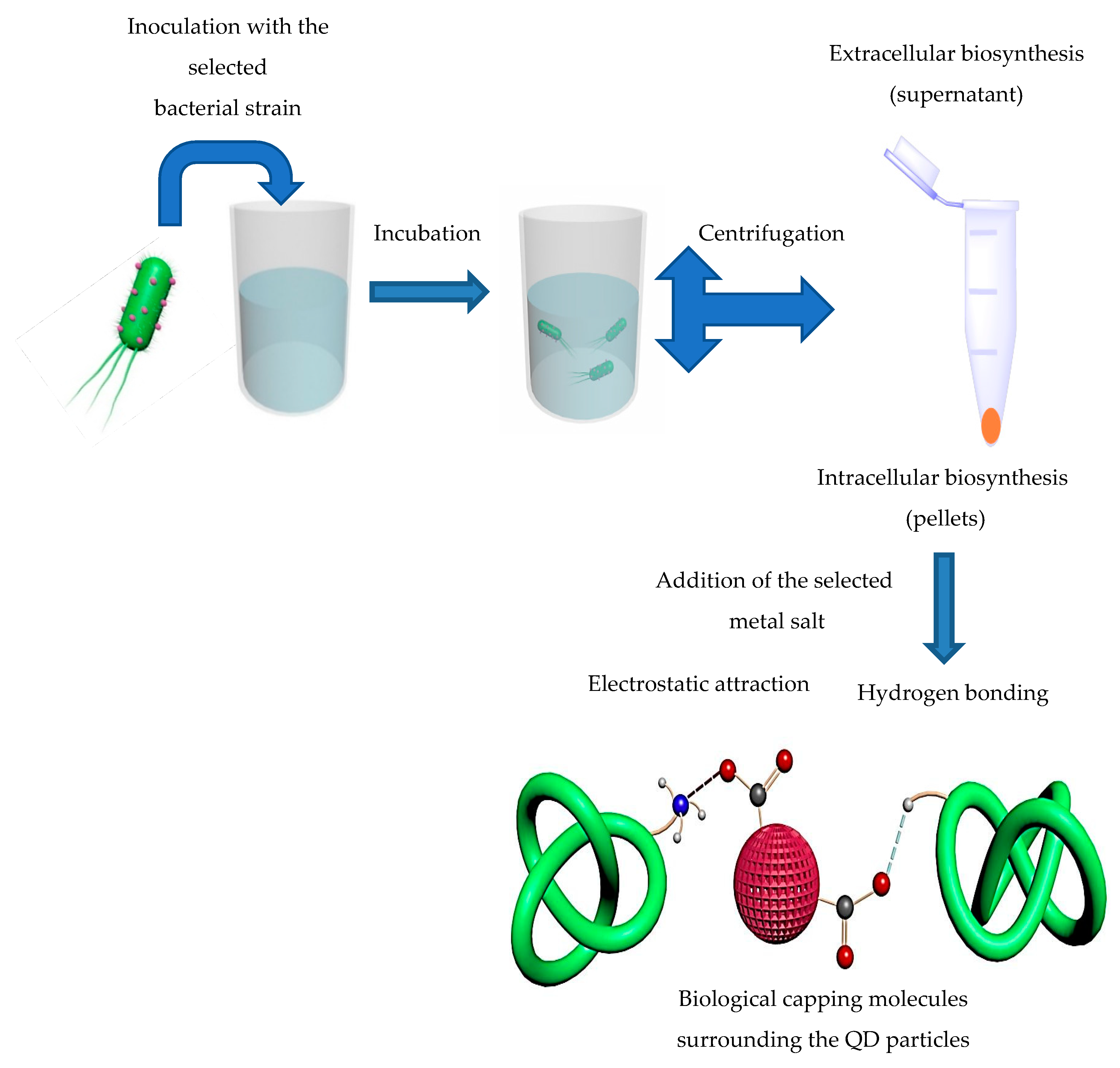
4.1. Mechanisms of Microbial Synthesis of QDs
4.1.1. Intracellular Microbial Synthesis of QDs
4.1.2. Extracellular Microbial Synthesis of QDs
4.2. Different Types of Microbially Fabricated QDs
4.2.1. Bacterial-Mediated Synthesis of QDs
4.2.2. Fungal-Mediated Synthesis of QDs
4.2.3. Yeast-Mediated Synthesis of QDs
5. Biomedical Applications of QDs
5.1. Applications of QDs in Tumor Research
5.2. Applications of QDs in Drug Delivery as Drug Carriers
5.3. Applications of QDs in Photodynamic Therapy
5.4. Applications of QDs in Microbial Labeling and Tracking
5.4.1. Single-Virus Labeling and Tracking
5.4.2. Bacterial Labeling
5.4.3. Fungal Labeling
5.5. MicroRNAs (miRNA) Detection
6. Conclusions, Challenges and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Xue, J.; Wang, X.; Hyun, J.; Yan, X. Fabrication, photoluminescence and applications of quantum dots embedded glass ceramics. Chem. Eng. J. 2020, 383, 123082–123115. [Google Scholar] [CrossRef]
- Jefferson, J.H.; Häusler, W. Quantum dots and artificial atoms. arXiv 1997, arXiv:cond-mat/9705012. [Google Scholar]
- Suri, S.; Ruan, G.; Winter, J.; Schmidt, C.E. Microparticles and nanoparticles. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 360–388. [Google Scholar]
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in three-dimensional microscopic semiconductor crystals. Jetp Lett. 1981, 34, 345–349. [Google Scholar]
- Dong, G.; Wang, H.; Chen, G.; Pan, Q.; Qiu, J. Quantum dot-doped glasses and fibers: Fabrication and optical properties. Front. Mater. 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Ren, J.; Fang, Z.; Lu, X.; Lewis, E.; Farrell, G.; Yang, J.; Wang, P. Selective doping of Ni2+ in highly transparent glass-ceramics containing nano-spinels ZnGa2O4 and Zn1+x Ga2−2x GexO4 for broadband near-infrared fiber amplifiers. Sci. Rep. 2017, 7, 1783. [Google Scholar] [CrossRef]
- Li, P.; Duan, Y.; Lu, Y.; Xiao, A.; Zeng, Z.; Xu, S.; Zhang, J. Nanocrystalline structure control and tunable luminescence mechanism of Eu-doped CsPbBr3 quantum dot glass for WLEDs. Nanoscale 2020, 12, 6630–6636. [Google Scholar] [CrossRef]
- Dong, G.; Wu, G.; Fan, S.; Zhang, F.; Zhang, Y.; Wu, B.; Ma, Z.; Peng, M.; Qiu, J. Formation, near-infrared luminescence and multi-wavelength optical amplification of PbS quantum dot-embedded silicate glasses. J. Non-Cryst. Solids 2014, 383, 192–195. [Google Scholar] [CrossRef]
- Reed, M.A.; Randall, J.N.; Aggarwal, R.J.; Matyi, R.J.; Moore, T.M.; Wetsel, A.E. Observation of discrete electronic states in a zero-dimensional semiconductor nanostructure. Phys. Rev. Lett. 1988, 60, 535–540. [Google Scholar] [CrossRef]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Weller, H. Colloidal semiconductor q-particles: Chemistry in the transition region between solid state and molecules. Angew. Chem. Int. Ed. 1993, 32, 41–53. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Mai, Y. Size-dependent absorption properties of CdX (X = S, Se, Te) quantum dots. Chem. Phys. Lett. 2012, 535, 91–93. [Google Scholar] [CrossRef]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Jacob, J.M.; Lens, P.N.L.; Balakrishnan, R.M. Microbial synthesis of chalcogenide semiconductor nanoparticles: A review. Microb. Biotechnol. 2016, 9, 11–21. [Google Scholar] [CrossRef]
- Sirinakis, G.; Zhao, Z.Y.; Sevryugina, Y.; Tayi, A.; Carpenter, M. Tailored Nanomaterials: Selective & Sensitive Chemical Sensors for Hydrocarbon Analysis; School of NanoSciences and NanoEngineering, University at Albany, SUNY: Albany, NY, USA, 2003; Available online: http://eqs.syr.edu/documents/research (accessed on 15 June 2003).
- Fariq, A.; Khan, T.; Yasmin, A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. J. Appl. Biomed. 2017, 15, 241–248. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Saklani, V.; Suman, J.V.K.; Jain, K. Microbial synthesis of silver nanoparticles: A review. J. Biotechnol. Biomater. 2012, S13, 7–10. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A.; Mehryar, P.; Pakshir, K.; Rahimi, M.J.; Arabi Monfared, A. Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Madakka, M.; Jayaraju, N.; Rajesh, N. Mycosynthesis of silver nanoparticles and their characterization. MethodsX 2018, 5, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, B.; Zheng, Z.; Liu, T. Semiconductor quantum dots in tumor research. J. Lumin. 2019, 209, 61–68. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Balakrishna, R.G. Quantum dots as fluorescent probes: Synthesis, surface chemistry, energy transfer mechanisms, and applications. Sens. Actuators B Chem. 2018, 258, 1191–1214. [Google Scholar] [CrossRef]
- Pandey, S.; Bodas, D. High-quality quantum dots for multiplexed bioimaging: A critical review. Adv. Colloid Interface Sci. 2020, 278, 102137–102153. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Rizvi, S.B.; Yildirimer, L.; Ghaderi, S.; Ramesh, B.; Seifalian, A.M.; Keshtgar, M. A novel POSS-coated quantum dot for biological application. Int. J. Nanomed. 2012, 7, 3915–3927. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Clapp, A. Overview of stabilizing ligands for biocompatible quantum. Sensors 2011, 11, 11036–11055. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, I.V.; Litvin, A.P.; Purcell-Milton, F.; Baranov, A.V.; Fedorov, A.V.; Gun’Ko, Y.K. Application of semiconductor quantum dots in bioimaging and biosensing. J. Mater. Chem. B 2017, 5, 6701–6727. [Google Scholar] [CrossRef] [PubMed]
- Credi, A. Photoactive Semiconductor Nanocrystal Quantum Dots: Fundamentals and Applications; Springer: Gewerbestrasse, Swizterland, 2017; ISBN 3319511920. [Google Scholar]
- Reiss, P.; Protiere, M.; Li, L. Core/shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Ziaudeen, S.A.; Gaddam, R.R.; Pallapothu, P.K.; Sugumar, M.K.; Rangarajan, J. Supra gap excitation properties of differently confined PbS-nano structured materials studied with opto-impedance spectroscopy. J. Nanophotonics 2013, 7, 73075–73088. [Google Scholar] [CrossRef]
- Mews, A.; Eychmüller, A.; Giersig, M.; Schooss, D.; Weller, H. Preparation, characterization, and photophysics of the quantum dot quantum well system cadmium sulfide/mercury sulfide/cadmium sulfide. J. Phys. Chem. 1994, 98, 934–941. [Google Scholar] [CrossRef]
- Cao, Y.; Banin, U. Synthesis and characterization of InAs/InP and InAs/CdSe core/shell nanocrystals. Angew. Chem. Int. Ed. 1999, 38, 3692–3694. [Google Scholar] [CrossRef]
- Battaglia, D.; Li, J.J.; Wang, Y.; Peng, X. Colloidal two-dimensional systems: CdSe quantum shells and wells. Angew. Chem. Int. Ed. 2003, 42, 5035–5039. [Google Scholar] [CrossRef]
- Zhong, X.; Xie, R.; Zhang, Y.; Basche, T.; Knoll, W. High-quality violet-to red-emitting ZnSe/CdSe core/shell nanocrystals. Chem. Mater. 2005, 17, 4038–4042. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, M.; Wu, W.; Yang, X.; Sun, X.W.; Zhang, J.; Wang, H.-C.; Liu, R.-S.; Han, C.-Y.; Yang, H. Recent advances in quantum dot-based light-emitting devices: Challenges and possible solutions. Mater. Today 2019, 24, 69–93. [Google Scholar] [CrossRef]
- Arya, H.; Kaul, Z.; Wadhwa, R.; Taira, K.; Hirano, T.; Kaul, S.C. Quantum dots in bio-imaging: Revolution by the small. Biochem. Biophys. Res. Commun. 2005, 329, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.B.; Ghaderi, S.; Keshtgar, M.; Seifalian, A.M. Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev. 2010, 1, 5161. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the potential of graphene quantum dots in biomedicine and neuroscience. Int. J. Mol. Sci. 2020, 21, 3712. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pal, P.; Gupta, V.; Yadav, T.P.; Gupta, V.; Singh, S.P. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater. Chem. Phys. 2018, 203, 40–48. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labeling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer Science & Business Media: Heidelberg, Germany, 2008; ISBN 140209003X. [Google Scholar]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. In vivo applications of quantum dot nanoparticles for optical diagnostics and therapy. Nanomedicine 2011, 2, 21–40. [Google Scholar]
- Ducheyne, P. Comprehensive Biomaterials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 0081006926. [Google Scholar]
- Cordones, A.A.; Leone, S.R. Mechanisms for charge trapping in single semiconductor nanocrystals probed by fluorescence blinking. Chem. Soc. Rev. 2013, 42, 3209–3221. [Google Scholar] [CrossRef]
- Mahler, B.; Spinicelli, P.; Buil, S.; Quelin, X.; Hermier, J.; Dubertret, B.; Brossel, L.K.; Umr, C.; Pierre, U.; Cedex, P.; et al. Towards non-blinking quantum dots: The effect of thick shell. Proc. SPIE 2009, 7189, 1–9. [Google Scholar] [CrossRef]
- Mingqian, T.; Aiguo, W. Nanomaterials for Tumor Targeting Theranostics: A Proactive Clinical Perspective; World Scientific: Rosewood Drive, SC, USA, 2016; ISBN 981463543X. [Google Scholar]
- Stokes, G.G. On the change of refrangibility of light. Philos. Trans. R. Soc. 1852, 142, 463–562. [Google Scholar] [CrossRef]
- McCartney, M.; Whitaker, A.; Wood, A. George Gabriel Stokes: Life, Science and Faith; Oxford University Press: New York, NY, USA, 2019; ISBN 0198822863. [Google Scholar]
- Zhou, R.; Lu, X.; Yang, Q.; Wu, P. Nanocrystals for large Stokes shift-based optosensing. Chin. Chem. Lett. 2019, 30, 1843–1848. [Google Scholar] [CrossRef]
- Vollmer, F.; Rettig, W.; Birckner, E. Photochemical mechanisms producing large fluorescence stokes shifts. J. Fluoresc. 1994, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.B.; Rouhi, S.; Taniguchi, S.; Yang, S.Y.; Green, M.; Keshtgar, M.; Seifalian, A.M. Near-infrared quantum dots for HER2 localization and imaging of cancer cells. Int. J. Nanomed. 2014, 9, 1323–1337. [Google Scholar] [CrossRef]
- Jung, J.; Lin, C.H.; Yoon, Y.J.; Malak, S.T.; Zhai, Y.; Thomas, E.L.; Vardeny, V.; Tsukruk, V.V.; Lin, Z. Crafting core/graded shell–shell quantum dots with suppressed re-absorption and tunable Stokes shift as high optical gain materials. Angew. Chem. Int. Ed. 2016, 55, 5071–5075. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.; Shi, Y.; Wang, J.; Cheng, Y.; Li, Y.; Gao, X.; Yan, Y.; Sun, J.Z.; Qin, A. Malonitrile-functionalized tetraphenylpyrazine: Aggregation-induced emission, ratiometric detection of hydrogen sulfide, and mechanochromism. Adv. Funct. Mater. 2018, 28, 1704689–1704699. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, J.; Xie, Y.-N.; Zhang, X.; Jiang, X.; Hou, X.; Wu, P. Ratiometric phosphorescent probe for thallium in serum, water, and soil samples based on long-lived, spectrally resolved, Mn-doped ZnSe quantum dots and carbon dots. Anal. Chem. 2018, 90, 2939–2945. [Google Scholar] [CrossRef]
- Ca, N.X.; Hien, N.T.; Luyen, N.T.; Lien, V.T.K.; Thanh, L.D.; Do, P.V.; Bau, N.Q.; Pham, T.T. Photoluminescence properties of CdTe/CdTeSe/CdSe core/alloyed/shell type-II quantum dots. J. Alloys Compd. 2019, 787, 823–830. [Google Scholar] [CrossRef]
- Aikens, C.M. Electronic and geometric structure, optical properties, and excited state behavior in atomically precise thiolate-stabilized noble metal nanoclusters. Acc. Chem. Res. 2018, 51, 3065–3073. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Ramasamy, K.P.; Mancini, A.; Giuli, G.; Natalello, A.; Ballarini, P.; Miceli, C.; Pucciarelli, S. Synthesis of bioactive silver nanoparticles by a Pseudomonas strain associated with the antarctic psychrophilic protozoon Euplotes focardii. Mar. Drugs 2020, 18, 38. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Physiochemical properties of Trichoderma longibrachiatum DSMZ 16517-synthesized silver nanoparticles for the mitigation of halotolerant sulphate-reducing bacteria. J. Appl. Microbiol. 2019, 126, 138–154. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656–105680. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K.L. Repurposing plant virus nanoparticles. Vaccines 2018, 6, 11. [Google Scholar] [CrossRef]
- Seifipour, R.; Nozari, M.; Pishkar, L. Green synthesis of silver nanoparticles using Tragopogon collinus leaf extract and study of their antibacterial effects. J. Inorg. Organomet. Polym. Mater. 2020. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Waste upcycling of Citrus sinensis peels as a green route for the synthesis of silver nanoparticles. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Jacob, J.M.; Balakrishnan, R.M.; Kumar, U.B. Biosynthesis of lead selenide quantum rods in marine Aspergillus terreus. Mater. Lett. 2014, 124, 279–281. [Google Scholar] [CrossRef]
- Mi, C.; Wang, Y.; Zhang, J.; Huang, H.; Xu, L.; Wang, S.; Fang, X.; Fang, J.; Mao, C.; Xu, S. Biosynthesis and characterization of CdS quantum dots in genetically engineered Escherichia coli. J. Biotechnol. 2011, 153, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, L.; Berard, V.F.; He, Q.; Kiely, C.J.; Berger, B.W.; McIntosh, S. Biomanufacturing of CdS quantum dots. Green Chem. 2015, 17, 3775–3782. [Google Scholar] [CrossRef]
- Yue, L.; Wang, J.; Zhang, Y.; Qi, S.; Xin, B. Controllable biosynthesis of high-purity lead-sulfide (PbS) nanocrystals by regulating the concentration of polyethylene glycol in microbial system. Bioprocess Biosyst. Eng. 2016, 39, 1839–1846. [Google Scholar] [CrossRef]
- Bruna, N.; Collao, B.; Tello, A.; Caravantes, P.; Díaz-Silva, N.; Monrás, J.P.; Órdenes-Aenishanslins, N.; Flores, M.; Espinoza-Gonzalez, R.; Bravo, D.; et al. Synthesis of salt-stable fluorescent nanoparticles (quantum dots) by polyextremophile halophilic bacteria. Sci. Rep. 2019, 9, 1953. [Google Scholar] [CrossRef]
- Oliva-Arancibia, B.; Órdenes-Aenishanslins, N.; Bruna, N.; Ibarra, P.S.; Zacconi, F.C.; Pérez-Donoso, J.M.; Poblete-Castro, I. Co-synthesis of medium-chain-length polyhydroxyalkanoates and CdS quantum dots nanoparticles in Pseudomonas putida KT2440. J. Biotechnol. 2017, 264, 29–37. [Google Scholar] [CrossRef]
- Venegas, F.A.; Saona, L.A.; Monrás, J.P.; Órdenes-Aenishanslins, N.; Giordana, M.F.; Ulloa, G.; Collao, B.; Bravo, D.; Pérez-Donoso, J.M. Biological phosphorylated molecules participate in the biomimetic and biological synthesis of cadmium sulphide quantum dots by promoting H2S release from cellular thiols. RSC Adv. 2017, 7, 40270–40278. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Du, Q.Q.; Qian, J.; Wan, D.Y.; Wu, S.M. Eco-friendly intracellular biosynthesis of CdS quantum dots without changing Escherichia coli’s antibiotic resistance. Enzym. Microb. Technol. 2017, 96, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kominkova, M.; Milosavljevic, V.; Vitek, P.; Polanska, H.; Cihalova, K.; Dostalova, S.; Hynstova, V.; Guran, R.; Kopel, P.; Richtera, L.; et al. Comparative study on toxicity of extracellularly biosynthesized and laboratory synthesized CdTe quantum dots. J. Biotechnol. 2017, 241, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ashengroph, M.; Khaledi, A.; Bolbanabad, E.M. Extracellular biosynthesis of cadmium sulphide quantum dot using cell-free extract of Pseudomonas chlororaphis CHR05 and its antibacterial activity. Process Biochem. 2020, 89, 63–70. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Sun, J.; Zhang, Y.; Pu, M.; Zhang, G.; He, N.; Zeng, X. Effective integration of targeted tumor imaging and therapy using functionalized InP QDs with VEGFR2 monoclonal antibody and miR-92a inhibitor. ACS Appl. Mater. Interfaces 2017, 9, 13068–13078. [Google Scholar] [CrossRef]
- Sandana Mala, J.G.; Rose, C. Facile production of ZnS quantum dot nanoparticles by Saccharomyces cerevisiae MTCC 2918. J. Biotechnol. 2014, 170, 73–78. [Google Scholar] [CrossRef]
- Brooks, J.; Lefebvre, D.D. Optimization of conditions for cadmium selenide quantum dot biosynthesis in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2735–2745. [Google Scholar] [CrossRef]
- Al-Shalabi, Z.; Doran, P.M. Biosynthesis of fluorescent CdS nanocrystals with semiconductor properties: Comparison of microbial and plant production systems. J. Biotechnol. 2016, 223, 13–23. [Google Scholar] [CrossRef]
- Cao, K.; Chen, M.M.; Chang, F.Y.; Cheng, Y.Y.; Tian, L.J.; Li, F.; Deng, G.Z.; Wu, C. The biosynthesis of cadmium selenide quantum dots by Rhodotorula mucilaginosa PA-1 for photocatalysis. Biochem. Eng. J. 2020, 156, 107497. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of CdTe quantum dots by the fungus Fusarium oxysporum and their anti-bacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Chen, G.; Yi, B.; Zeng, G.; Niu, Q.; Yan, M.; Chen, A.; Du, J.; Huang, J.; Zhang, Q. Facile green extracellular biosynthesis of CdS quantum dots by white rot fungus Phanerochaete chrysosporium. Colloids Surf. B Biointerfaces 2014, 117, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Borovaya, M.; Pirko, Y.; Krupodorova, T.; Naumenko, A.; Blume, Y.; Yemets, A. Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. Kumm. Biotechnol. Biotechnol. Equip. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Mareeswari, P.; Brijitta, J.; Etti, S.H.; Meganathan, C.; Kaliaraj, G.S. Rhizopus stolonifer mediated biosynthesis of biocompatible cadmium chalcogenide quantum dots. Enzym. Microb. Technol. 2016, 95, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhou, N.; Liu, X.; Zhang, X.; Zhu, T.; Li, L. Fluorescence dynamics of the biosynthesized CdSe quantum dots in Candida utilis. Sci. Rep. 2017, 7, 2048–2054. [Google Scholar] [CrossRef]
- Cárdenas, S.; Issell, D.; Gomez-Ramirez, M.; Rojas-Avelizapa, N.G.; Vidales-Hurtado, M.A. Synthesis of cadmium sulfide nanoparticles by biomass of Fusarium oxysporum f. sp. lycopersici. J. Nano Res. 2017, 46, 179–191. [Google Scholar] [CrossRef]
- Jacob, J.M.; Rajan, R.; Kurup, G.G. Biologically synthesized ZnS quantum dots as fluorescent probes for lead (II) sensing. Luminescence 2020, 1–10. [Google Scholar] [CrossRef]
- Jacob, J.M.; Rajan, R.; Aji, M.; Kurup, G.G.; Pugazhendhi, A. Bio-inspired ZnS quantum dots as efficient photo catalysts for the degradation of methylene blue in aqueous phase. Ceram. Int. 2019, 45, 4857–4862. [Google Scholar] [CrossRef]
- Qin, Z.; Yue, Q.; Liang, Y.; Zhang, J.; Zhou, L.; Hidalgo, O.B.; Liu, X. Extracellular biosynthesis of biocompatible cadmium sulfide quantum dots using Trametes versicolor. J. Biotechnol. 2018, 284, 52–56. [Google Scholar] [CrossRef]
- Bai, H.J.; Zhang, Z.M.; Guo, Y.; Yang, G.E. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf. B Biointerfaces 2009, 70, 142–146. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Deepak, V.; Kalishwaralal, K.; Gurunathan, S. Biologically synthesized fluorescent CdS NPs encapsulated by PHB. Enzym. Microb. Technol. 2011, 48, 319–325. [Google Scholar] [CrossRef]
- Raouf Hosseini, M.; Nasiri Sarvi, M. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, G.; Collao, B.; Araneda, M.; Escobar, B.; Álvarez, S.; Bravo, D.; Pérez-Donoso, J.M. Use of acidophilic bacteria of the genus Acidithiobacillus to biosynthesize CdS fluorescent nanoparticles (quantum dots) with high tolerance to acidic pH. Enzym. Microb. Technol. 2016, 95, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Baesman, S.M.; Bullen, T.D.; Dewald, J.; Zhang, D.; Curran, S.; Islam, F.S.; Beveridge, T.J.; Oremland, R.S. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl. Environ. Microbiol. 2007, 73, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Forootanfar, H.; Amirpour-Rostami, S.; Jafari, M.; Forootanfar, A.; Yousefizadeh, Z.; Shakibaie, M. Microbial-assisted synthesis and evaluation the cytotoxic effect of tellurium nanorods. Mater. Sci. Eng. C 2015, 49, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K. Extracellular bio-production and characterization of small monodispersed CdSe quantum dot nanocrystallites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Yang, Y.; Commonwealth, T. Biosynthesis of biocompatible cadmium telluride quantum dots using yeast cells. Nano Res. 2010, 3, 481–489. [Google Scholar] [CrossRef]
- Naik, V.; Zantye, P.; Gunjal, D.; Gore, A.; Anbhule, P.; Kowshik, M.; Bhosale, S.V.; Kolekar, G. Nitrogen-doped carbon dots via hydrothermal synthesis: Naked eye fluorescent sensor for dopamine and used for multicolor cell imaging. ACS Appl. Bio Mater. 2019, 2, 2069–2077. [Google Scholar] [CrossRef]
- Ramalingam, G.; Saravanan, K.V.; Vizhi, T.K.; Rajkumar, M.; Baskar, K. Synthesis of water-soluble and bio-taggable CdSe@ZnS quantum dots. RSC Adv. 2018, 8, 8516–8527. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum dots—Characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Granada-Ramírez, D.A.; Arias-Cerón, J.S.; Rodriguez-Fragoso, P.; Vázquez-Hernández, F.; Luna-Arias, J.P.; Herrera-Perez, J.L.; Mendoza-Álvarez, J.G. Quantum dots for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing Elsevier: Chennai, India, 2017; pp. 411–436. [Google Scholar]
- Gui, R.; Jin, H.; Wang, Z.; Zhang, F.; Xia, J.; Yang, M.; Bi, S.; Xia, Y. Room-temperature phosphorescence logic gates developed from nucleic acid functionalized carbon dots and graphene oxide. Nanoscale 2015, 7, 8289–8293. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.N. Introduction to Nanomedicine and Nanobioengineering; John Wiley & Sons: New York, NY, USA, 2012; Volume 7, ISBN 1118093437. [Google Scholar]
- Jiang, P.; Zhu, C.-N.; Zhang, Z.-L.; Tian, Z.-Q.; Pang, D.-W. Water-soluble Ag2S quantum dots for near-infrared fluorescence imaging in vivo. Biomaterials 2012, 33, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Xu, L.; Zhang, D.; Liu, M.; Li, X.; Sun, H.; Lin, Q.; Yang, B. Facile aqueous-phase synthesis of biocompatible and fluorescent Ag2S nanoclusters for bioimaging: Tunable photoluminescence from red to near infrared. Small 2012, 8, 3137–3142. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S quantum dot: A bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.P. Fabrication of vascular endothelial growth factor antibody bioconjugated ultrasmall near-infrared fluorescent Ag2S quantum dots for targeted cancer imaging in vivo. Chem. Commun. 2013, 49, 3324–3326. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–72. [Google Scholar] [CrossRef]
- Erdem, T.; Nizamoglu, S.; Demir, H.V. Computational study of power conversion and luminous efficiency performance for semiconductor quantum dot nanophosphors on light-emitting diodes. Opt. Express 2012, 20, 3275–3295. [Google Scholar] [CrossRef]
- Zhao, M.X.; Zeng, E.Z. Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Xu, M.; Wang, J.; Hu, X.; Anwar, S.; Tedesco, A.C.; Morais, P.C.; Bi, H. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mater. Chem. B 2020, 8, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 178592–178630. [Google Scholar] [CrossRef]
- Miyashita, M.; Gonda, K.; Tada, H.; Watanabe, M.; Kitamura, N.; Kamei, T.; Sasano, H.; Ishida, T.; Ohuchi, N. Quantitative diagnosis of HER2 protein expressing breast cancer by single-particle quantum dot imaging. Cancer Med. 2016, 5, 2813–2824. [Google Scholar] [CrossRef]
- Trapiella-Alfonso, L.; Pons, T.; Lequeux, N.; Leleu, L.; Grimaldi, J.; Tasso, M.; Oujagir, E.; Seguin, J.; d’Orlyé, F.; Girard, C. Clickable-zwitterionic copolymer capped-quantum dots for in vivo fluorescence tumor imaging. ACS Appl. Mater. Interfaces 2018, 10, 17107–17116. [Google Scholar] [CrossRef]
- Voura, E.B.; Jaiswal, J.K.; Mattoussi, H.; Simon, S.M. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med. 2004, 10, 993–998. [Google Scholar] [CrossRef]
- Gazouli, M.; Bouziotis, P.; Lyberopoulou, A.; Ikonomopoulos, J.; Papalois, A.; Anagnou, N.P.; Efstathopoulos, E.P. Quantum dots-bevacizumab complexes for in vivo imaging of tumors. In Vivo 2014, 28, 1091–1095. [Google Scholar]
- Yao, J.; Li, P.; Li, L.; Yang, M. Biochemistry and biomedicine of quantum dots: From biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018, 74, 36–55. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO quantum dots–doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Penjweini, R.; Liu, B.; Kim, M.M.; Zhu, T.C. Explicit dosimetry for 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a-mediated photodynamic therapy: Macroscopic singlet oxygen modeling. J. Biomed. Opt. 2015, 20, 128003–128013. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers used in the photodynamic therapy of rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef]
- Fakayode, O.J.; Tsolekile, N.; Songca, S.P.; Oluwafemi, O.S. Applications of functionalized nanomaterials in photodynamic therapy. Biophys. Rev. 2018, 10, 49–67. [Google Scholar] [CrossRef]
- Agrawal, A.; Tripp, R.A.; Anderson, L.J.; Nie, S. Real-time detection of virus particles and viral protein expression with two-color nanoparticle probes. J. Virol. 2005, 79, 8625–8628. [Google Scholar] [CrossRef]
- Joo, K.-I.; Lei, Y.; Lee, C.-L.; Lo, J.; Xie, J.; Hamm-Alvarez, S.F.; Wang, P. Site-specific labeling of enveloped viruses with quantum dots for single virus tracking. ACS Nano 2008, 2, 1553–1562. [Google Scholar] [CrossRef]
- Wen, L.; Lin, Y.; Zheng, Z.-H.; Zhang, Z.-L.; Zhang, L.-J.; Wang, L.-Y.; Wang, H.-Z.; Pang, D.-W. Labeling the nucleocapsid of enveloped baculovirus with quantum dots for single-virus tracking. Biomaterials 2014, 35, 2295–2301. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, J.; Kim, J.; Huang, J.; Zhang, S.N.; Kang, Y.; Kim, H.; Yun, C. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: Effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008, 16, 635–651. [Google Scholar] [CrossRef]
- Luo, K.; Li, S.; Xie, M.; Wu, D.; Wang, W.; Chen, R.; Huang, L.; Huang, T.; Pang, D.; Xiao, G. Real-time visualization of prion transport in single live cells using quantum dots. Biochem. Biophys. Res. Commun. 2010, 394, 493–497. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef]
- Jia, H.-R.; Zhu, Y.-X.; Chen, Z.; Wu, F.-G. Cholesterol-assisted bacterial cell surface engineering for photodynamic inactivation of Gram-positive and Gram-negative bacteria. ACS Appl. Mater. Interfaces 2017, 9, 15943–15951. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef]
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon dots: Current advances in pathogenic bacteria monitoring and prospect applications. Biosens. Bioelectron. 2020, 156, 112085–112098. [Google Scholar] [CrossRef]
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Fu, Z.; Gao, W.; Zhang, C.; Zhao, Q.; Deng, F.; Lu, X. Review on carbon dots in food safety applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Devi, P.; Thakur, A.; Bhardwaj, S.K.; Saini, S.; Rajput, P.; Kumar, P. Metal ion sensing and light activated antimicrobial activity of Aloe vera derived carbon dots. J. Mater. Sci. Mater. Electron. 2018, 29, 17254–17261. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids Surf. B Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xie, H.; Li, Y.; Zhang, J.K.; Su, B.L. Direct and rapid quantum dots labeling of Escherichia coli cells. J. Colloid Interface Sci. 2013, 393, 438–444. [Google Scholar] [CrossRef]
- Bae, P.K.; So, H.M.; Kim, K.N.; You, H.S.; Choi, K.S.; Kim, C.H.; Park, J.K.; Lee, J.O. Simple route for the detection of Escherichia coli using quantum dots. Biochip J. 2010, 4, 129–133. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Sun, W.; Wu, F.-G. Fluorescent quantum dots for microbial imaging. Chin. Chem. Lett. 2018, 29, 1475–1485. [Google Scholar] [CrossRef]
- Rispail, N.; De Matteis, L.; Santos, R.; Miguel, A.S.; Custardoy, L.; Testillano, P.S.; Risueño, M.C.; Pérez-de-Luque, A.; Maycock, C.; Fevereiro, P. Quantum dot and superparamagnetic nanoparticle interaction with pathogenic fungi: Internalization and toxicity profile. ACS Appl. Mater. Interfaces 2014, 6, 9100–9110. [Google Scholar] [CrossRef]
- Wang, X.; van de Veerdonk, F.L.; Netea, M.G. Basic genetics and immunology of Candida infections. Infect. Dis. Clin. N. Am. 2016, 30, 85–102. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Cabrera, M.P.; Santos, N.R.M.; Napoleão, T.H.; Paiva, P.M.G.; Neves, R.P.; Silva, M.V.; Santos, B.S.; Coelho, L.C.B.B.; Cabral Filho, P.E.; et al. Evaluating glucose and mannose profiles in Candida species using quantum dots conjugated with Cramoll lectin as fluorescent nanoprobes. Microbiol. Res. 2020, 230, 126330–126336. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Feinbaum, R.; Ambros, V.; Lee, R. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 2004, 116, 843–854. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Mishra, P.K.; Goryacheva, I.Y. Luminescent quantum dots for miRNA detection. Talanta 2018, 179, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Tang, D.; Lai, W.; Chen, G.; Yang, H. Immobilization-free programmable hairpin probe for ultrasensitive electronic monitoring of nucleic acid based on a biphasic reaction mode. Anal. Chem. 2014, 86, 8400–8407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, Y.; Yuan, R.; Chai, Y. Intercalation of quantum dots as the new signal acquisition and amplification platform for sensitive electrochemiluminescent detection of microRNA. Anal. Chim. Acta 2015, 891, 130–135. [Google Scholar] [CrossRef] [PubMed]

| Microorganisms | QDs | Factors Optimization | References |
|---|---|---|---|
| Bacteria | |||
| Desulfovibrio desulfuricans NCIMB 8307 | ZnS | - | [12] |
| Genetically engineered Escherichia coli | CdS | Reactant concentrations, reaction time | [73] |
| Stenotrophomonas maltophilia | CdS | Reaction time | [74] |
| Clostridiaceae sp. | ZnS | - | [75] |
| Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus | CdS | pH | [76] |
| Pseudomonas putida KT2440 | CdS | CdSO4 concentration and exposure time | [77] |
| E. coli BW25113 | CdS | - | [78] |
| E. coli | CdS | Reaction time | [79] |
| E. coli | CdTe | - | [80] |
| P. chlororaphis CHR05 | CdS | CdSO4 concentration, temperature, time and pH | [81] |
| Yeast | |||
| Saccharomyces cerevisiae | CdS | - | [82] |
| S. cerevisiae MTCC 2918 | ZnS | Reaction time and different concentrations of yeast biomass and ZnSO4 | [83] |
| S. cerevisiae | CdSe | Effect of S. cerevisiae growth phase, selenite concentration, cadmium concentration, effects of selenite and cadmium incubating time | [84] |
| Schizosaccharomyces pombe | CdS | - | [85] |
| Rhodotorula mucilaginosa | CdSe | Different concentrations of Na2SeO3 and CdCl2 and pH | [86] |
| Fungi | |||
| Fusarium oxysporum | CdTe | - | [87] |
| Phanerochaete chrysosporium | CdS | - | [88] |
| F. oxysporum f. sp. lycopersici | CdS | Reaction time | [89] |
| Rhizopus stolonifera | CdTe and CdS | - | [90] |
| Pleurotus ostreatus | CdS | - | [91] |
| Aspergillus terreus | PbSe | - | [92] |
| Aspergillus sp. | ZnS | Reaction time, temperature, pH | [93] |
| Penicillium sp. | ZnS | - | [94] |
| Trametes versicolor | CdS | - | [95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Salam, M.; Omran, B.; Whitehead, K.; Baek, K.-H. Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots. Molecules 2020, 25, 4486. https://doi.org/10.3390/molecules25194486
Abdel-Salam M, Omran B, Whitehead K, Baek K-H. Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots. Molecules. 2020; 25(19):4486. https://doi.org/10.3390/molecules25194486
Chicago/Turabian StyleAbdel-Salam, Mohamed, Basma Omran, Kathryn Whitehead, and Kwang-Hyun Baek. 2020. "Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots" Molecules 25, no. 19: 4486. https://doi.org/10.3390/molecules25194486
APA StyleAbdel-Salam, M., Omran, B., Whitehead, K., & Baek, K.-H. (2020). Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots. Molecules, 25(19), 4486. https://doi.org/10.3390/molecules25194486







