Protective Effects of Traditional Polyherbs on Cisplatin-Induced Acute Kidney Injury Cell Model by Inhibiting Oxidative Stress and MAPK Signaling Pathway
Abstract
:1. Introduction
2. Results
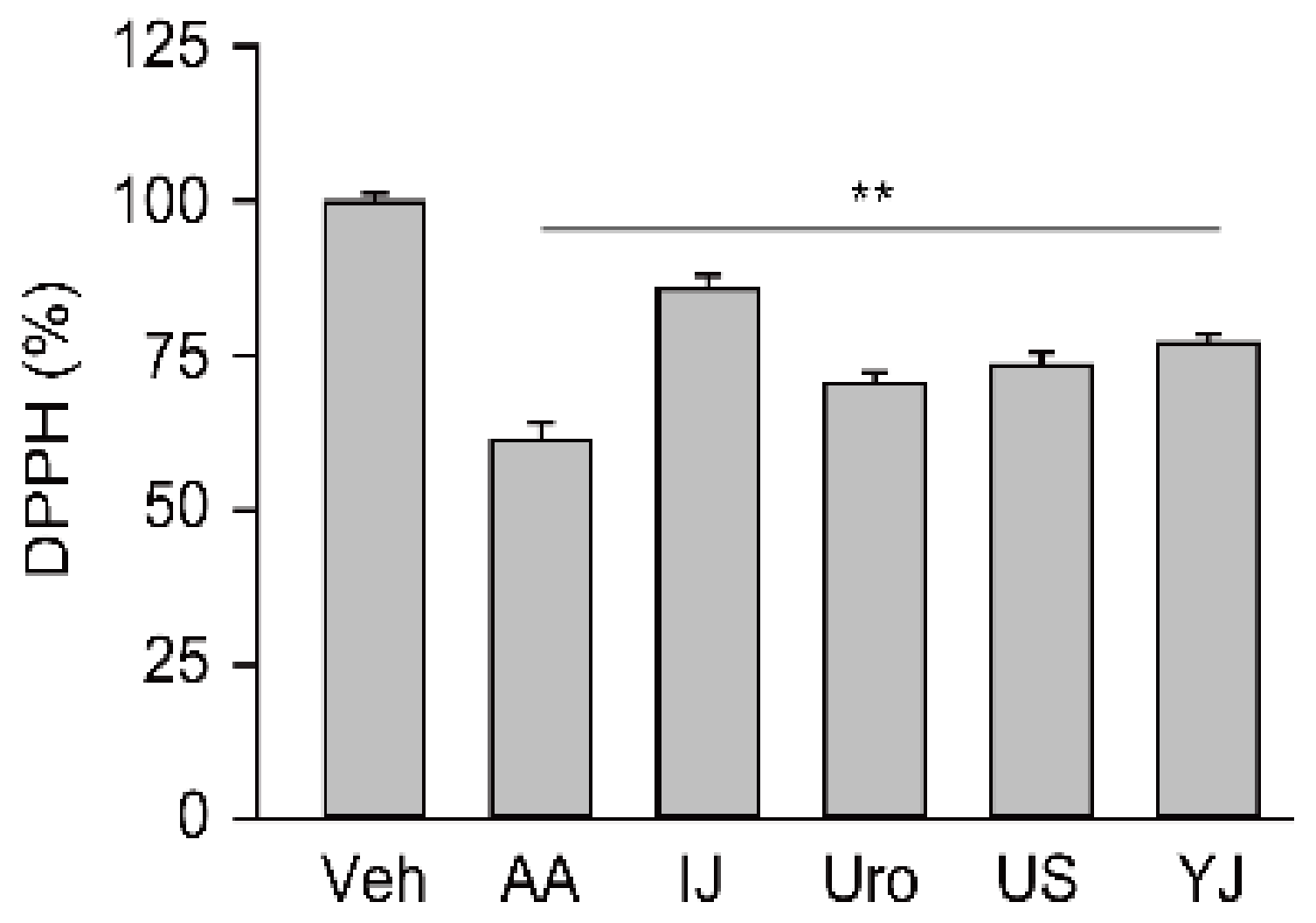
2.1. Free Radical Scavenging Activities of Traditional Polyherbs
2.2. Effects on Cell Viabilities in Cisplatin-Induced Nephrotoxicity
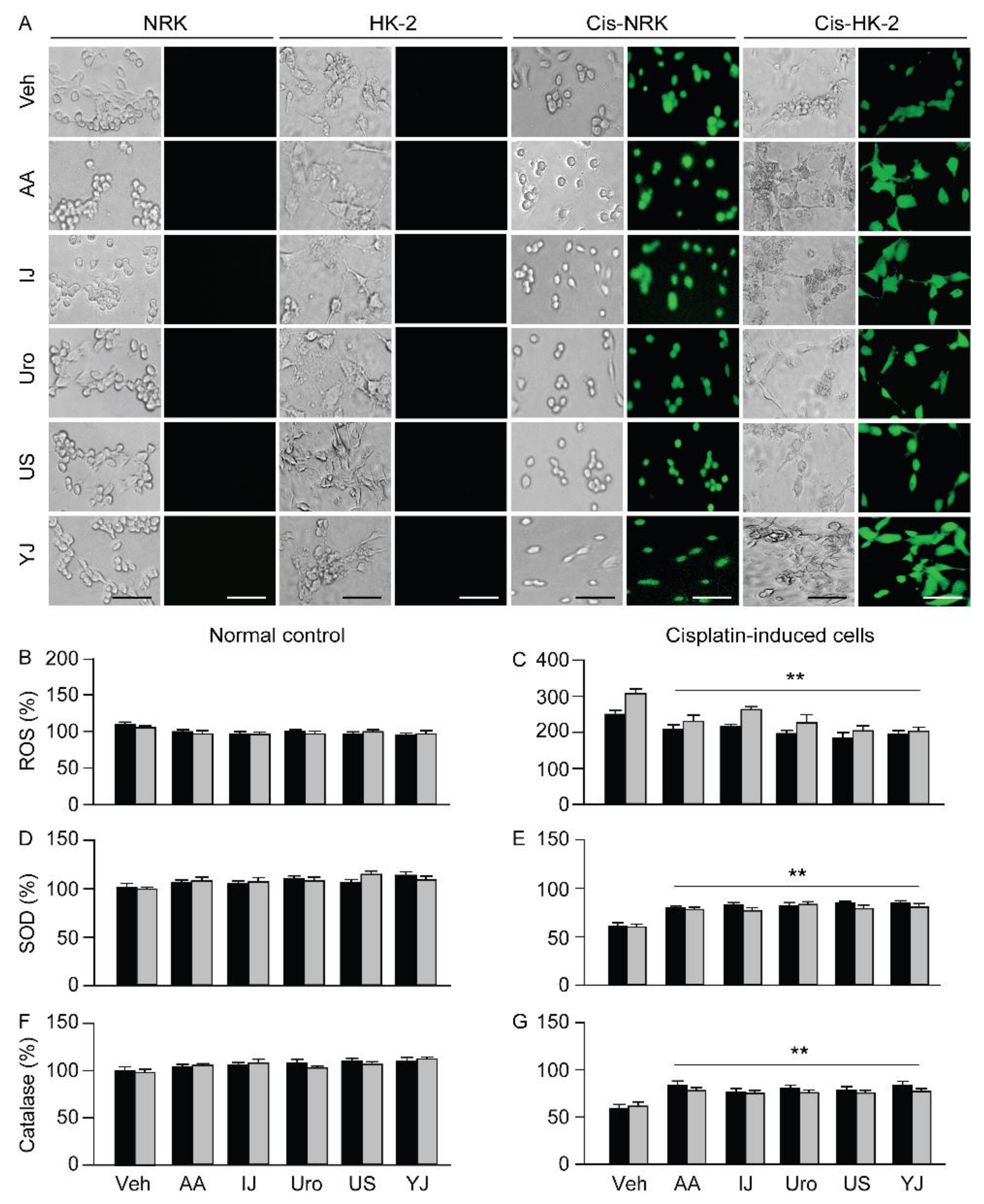
2.3. Effects on ROS Production and Antioxidant Activities
2.4. Effects on Apoptotic Changes
2.5. Effects on Cell Proliferation
2.6. Effects on Expression of Caspase-3 and MAPK Signaling Proteins
3. Discussion
4. Materials and Methods
4.1. Preparation of Traditional Polyherbs
4.2. Free Radical Scavenging Activity
4.3. Cell Culture
4.4. Cisplatin-Induced Acute Kidney Cell Model and Treatments
4.5. Cell Viability Assay
4.6. Assessment of ROS Levels
4.7. Assessment of Activities of Antioxidant Enzymes
4.8. Flow Cytometric Analysis
4.9. Immunoblotting
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharm. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nematbakhsh, M.; Nasri, H. The effects of vitamin E and selenium on cisplatin-induced nephrotoxicity in cancer patients treated with cisplatin-based chemotherapy: A randomized, placebo-controlled study. J. Res. Med. Sci. 2013, 18, 625. [Google Scholar]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Song, K.I.; Park, J.Y.; Lee, S.; Lee, D.; Jang, H.J.; Kim, S.N.; Ko, H.; Kim, H.Y.; Lee, J.W.; Hwang, G.S.; et al. Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: In vitro and in vivo studies. Planta Med. 2015, 81, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Asehnoune, K.; Strassheim, D.; Mitra, S.; Kim, J.Y.; Abraham, E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J. Immunol. 2004, 172, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C. Specificity of a third kind: Reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Investig. 2003, 111, 769–778. [Google Scholar] [CrossRef]
- Arany, I.; Megyesi, J.K.; Kaneto, H.; Price, P.M.; Safirstein, R.L. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2004, 287, F543–F549. [Google Scholar] [CrossRef] [Green Version]
- Dennis, J.M.; Witting, P.K. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Maliakel, D.M.; Kagiya, T.V.; Nair, C.K.K. Prevention of cisplatin-induced nephrotoxicity by glucosides of ascorbic acid and α-tocopherol. Exp. Toxicol. Pathol. 2008, 60, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abushouk, A.I.; Donia, T.; Alarifi, S.; Alkahtani, S.; Aleya, L.; Bungau, S.G. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ. Sci. Pollut. Res. 2019, 26, 13502–13509. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.K. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch. Pharm. 2007, 376, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese Herbal Medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Kim, D.Y.; Chung, S.H. Korean red ginseng extract alleviates advanced glycation end product-mediated renal injury. J. Ginseng Res. 2013, 37, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Busse, L.W. Novel Therapies for Acute Kidney Injury. Kidney Int. Rep. 2017, 2, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Baboota, S.; Amin, S.; Mir, S.R. Ameliorative effect of a standardized polyherbal combination in methotrexate-induced nephrotoxicity in the rat. Pharm. Biol. 2020, 58, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, J.; Zhang, L.; Chen, X.; Pan, Y.; Chen, S.S.; Zhang, S.; Wang, Z.; Xiao, W.; Yang, L.; et al. Systems pharmacology to decipher the combinational anti-migraine effects of Tianshu formula. J. Ethnopharmacol. 2015, 174, 45–56. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Zhang, Y. A systems pharmacology approach to decipher the mechanism of danggui-shaoyao-san decoction for the treatment of neurodegenerative diseases. J. Ethnopharmacol. 2016, 178, 66–81. [Google Scholar] [CrossRef]
- Huh, J. Dong Ui Bo Gam; Dong Ui Bo Gam Publisher: Seoul, Korea, 2005. [Google Scholar]
- Jeong, J.J.; Park, N.; Kwon, Y.J.; Ye, D.J.; Moon, A.; Chun, Y.J. Role of annexin A5 in cisplatin-induced toxicity in renal cells: Molecular mechanism of apoptosis. J. Biol. Chem. 2014, 289, 2469–2481. [Google Scholar] [CrossRef] [Green Version]
- Dogra, S.; Bandi, S.; Viswanathan, P.; Gupta, S. Arsenic trioxide amplifies cisplatin toxicity in human tubular cells transformed by HPV-16 E6/E7 for further therapeutic directions in renal cell carcinoma. Cancer Lett. 2015, 356, 953–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dachuri, V.; Song, P.H.; Ku, S.K.; Song, C.H. Protective Effects of Traditional Herbal Formulas on Cisplatin-Induced Nephrotoxicity in Renal Epithelial Cells via Antioxidant and Antiapoptotic Properties. Evid. Based Complement. Altern. Med. 2020, 2020, 5807484. [Google Scholar] [CrossRef] [PubMed]
- Baliga, R.; Ueda, N.; Walker, P.D.; Shah, S.V. Oxidant mechanisms in toxic acute renal failure. Drug Metab. Rev. 1999, 31, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Majed, A.A.; Sayed-Ahmed, M.M.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Shabanah, O.A. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharm. Res. 2006, 53, 278–286. [Google Scholar] [CrossRef]
- Benedetti, G.; Fredriksson, L.; Herpers, B.; Meerman, J.; van de Water, B.; de Graauw, M. TNF-α-mediated NF-κB survival signaling impairment by cisplatin enhances JNK activation allowing synergistic apoptosis of renal proximal tubular cells. Biochem. Pharmacol. 2013, 85, 274–286. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.E.; Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Tapia, E.; García-Torres, I.; Pedraza-Chaverri, J.; Pacher, P. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J. Nutr. Biochem. 2012, 23, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-κB signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408. [Google Scholar] [CrossRef]
- Cui, X.-L.; Douglas, J.G. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc. Natl. Acad. Sci. USA 1997, 94, 3771–3776. [Google Scholar] [CrossRef] [Green Version]
- Tsuruya, K.; Tokumoto, M.; Ninomiya, T.; Hirakawa, M.; Masutani, K.; Taniguchi, M.; Fukuda, K.; Kanai, H.; Hirakata, H.; Iida, M. Antioxidant ameliorates cisplatin-induced renal tubular cell death through inhibition of death receptor-mediated pathways. Am. J. Physiol. Ren. Physiol. 2003, 285, F208–F218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Okubo, T.; Juneja, L.R.; Yokozawa, T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br. J. Nutr. 2010, 103, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Huang, Z.; Zou, X.; Yang, Y.; Qiu, Y.; Wen, Y. Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8391. [Google Scholar]
- Cai, B.; Jiang, T. Study on preventive and curative effects of liu wei di huang tang on tumors. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 1994, 14, 207–211. [Google Scholar] [PubMed]
- Kang, D.G.; Sohn, E.J.; Moon, M.K.; Mun, Y.J.; Woo, W.H.; Kim, M.K.; Lee, H.S. Yukmijihwang-tang ameliorates ischemia/reperfusion-induced renal injury in rats. J. Ethnopharmacol. 2006, 104, 47–53. [Google Scholar] [CrossRef]
- Kim, J.S.; Na, C.S.; Pak, S.C.; Kim, Y.G. Effects of yukmi, an herbal formula, on the liver of senescence accelerated mice (SAM) exposed to oxidative stress. Am. J. Chin. Med. 2000, 28, 343–350. [Google Scholar] [CrossRef]
- Wu, C.-T.; Tsai, Y.-T.; Lin, J.-G.; Fu, S.-l.; Lai, J.-N. Chinese herbal products and the reduction of risk of breast cancer among females with type 2 diabetes in Taiwan: A case–control study. Medicine 2018, 97, e11600. [Google Scholar] [CrossRef]
- Suzuki, Y.; Goto, K.; Ishige, A.; Komatsu, Y.; Kamei, J. Effect of Gosha-jinki-gan, a Kampo medicine, on enhanced platelet aggregation in streptozotocin-induced diabetic rats. Jpn. J. Pharm. 1998, 78, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Chen, H.; Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158, 373–387. [Google Scholar] [CrossRef]
- Fu, P.K.; Yang, C.Y.; Tsai, T.H.; Hsieh, C.L. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine 2012, 19, 1206–1215. [Google Scholar] [CrossRef]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-C.; Suzuki, M.; Ohnishi-Kameyama, M.; Oh, M.-J.; Kim, H.-R.; Kim, J.-H.; Nagata, T. Extraction and identification of antioxidant components from Artemisia capillaris herba. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Han, K.-H.; Jeon, Y.-J.; Athukorala, Y.; Choi, K.-D.; Kim, C.-J.; Cho, J.-K.; Sekikawa, M.; Fukushima, M.; Lee, C.-H. A water extract of Artemisia capillaris prevents 2, 2′-azobis (2-amidinopropane) dihydrochloride-induced liver damage in rats. J. Med. Food 2006, 9, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Huang, H.H.; Yang, C.M.; Lin, L.T.; Lin, C.C. The in vitro anti-herpes simplex virus type-1 and type-2 activity of Long Dan Xie Gan Tan, a prescription of traditional Chinese medicine. Chemotherapy 2008, 54, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, K.; Lee, D.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Protective effect and mechanism of action of lupane triterpenes from Cornus walteri in cisplatin-induced nephrotoxicity. Bioorg. Med. Chem. Lett. 2015, 25, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, Y.J.; Wang, M.Z.; Sun, Y.; Li, G.Y.; Wang, Q.L.; Yao, J.C.; Yue, J.; Liu, Z.; Zhang, G.M.; et al. Loganetin protects against rhabdomyolysis-induced acute kidney injury by modulating the toll-like receptor 4 signalling pathway. Br. J. Pharm. 2019, 176, 1106–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Ingredients | |
|---|---|
| IJ | Artemisiae Capillaris Herba 2 g, Gardenia Fruit 1 g, Rhubarb 0.67 g |
| Uro | Akebiae Caulis 416.7 mg, Alisma Rhizome 250 mg, Angelica Gigas Root 416.7 mg, Cinnamon Bark 16.7 mg, Ephedra Herb 16.7 mg, Forsythia Fruit 16.7 mg, Gardenia Fruit 125 mg, Gentian Root 125 mg, Glycyrrhiza 125 mg, Plantago Seed 250 mg, Rhubarb 16.7 mg, Scutellaria Root 250 mg, Raw Ginger 16.7 mg, Rehmannia Root 416.7 mg |
| US | Achyranthes Root 3.0 mg, Alisma Rhizome 3.0 mg, Cinnamon Bark 1 g, Cornus Fruit 3.0 mg, Dioscorea Rhizome 3.0 mg, Hoelen 3 g, Moutan Root Bark 3.0 mg, Psyllium Husk 3 g, Pulvis Aconiti Tuberis Purificatum 1.0 mg, Rehmannia Root 5.0 mg |
| YJ | Alisma Rhizome 240 mg, Cornus Fruit 320 mg, Dioscorea Rhizome 320 mg, Hoelen 240 mg, Moutan Root Bark 240 mg, Steamed Rehmannia Root 640 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dachuri, V.; Song, P.H.; Kim, Y.W.; Ku, S.-K.; Song, C.-H. Protective Effects of Traditional Polyherbs on Cisplatin-Induced Acute Kidney Injury Cell Model by Inhibiting Oxidative Stress and MAPK Signaling Pathway. Molecules 2020, 25, 5641. https://doi.org/10.3390/molecules25235641
Dachuri V, Song PH, Kim YW, Ku S-K, Song C-H. Protective Effects of Traditional Polyherbs on Cisplatin-Induced Acute Kidney Injury Cell Model by Inhibiting Oxidative Stress and MAPK Signaling Pathway. Molecules. 2020; 25(23):5641. https://doi.org/10.3390/molecules25235641
Chicago/Turabian StyleDachuri, VinayKumar, Phil Hyun Song, Young Woo Kim, Sae-Kwang Ku, and Chang-Hyun Song. 2020. "Protective Effects of Traditional Polyherbs on Cisplatin-Induced Acute Kidney Injury Cell Model by Inhibiting Oxidative Stress and MAPK Signaling Pathway" Molecules 25, no. 23: 5641. https://doi.org/10.3390/molecules25235641







