Abstract
Efforts are described towards the total synthesis of the bacterial macrolide rhizoxin F, which is a potent tubulin assembly and cancer cell growth inhibitor. A significant amount of work was expanded on the construction of the rhizoxin core macrocycle by ring-closing olefin metathesis (RCM) between C(9) and C(10), either directly or by using relay substrates, but in no case was ring-closure achieved. Macrocycle formation was possible by ring-closing alkyne metathesis (RCAM) at the C(9)/C(10) site. The requisite diyne was obtained from advanced intermediates that had been prepared as part of the synthesis of the RCM substrates. While the direct conversion of the triple bond formed in the ring-closing step into the C(9)-C(10) E double bond of the rhizoxin macrocycle proved to be elusive, the corresponding Z isomer was accessible with high selectivity by reductive decomplexation of the biscobalt hexacarbonyl complex of the triple bond with ethylpiperidinium hypophosphite. Radical-induced double bond isomerization, full elaboration of the C(15) side chain, and directed epoxidation of the C(11)-C(12) double bond completed the total synthesis of rhizoxin F.
1. Introduction
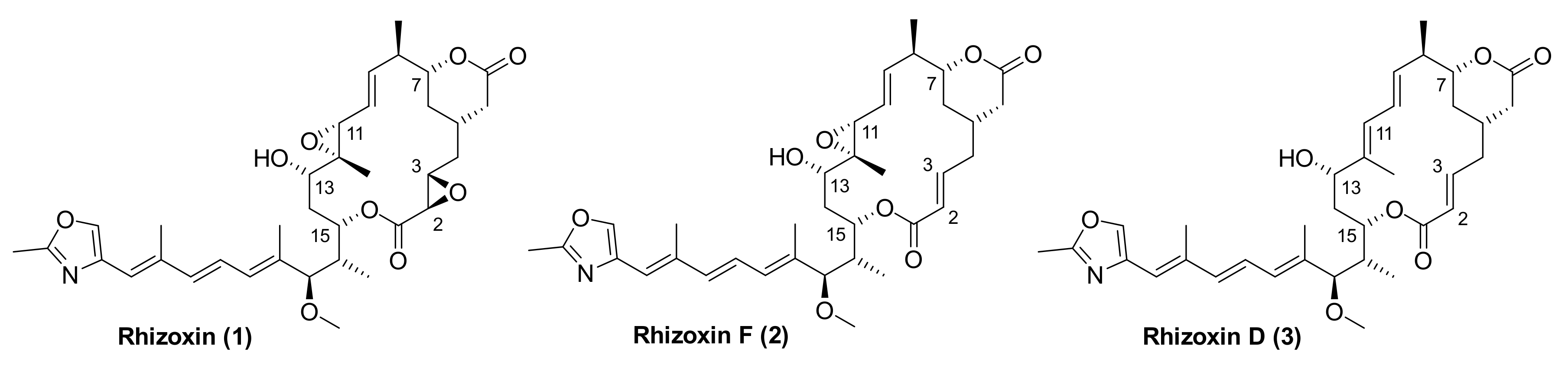
Rhizoxin (1) (Figure 1) is a 16-membered macrolide that was first isolated in 1984 from the plant pathogenic fungus Rhizopus chinensis by Iwasaki and co-workers [1] (for the elucidation of the absolute configuration of rhizoxin (1) by means of X-ray crystallography cf. [2]). Fungi of the genus Rhizopus are responsible for the rice seedling blight and rhizoxin (1) is the causative phytotoxin responsible for the disease [1].
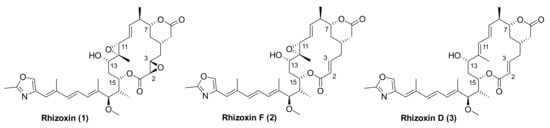
Figure 1.
Molecular structures of rhizoxin (1), rhizoxin F (2), and rhizoxin D (3).
Mechanistically, rhizoxin (1) binds to rice β-tubulin [3,4], which leads to inhibition of mitosis, cell cycle arrest, and, ultimately, death of the plant. Rhizopus sp. were believed to be the producers of rhizoxin (1) until 2005, when Partida-Martinez and Hertweck demonstrated that rhizoxin in Rhizopus microsporus is not produced by the fungus itself, but by endosymbiotic bacteria of the genus Burkholderia that were termed “Burkholderia rhizoxina” [5]. Partida-Martinez and Hertweck also located, cloned, and sequenced the entire gene locus that encodes rhizoxin biosynthesis in Burkholderia rhizoxina [6]. In addition, the endosymbiont was isolated in pure form and fermented on large scale, resulting in the recovery of a number of rhizoxin variants with highly potent biological activity, including WF-1360F (2) [7] (Figure 1), which we have termed rhizoxin F [8]. The isolation of rhizoxin F (2) had also been reported previously by Iwasaki et al. [9] and Kiyoto et al. [10] from Rhizopus chinensis and Rhizopus sp., respectively. In addition, rhizoxin variants have been isolated from fermentations of Pseudomonas fluorescens Pf-5 [11,12] (see also in [13]).
Rhizoxin (1) also binds to eukaryotic tubulin and prevents its assembly (“polymerization”) into microtubules [14,15]; in addition, preformed microtubules are depolymerized by the compound. As a consequence, rhizoxin (1) is a potent inhibitor of cancer cell growth in vitro with IC50 values in the single digit nM or even sub-nM range [11,16]. (For example, the IC50 value of 1 against the mouse P388 leukemia cell line is 0.16 nM [10].) Rhizoxin (1) had been suggested to bind to the β-subunit of the α/β-tubulin heterodimer at the same site as maytansine, and this binding site was proposed to be distinct from either the vinblastine or the colchicine site [14,15]. These hypotheses have recently been confirmed experimentally by means of X-ray crystallography on complexes of α/β-tubulin with rhizoxin F (2) and maytansine, respectively [8]. Importantly, compared to rhizoxin (1), monoepoxy-based rhizoxin F (2) has been reported to exhibit similar [10] or even significantly higher [8] in vitro antiproliferative activity.
Rhizoxin (1) has shown significant in vivo antitumor activity in animal models of cancer [11,16,17] and the compound has been evaluated in humans in a number of Phase I and Phase II clinical studies. However, the outcome of these trials was generally disappointing [18], although the reasons for the lack of clinical efficacy of rhizoxin (1) have not been elucidated in detail (based on public literature). It has been suggested that “further studies of this class of agents [rhizoxins] will depend on the identification of more efficacious analogs in preclinical models” [18].
The chemistry of rhizoxins has been explored extensively and as of today, 10 total syntheses of a naturally occurring rhizoxin have been reported in the literature. Out of these 10 syntheses, eight have targeted the epoxide-free rhizoxin variant rhizoxin D (3) (Figure 1) and only Ohno and co-workers have described the final conversion of 3 into rhizoxin [19]. In addition, we have reported the total synthesis of the monoepoxy variant rhizoxin F (2) [20]. Early work in the field (up to 2004) has been reviewed in detail in an excellent account by Hong and White [21] and shall not be discussed here, except to note that a recurring theme in the majority of these syntheses is the closure of the macrolactone ring through intramolecular Horner-Wittig-Emmons (HWE) reaction. Alternative approaches to ring-closure have been reported by Pattenden and co-workers (Stille coupling between C(10) and C(11)) [22] and by our own group (ring-closing alkyne metathesis (RCAM) between C(9) and C(10)) [20]. Most recently, Fürstner and co-workers have described an intriguing formal total synthesis of rhizoxin D (3) that included ring-closure by RCAM between C(11) and C(12) (!) [23]. The resulting triple bond was then efficiently transformed into the required trisubstituted trans double bond by trans-stannylation and subsequent C-methylation with excellent selectivity.
Our group has a long-standing interest in the chemistry, medicinal chemistry, and pharmacology of bioactive macrocyclic natural products. In this context, we were interested to develop a new synthetic approach towards rhizoxins that would be based on ring-closing olefin metathesis (RCM), which, somewhat surprisingly, had not been investigated as part of any of the previous total syntheses of a rhizoxin variant. The specific target to be pursued in our work was rhizoxin F (2); as alluded to above, this monoepoxy rhizoxin variant has been reported to be at least equipotent with rhizoxin (1) with regard to cancer cell proliferation in vitro. In a previous communication, we have reported the total synthesis of rhizoxin F (2) via RCAM [20]; in this communication, we also briefly touched upon our numerous futile attempts at RCM-based ring closure. In the current paper, we now disclose full details on these attempts and on the successful total synthesis of 2 based on macrocyclization by RCAM.
2. Results and Discussion
2.1. Synthetic Planning
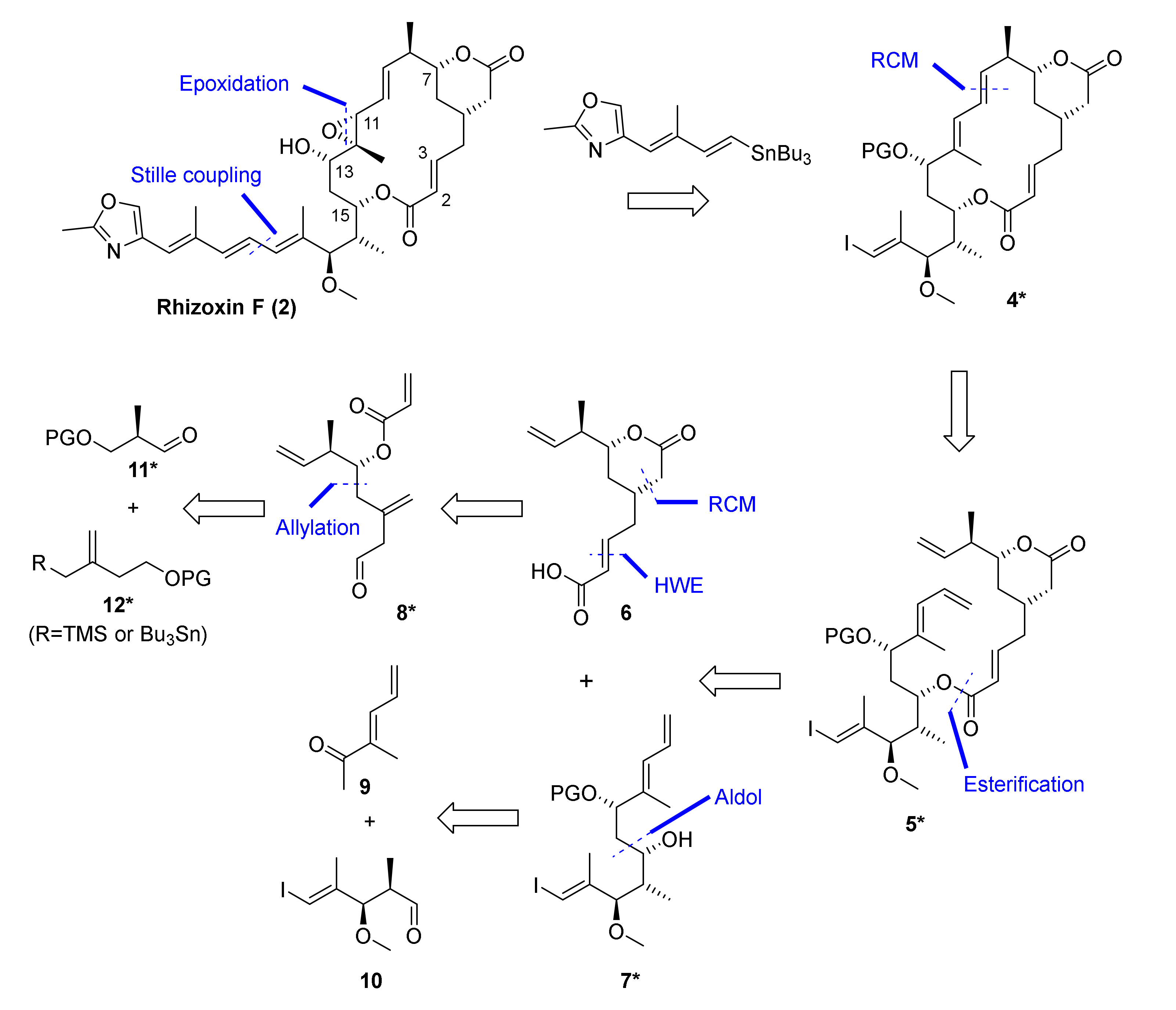
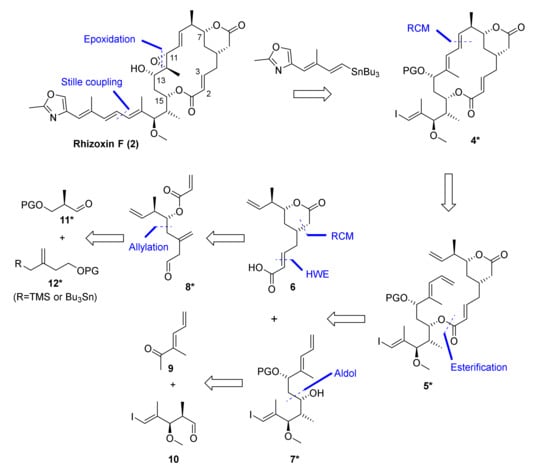
Apart from the exploration of the feasibility of an RCM-based macrocyclization strategy, our total synthesis of 2 was also to provide a platform for the subsequent synthesis of side chain-modified rhizoxin F analogs for SAR studies. In this context, the intermediate formed in the ring-closure reaction was also meant to serve as a central node and point of diversification for the elaboration of differently modified side chains in a single step. One possible strategy for the synthesis of rhizoxin F (2) that would meet our predefined boundary conditions is outlined in Scheme 1. This strategy envisioned the late stage elaboration of the C15-side chain from macrocyclic vinyl iodide 4* by means of Stille coupling. Vinyl iodide 4* could also serve as a versatile common intermediate for the synthesis of differently modified side chain analogs by employing alternative vinyl stannanes as building blocks. Stille coupling was to be followed by deprotection of the C13 hydroxy group and directed epoxidation of the C(11)-C(12) double bond to deliver 2; the feasibility of this latter transformation had already been demonstrated by Ohno and co-workers as part of their synthesis of rhizoxin (1), although for a differently decorated macrocycle [19].
Scheme 1.
Retrosynthesis of rhizoxin F (2) based on ring-closing olefin metathesis (RCM) of diene 5*. PG, protecting group. The suffix “*” indicates that differently protected versions of the corresponding intermediates were contemplated at the planning stage.
Out the three possible diene precursors, our RCM-based disconnection of macrocycle 4* led to diene 5* as the most promising substrate, as it presents two unencumbered monosubstituted terminal double bonds as the sites of reaction. At the same time, 5* was envisaged to be accessible by the esterification of two fragments of similar size and structural complexity, i.e., acid 6 and alcohol 7*; therefore, this approach also offered the highest level of convergency for the total synthesis. The δ-lactone-containing carboxylic acid 6 was envisioned to be accessed by a diastereoselective allylation of aldehyde 11*, followed by RCM to close the δ-lactone ring. Alcohol 7* was planned to be obtained by the substrate-controlled 1,3-anti reduction of a β-hydroxy ketone precursor, which in turn was to be furnished by stereoselective aldol reaction between known aldehyde 10 and methyl ketone 11, either under Mukaiyama [24,25] or Paterson [26] conditions. Acid 6 was envisioned to be accessed by two-carbon extension of aldehyde 8* via HWE olefination with an alkyl dialkylphosphono acetate; the former was envisaged to result from the stereoselective allylation of an aldehyde 11* with an allylsilane/allyl stannane 12* in a Hosomi-Sakurai- [27] or Keck-type [28] allylation reaction and subsequent functional group adjustment.
2.2. Synthesis of Alcohol 7*
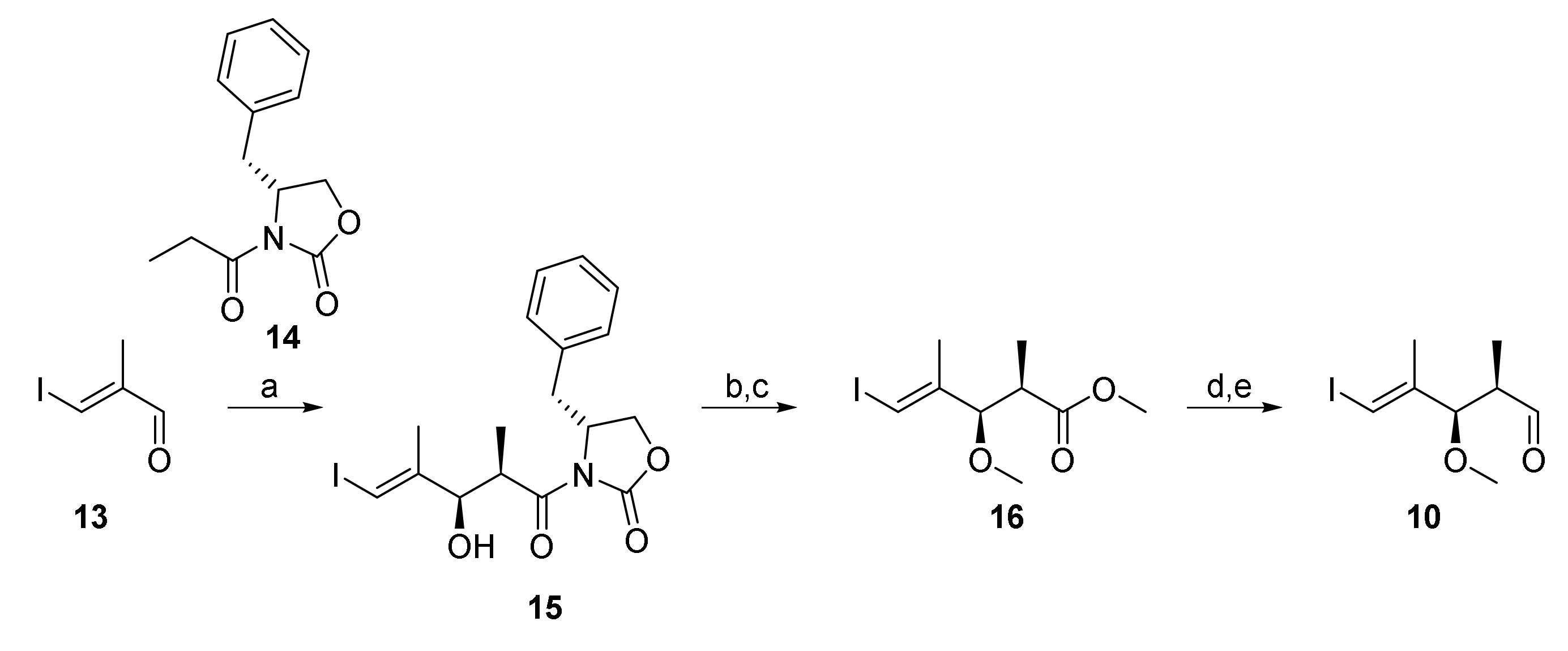
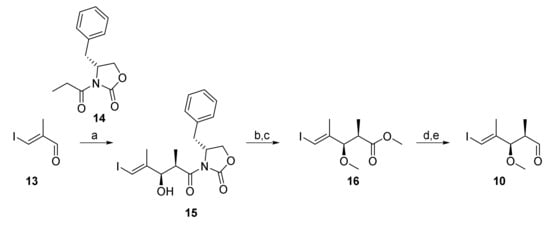
As illustrated in Scheme 2, the synthesis of aldehyde 10 as one of the building blocks for the construction of 7* was based on a high-yielding and highly stereoselective Evans aldol reaction between aldehyde 13 and N-propionyl-oxazolidinone 14 (92% yield of a 15 as a single isomer), as has been described previously by White and co-workers [29]; aldehyde 13 was obtained from diethyl methyl malonate according to Menche et al. [30]. Imide 15 was then cleanly converted into methyl ester 16 by treatment with sodium methoxide. The latter was elaborated into aldehyde 10 by methylation with NaH and MeI, followed by DIBAL reduction of the ester group (91% overall yield) and Dess-Martin periodinane (DMP) [31] oxidation of the ensuing primary alcohol. In order to prevent epimerization of 10 during chromatography, the crude aldehyde was directly used in the aldol step.
Scheme 2.
Reagents and conditions: (a) 14, nBu2BOTf, NEt3, CH2Cl2, 0 °C, then 13, −78 °C to 0 °C, 92%, single isomer; (b) NaOMe, CH2Cl2/MeOH (3:1), 0 °C, 95%; (c) NaH, MeI, THF/DMF (3:1), 0 °C, 94%; (d) DIBAL, CH2Cl2, −78 °C to 0 °C, 97%; (e) DMP, NaHCO3, 0 °C to rt. DIBAL, diisobutylaluminum hydride; DMP, Dess–Martin periodinane (1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one); Tf, trifluormethansulfonyl.
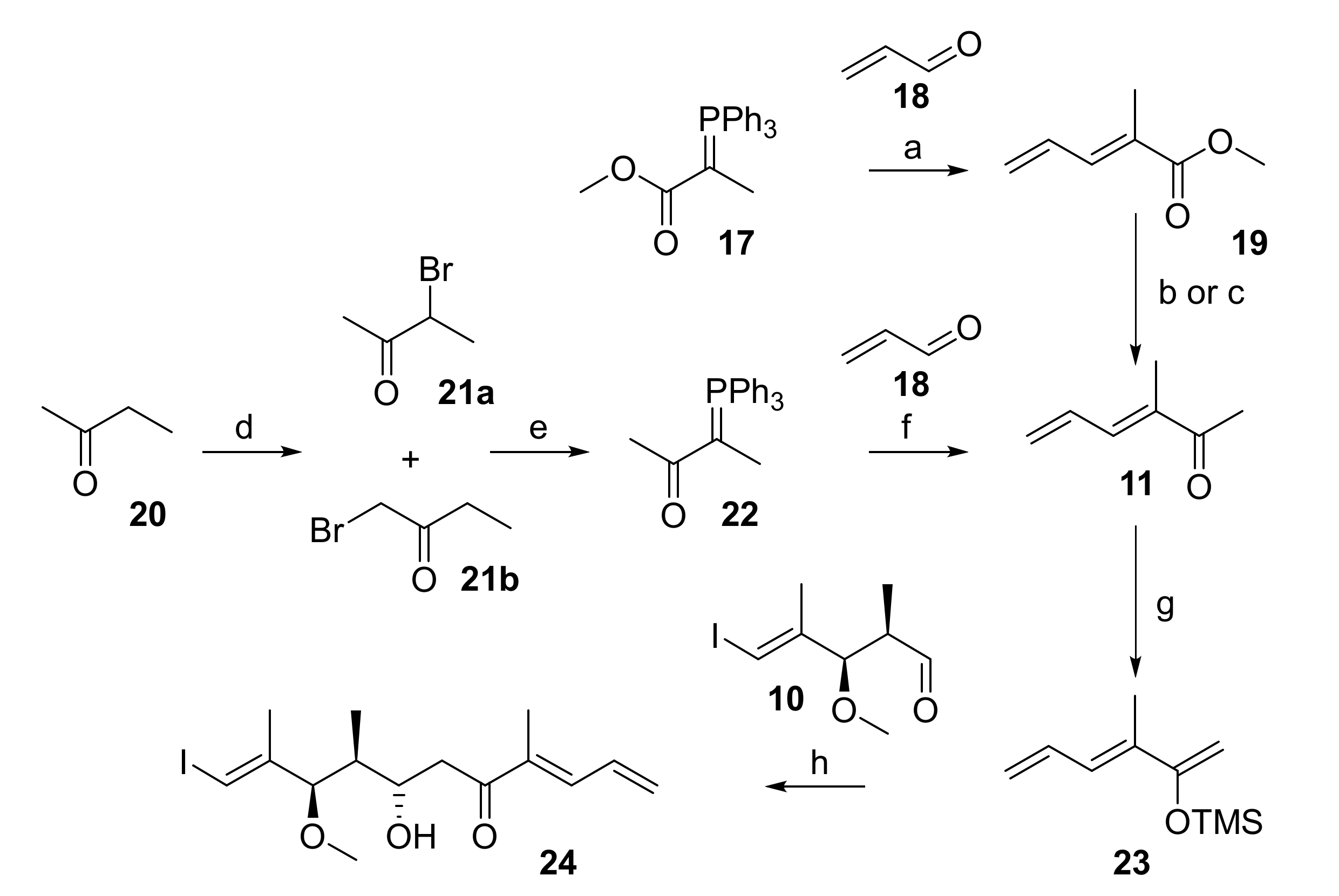
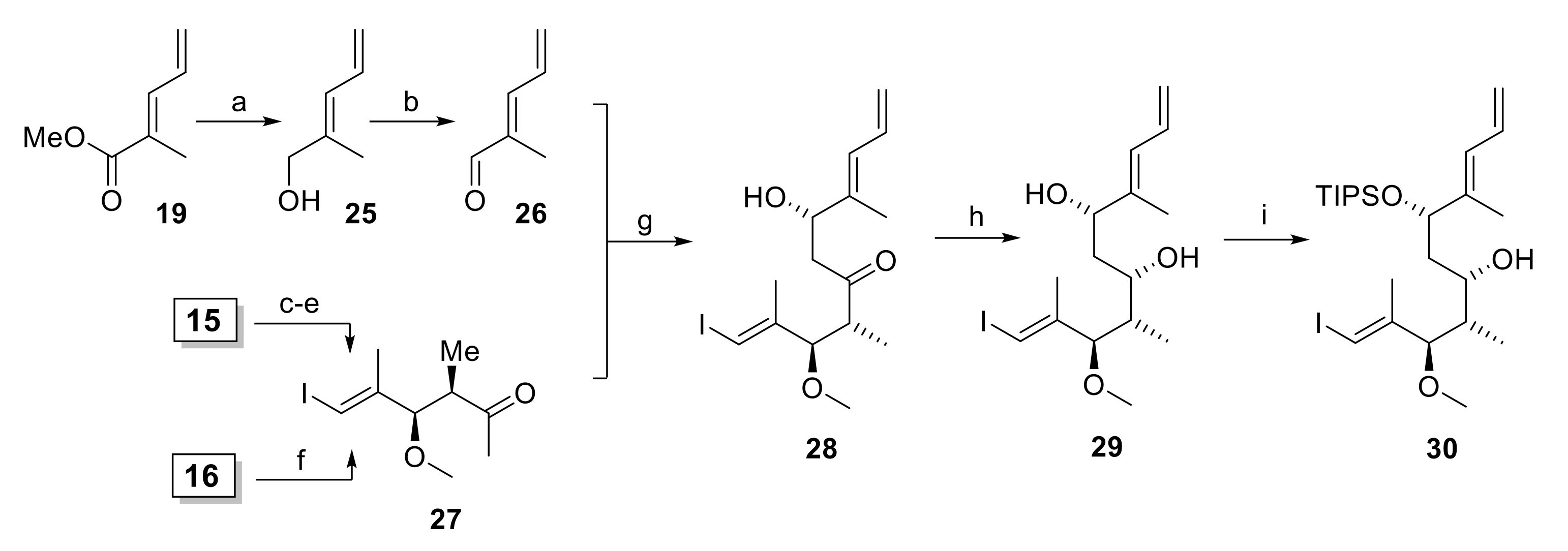
In initial experiments, methyl ketone 11 was prepared via the known ester 19 [32] (Scheme 3), which was obtained by Wittig reaction of phosphorane 17 with acrolein (18) in 70% yield. Ester 19 could be converted into 11 either by enolate trap Grignard addition or by one-pot Weinreb ketone synthesis [33]. However, for both approaches the yield of 11 was moderate at best and the reactions suffered from poor reproducibility. Alternatively, methyl ketone 11 was accessed more reliably from 2-butanone (20), which was α-brominated to yield an 11:1 mixture of bromides 21a and 21b in 47% yield (Scheme 3). Reaction of the mixture of 21a/21b with PPh3 in acetonitrile gave a crude phosphorane 22 that reacted with acrolein (18) to furnish 11 as a single isomer in 57% yield. Notably, the treatment of 21a/b with aqueous NaOH (which were the conditions used for the preparation of 17 from its phosphonium salt precursor in high yield) did not give any of the desired phosphorane 22.
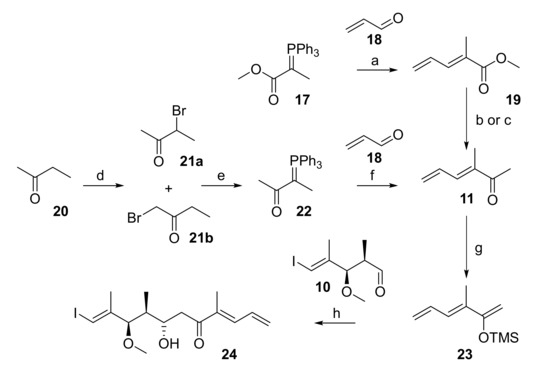
Scheme 3.
Reagents and conditions: (a) CH2Cl2, rt to rf, 70%; (b) Me(OMe)NH∙HCl, MeMgCl, THF, −15 °C to rt, 51%; (c) LiHMDS, MeMgCl, THF, −78 °C to +10 °C, 35%; (d) Br2, AcOH, H2O, 65 °C, 47%, 21a:21b = 11:1; (e) (i) PPh3, MeCN, 70 °C; (ii) NaOH, MeCN, rt, 79%; (f) CH2Cl2, rt to rf, 57%; (g) NEt3, TMSCl, NaI, CH3CN, 0 °C to 90 °C, 86%; (h) TiCl4, CH2Cl2, −78 °C, 12%, dr 4:1. LiHMDS, lithium bis(trimethylsilyl)amide; TMS, trimethylsilyl.
With aldehyde 10 and methyl ketone 11 in hand, the stage was set for the projected aldol reaction. To this end, ketone 11 was first transformed into its TMS-enol ether 23 as the necessary precursor for a Mukaiyama aldol reaction [24,25] (Scheme 3). The best conditions for the formation of 23 involved treatment of 11 with triethylamine, TMS-chloride, and sodium iodide in acetonitrile, a method developed by Cazeau et al. for the special purpose of forming TMS-enol ethers from α,β-unsaturated ketones and aldehydes [34]. In analogy to the work of Pattenden et al. [22], enol ether 23 was then submitted to reaction with aldehyde 10 in the presence of Me2AlCl; however, these conditions led to a mixture of at least three compounds, whose spectroscopic data could not be interpreted. If TiCl4 was used as a Lewis acid, the aldol product was indeed obtained, but only in 12% yield and with moderate diastereoselectivity (4:1).
In light of the disappointing findings on the Mukaiyama aldol reaction, we turned our attention to the possible construction of the C15 stereocenter (rhizoxin numbering, see Scheme 1) by a Paterson aldol reaction [26] between aldehyde 26 and methyl ketone 27 (Scheme 4). The successful Paterson aldol reaction between 27 and a more complex aldehyde comprising the C(3)–C(13) segment of rhizoxin had been reported by White and co-workers as part of their synthesis of rhizoxin D [29].
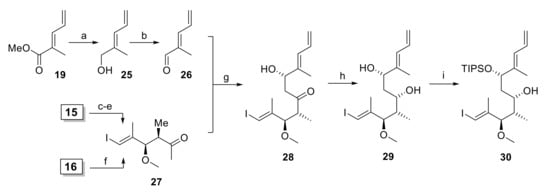
Scheme 4.
Reagents and conditions: (a) LiAlH4, Et2O, 0 °C; (b) activated MnO2, CH2Cl2, rt, 78% (2 steps); (c) Me(OMe)NH∙HCl, AlMe3, THF, 0 °C to rt, then 15, −40 °C to rt, 96%; (d) NaH, MeI, THF/DMF (3:1), 0 °C, 95%; (e) MeMgBr, THF, −20 °C to −5 °C, 93%; (f) Me(OMe)NH∙HCl, MeMgCl, THF, −10 °C to rt, 72%; (g) (i) 27, NEt3, (+)-DIPCl, CH2Cl2, −78 °C, then 26, −78 °C to −18 °C; (ii) H2O2, phosphate buffer (pH 7), MeOH, 0 °C to rt, 58%, dr 14.8:1; (h) NMe4BH(OAc)3, CH3CN/AcOH (2:1), −40 °C to rt, 92%, single isomer; (i) TIPSOTf, 2,6-lutidine, 3 Å molecular sieves, CH2Cl2, −78 °C, 91%. DIPCl, B-chlorodiisopinocampheylborane; Tf, trifluormethansulfonyl; TIPS, triisopropylsilyl.
Aldehyde 26 was readily accessible from ester 19 by reduction to the alcohol and subsequent oxidation of the latter with activated MnO2 (Scheme 4). Methyl ketone 27 could be prepared in 3 steps from aldol product 15 (cf. Scheme 2) as described by White [29] (with improved conditions for the methylation step) in 85% overall yield. Alternatively, ester 16 (cf. Scheme 2) could be converted into 27 by one-pot Weinreb amide ketone synthesis in 72% yield.
Satisfyingly, the reaction of the boron enolate of ketone 27 (formed with NEt3 and (+)-DIPCl in dichloromethane) with aldehyde 26 afforded β-hydroxy ketone 28 in 58% yield with a dr of 14.8:1. While the aldol product was inseparable from the isopinocampheol formed upon oxidative workup, the latter did not interfere with the subsequent reaction step and could then be easily removed by chromatography. Importantly, a control reaction with (−)-DIPCl proceeded without significant stereoselectivity (dr 1.3:1), thus indicating that the combination of α,β-chiral ketone 27 and (−)-DIPCl represents a mismatched case for the Paterson aldol reaction. Reduction of β-hydroxy ketone 28 with NMe4BH(OAc)3 [35] gave diol 29 in 92% yield as a single isomer (Scheme 4). While attempts at the regioselective TBS protection of the C(13)-OH group (rhizoxin numbering) returned only mixtures of regioisomers, treatment of 29 with TIPS-OTf and 2,6-lutidine at −78 °C gave silyl ether 30 in 91% yield as a single regioisomer.
2.3. Synthesis of Acid 6
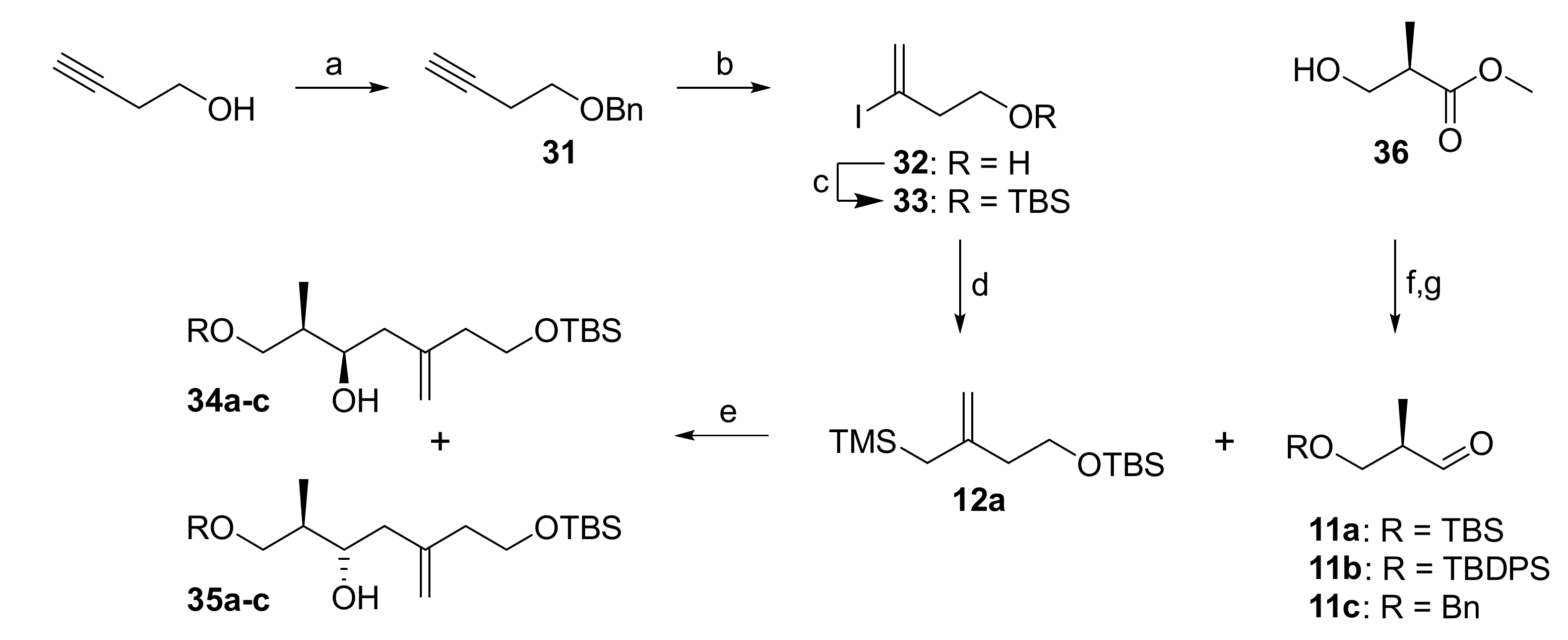
The synthesis of acid building block 6 at an early stage of our work involved exploration of the stereoselective allylation of an aldehyde 11 with allylsilane 12a in a Hosomi-Sakurai reaction [27]. As depicted in Scheme 5, the preparation of allylsilane 12a commenced with the benzylation of commercially available 3-butynol with BnBr/NaH, which delivered benzyl ether 31 in quantitative yield. Hydroiodination of 31 with in situ formed HI (from TMSCl, NaI and water) [36] in the presence of 0.6 equivalents of water at 0 °C led to concomitant loss of the benzyl group and gave the free alcohol 32 in 40% yield. These optimized hydroiodination conditions do not generate more than 0.6 equivalents of HI, thereby effectively preventing diiodination.
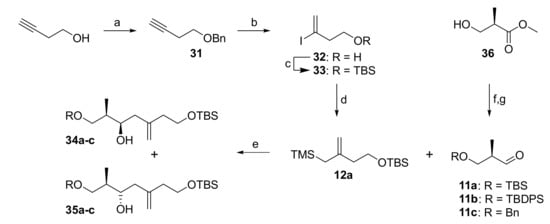
Scheme 5.
Reagents and conditions: (a) NaH, BnBr, TBAI, THF, 0 °C to rt, quant.; (b) TMSCl, NaI, H2O (0.6 equiv), MeCN, 0 °C to rt, 40%, 96% pure; (c) TBSCl, imidazole, DMF, rt, 98%; (d) TMSCH2MgCl, Pd(PPh3)4 (0.5 mol%), LiCl, Et2O, rt, 93%; (e) 34/35a (R = TBS): (i) 12a (2.1 equiv), 4 Å molecular sieves, SnCl4 (2.0 equiv), CH2Cl2, −78 °C; (ii) 11a (1.0 equiv), CH2Cl2, –78 °C, 71% (2 steps, including step g)), 34a/35a = 1.7:1; 34/35b (R = TBDPS): 11b (1.0 equiv), 12a (2.2 equiv), TiCl4 (1.1 equiv), CH2Cl2, −78 °C; 37% (2 steps, including step g)), 34b/35b = 1.74:1; 34/35c (R = Bn): 11c (1.0 equiv), 12a (2.6 equiv), TiCl4 (1.4 equiv), CH2Cl2, −78 °C, 50% (2 steps, including step g)), 34c/35c = 1:3.3; (f) a: TBSCl, imidazole, CH2Cl2, rt, 99%; b: TBDPSCl, imidazole, DMF, rt, 92%; c: benzyl 2,2,2-trichloroacetimidate, TfOH (cat.), CH2Cl2/cyclohexane (1:1), rt, 77%; (g) DIBAL (1.05 equiv), CH2Cl2, −78 °C, crude. TBAI, tetra-n-butylammonium iodide; TBS, tert-butyldimethylsilyl; TBDPS, tert-butyldiphenylsilyl; TMS, trimethylsilyl.
Hydroiodination was also possible with free butyn-3-ol, but the desired iodo alcohol 32 was only obtained in 22% isolated yield. Attempts to (re)protect 32 as a benzyl ether under basic conditions (BnBr, NaH) predominantly led to elimination and gave an inseparable mixture of the desired product and benzyl ether 31 in a combined yield of 41%. Attempts to conduct the benzylation under neutral (Dudley’s reagent [37]) or acidic conditions (benzyl trichloroacetimidate/catalytic acid [38]) resulted in complex reaction mixtures, from which the desired product could not be purified to homogeneity. In contrast, treatment of 32 with TBSCl cleanly furnished TBS-ether 33 in 98% isolated yield. Subsequent cross-coupling with TMS-methylmagnesium chloride either under Negishi [39] or Kumada [40,41] (Ni and Pd) conditions generally worked well. In particular, the use of Pd(PPh3)4 and LiCl gave excellent yields of allyl silane 12a at very low catalyst loadings (down to 0.5 mol%) even on large scale. Allylsilane 12a was found to be highly acid sensitive and prone to protodesilylation. Thus, the eluent for silica gel chromatography was buffered with 0.5% of NEt3 and deuterated benzene was employed as the NMR solvent instead of chloroform. These conditions effectively prevented degradation of 12a during purification and analysis.
The Hosomi-Sakurai reaction between 12a and aldehydes 11 was then investigated for a range of Lewis acids. As expected, the non-coordinating aldehydes 11a and 11b gave the desired Felkin-Anh (syn) products 34a or 34b, respectively, preferentially, but yields were generally low to moderate and even in the most favorable case (aldehyde 11a in combination with BF3•Et2O) the dr did not exceed 4:1. As expected, benzyl-protected aldehyde 11c reacted with chelation control, thus delivering the anti product 35c in excess.
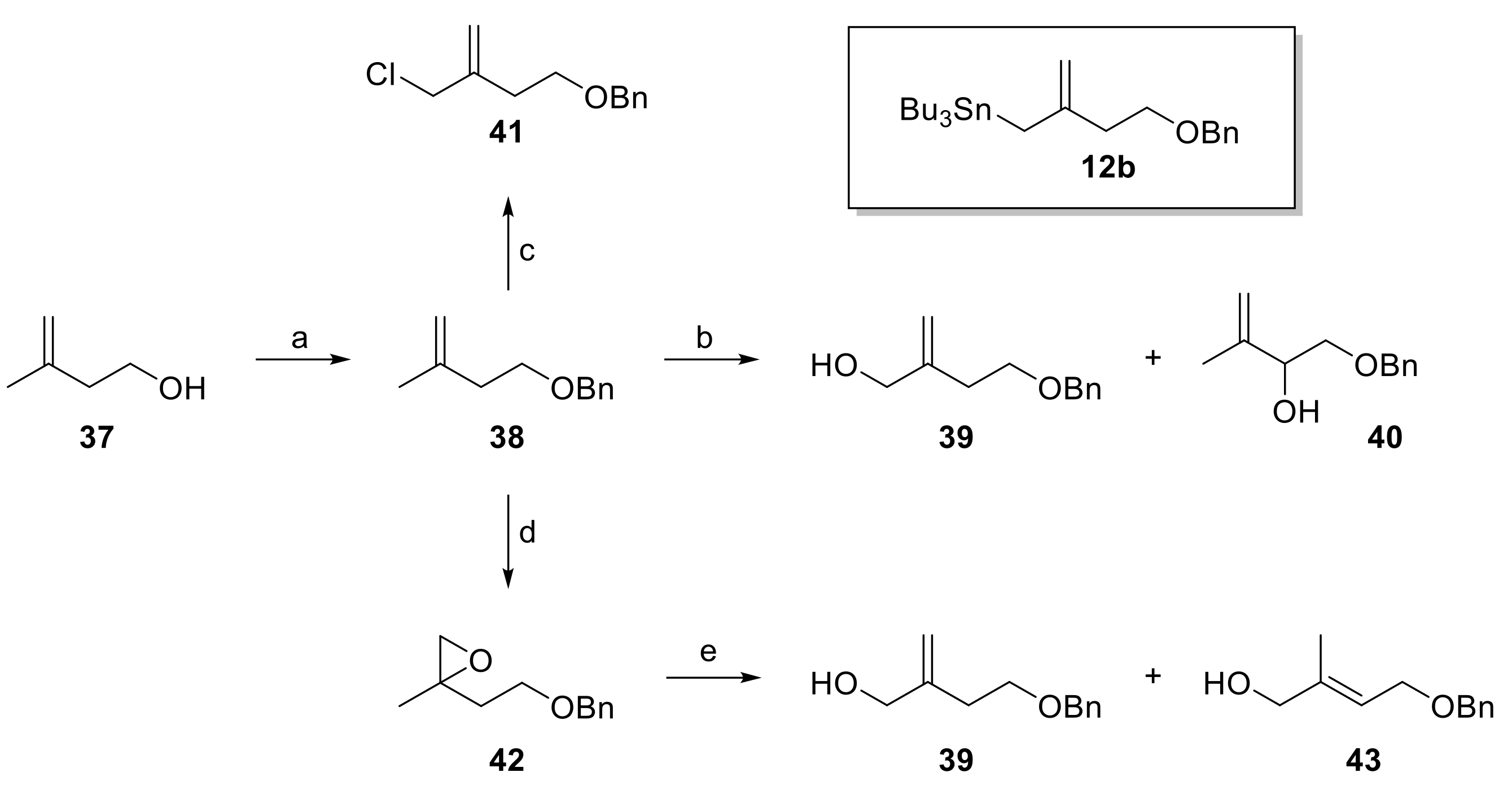
Rather than trying to improve the selectivity of the Sakurai reaction by using chiral Lewis acid as, for example, developed by Yamamoto [42], we considered it more promising to assess the stereoselective Keck-type allylation [28] of aldehydes 11 with allyl stannane 12b. Different precursors were envisioned for the preparation of 12b by reaction with tributyltin chloride, including benzyl ether 38 (via the derived anion), allylic alcohol 39 (e.g., via the corresponding mesylate) or allylic chloride 41; in principle, 38 should also serve as a precursor for 39 and 41 (Scheme 6). Benzyl ether 38 was readily accessible from 2-methylbut-1-en-4-ol (37) in excellent yield (97%) by benzylation with benzyl bromide under basic conditions. Unfortunately, however, deprotonation of 38 with nBuLi/TMEDA [43] or Schlosser’s base [44,45] followed by quenching of the resulting anion with tributyltin chloride failed to yield allyl stannane 12b. Allylic chlorination [46] of 38 was non-selective and the desired isomer 41 could not be separated from isomeric impurities. In contrast, Riley oxidation [47] furnished a separable mixture of allylic alcohols 39 and 40, from which the desired isomer 39 could isolated in 23% yield; 40 was obtained in 28% yield.
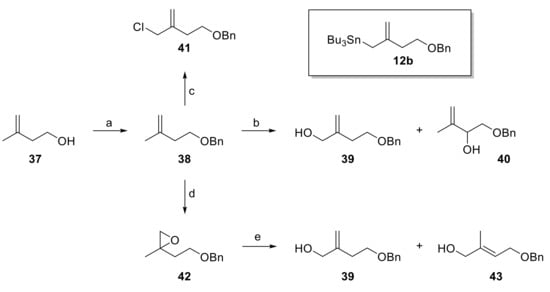
Scheme 6.
Reagents and conditions: (a) NaH, BnBr, TBAI, THF, 0 °C to rt, 97%; (b) (i) SeO2 (10 mol%), tBuOOH, CH2Cl2, rt; (ii) NaBH4, MeOH, 0 °C, 39: 23%, 38% brsm, 40: 28%; (c) CeCl3·7H2O, NaClO, CH2Cl2/H2O (1:1), rt, 58% + inseparable regioisomers; (d) mCPBA, CHCl3, rt, 95%; (e) TMPAlEt2, toluene, 0 °C, 39: 48%, 43: 16%. TBAI, tetra-n-butylammonium iodide; TMP = 2,2,6,6-tetramethylpiperidine. Brsm, based on recovered starting material.
As an alternative to direct allylic hydroxylation, we also explored the regioselective β-elimination from gem-disubstituted epoxide 42 by means of a sterically hindered aluminium amide base, following an approach developed by Brückner [48]. However, while the treatment of the TBS-protected variant of 42 with TMPAlEt2 has been reported to yield the allylic alcohol corresponding to 39 in high yield as a single isomer [48], epoxide 42 furnished a ~3:1 mixture of allylic alcohols 39 and 43, which were isolated in 48% and 16% yield, respectively. Thus, while allylic alcohol 39 could be accessed from 38 as a single isomer, the yield was far from satisfactory.
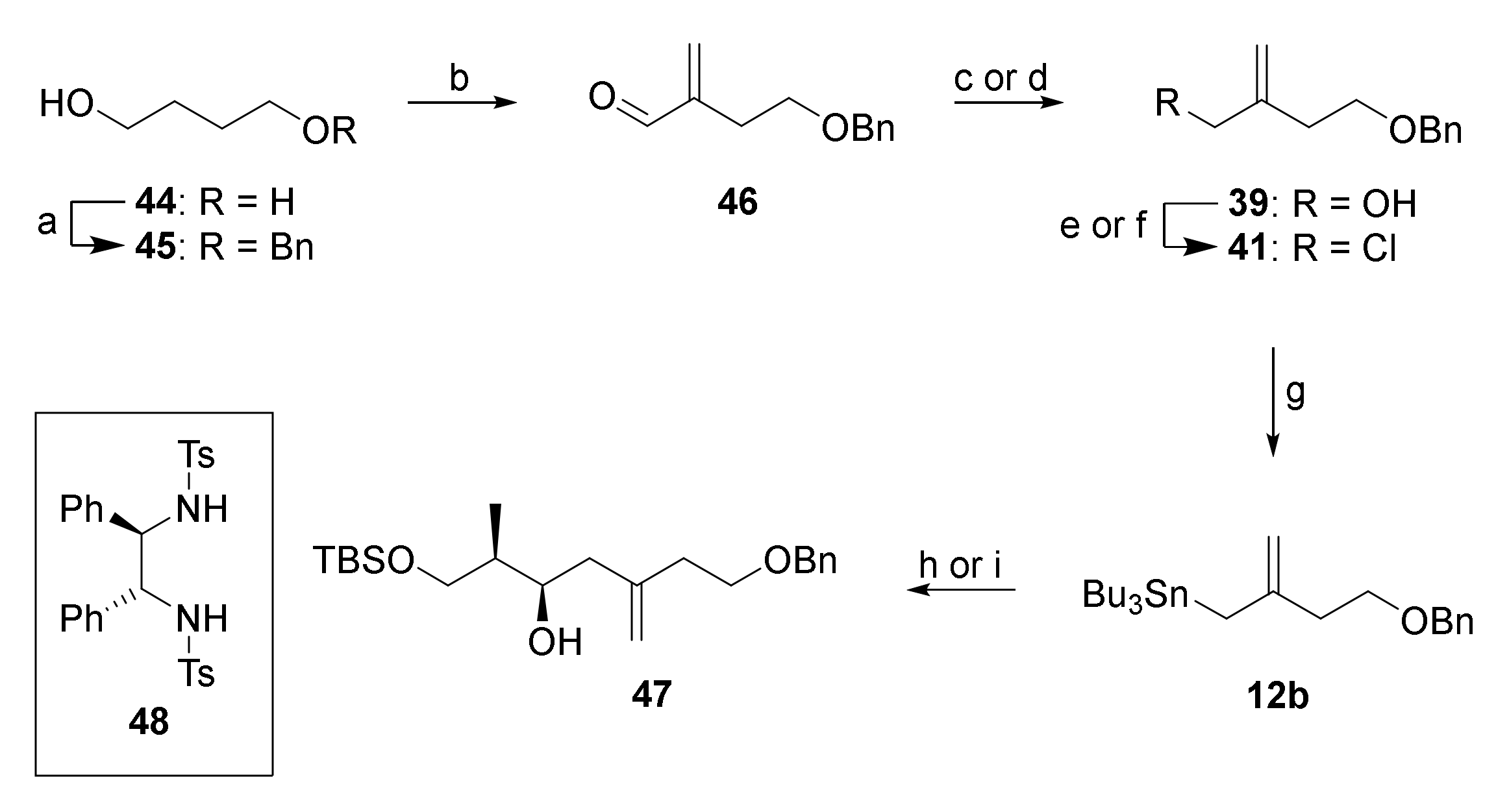
In light of the difficulties to access 39 directly from 38, an alternative route was developed from 1,4-butandiol (44) which delivered 39 with higher overall efficiency and without the need for isomer separation. As depicted in Scheme 7, monobenzylation of 44 followed by Swern oxidation furnished an aldehyde that was α-methylenated in situ (CH2NMe2Cl, DBU), employing a modification of a procedure originally developed by Ogasawara and co-workers [49]. The resulting α-methylene aldehyde 46 was obtained in 86% yield. Reduction of 46 with LiAlH4 gave alcohol 39, which was submitted to Appel reaction to furnish allylic chloride 41. In initial experiments, 46 was converted into 41 by NaBH4 reduction followed by Finkelstein reaction, but this approach proved to be inconvenient on larger scale, due to the use of DMF and EtOH in large quantities.
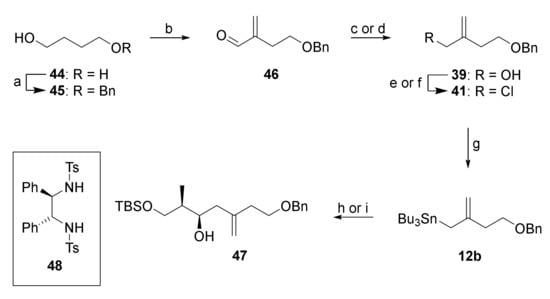
Scheme 7.
Reagents and conditions: (a) NaH, BnBr, THF, 0 °C to rt, 15 h, 97%; (b) (COCl)2, DMSO, NEt3, CH2Cl2, −78 °C to 0 °C, 2 h, then CH2NMe2Cl, DBU, rt, 22 h, 86%; (c) NaBH4, EtOH, 0 °C, 1 h, 94%; (d) LiAlH4, THF, 0 °C, 96%; (e) MsCl, 2,6-lutidine, LiCl, DMF, 0 °C to rt, 5 h, 88%; (f) CCl4, PPh3, MeCN, rt, 2 h, 95%; (g) Mg, Bu3SnCl, THF, ultrasound, 0 °C, 2h, then dibromoethane (cat.), refl., then rt, overnight, quant., 80% purity; (h) 11a, BF3·OEt2, CH2Cl2, −78 °C, 1 h, 65% (dr 1.9:1); (i) (i) BBr3, ligand 48, CH2Cl2, 0 °C to rt, 1 h, (ii) 12b, 0 °C to rt, 16 h, (iii) 11a, −78 °C, 2 h, 74% (2 steps) (dr 10:1). DBU, 1,8-diazabicyclo [5.4.0]undec-7-ene; Ms, mesyl.
Conversion of chloride 41 into stannane 12b was then achieved by Barbier-type reaction of 41, Mg turnings, and tributyltin chloride with ultrasound irradiation (quantitative yield, 80% purity by 1H-NMR analysis) [50]. The purity of the material obtained after simple extractive work-up was sufficient for the next step.
The BF3•OEt2-mediated allylation of aldehyde 11a with stannane 12b gave alcohol 47 only with poor diastereoselectivity (dr 1.9:1). However, transmetalation of 12b with bromoborane complex (prepared from BBr3 and bis-tosylated (R,R)-1,2-diphenylethylenediamine ((R,R)-DPEN) (48) [51] under conditions elaborated by Williams et al. [52] and subsequent reaction with 11a at −78 °C furnished secondary alcohol 47 in very good yield and with high diastereoselectivity (74% over 2 steps, dr 10:1, 94% recovery of 48). The configuration of the newly established stereocenter in 47 was confirmed by Mosher ester analysis [53].
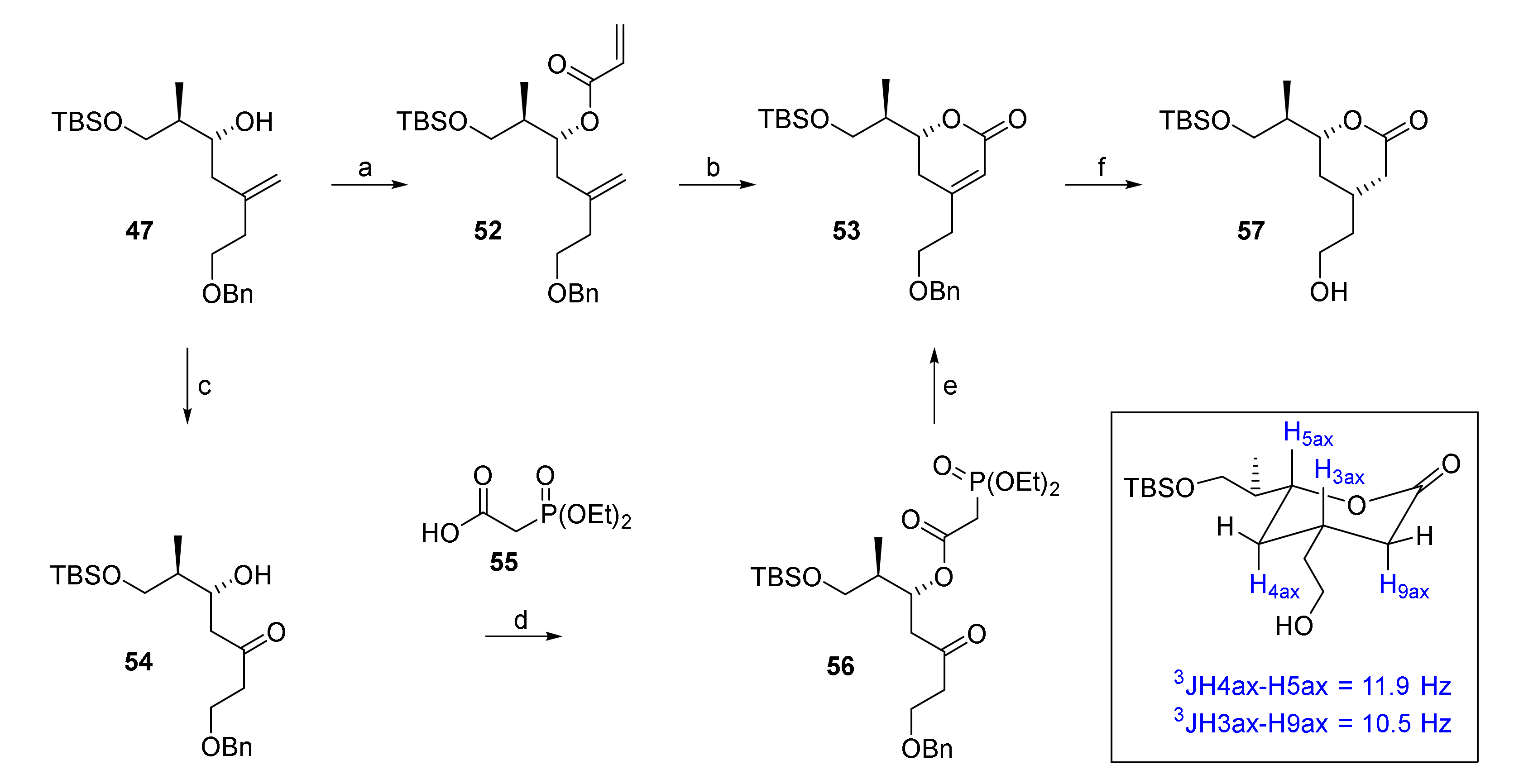
Esterification of 47 with acryloyl chloride afforded diene 52 in high yield (85%) as a single isomer (Scheme 8). RCM of 52 with 10 mol% of Hoveyda-Grubbs 2nd generation catalyst in refluxing dichloroethane delivered dihydropyrone 53 in an excellent 89% yield on small scale (44 mg of 52). However, the reaction was found to be rather unreliable on multigram scale, where it frequently did not proceed beyond 50% conversion, thus requiring multiple recyclings of the starting diene to achieve acceptable yields. Moreover, dimerization via the more reactive monosubstituted double bond started to compete with ring formation over time. Although this pathway could be partially suppressed by using higher dilution, this requirement imposed restrictions on scale-up. Therefore, an alternative three-step synthesis of 53 from 47 was elaborated that was based on an intramolecular HWE reaction for 6-membered ring formation, similar to what had been reported by Regan and co-workers as part of their synthesis of the C(1)–C(9) fragment of rhizoxin [54].
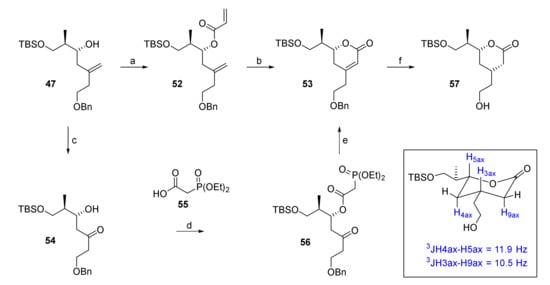
Scheme 8.
Reagents and conditions: (a) Acryloyl chloride, DIPEA, CH2Cl2, −50 °C, 85%; (b) Hoveyda-Grubbs 2nd generation catalyst (14 mol%), DCE, reflux, 89%; (c) OsO4 (cat.), NaIO4, 2,6-lutidine, dioxane/water, rt, 94%; (d) 55, CME-CDI, DMAP, CH2Cl2, 0 °C to rt, 93%; (e) NaH, THF, 0 °C, 83%; (f) H2 (9 bar), Pd(OH)2/C (5 mol%), EtOAc, rt, 98%. CDE-CDI = N-cyclohexyl-N′-(β-[N-methyl-morpholino]ethyl) carbodiimide p-toluenesulfonate; DMAP, 4-(dimethylamino)pyridine.
Thus, alkene 47 was subjected to Lemieux-Johnson oxidation, to furnish β-hydroxy ketone 54 in excellent 94% yield. In comparison, ozonolysis led to lower yields (76–84%) and the formation of inseparable side products (~5–10%). Steglich esterification of 54 with diethylphosphonoacetic acid (55) yielded phosphono ester 56, which underwent intramolecular HWE reaction upon treatment with NaH, to yield the desired dihydropyrone 53 in 83% yield. The stereoselective hydrogenation of the double bond in 53 with Pearlman’s catalyst, according to the procedure reported by Regan [54] proceeded with concomitant cleavage of the benzyl ether moiety, thus furnishing lactone 57 as a single isomer in 98% yield. The large trans-diaxial coupling constants of 11.9 Hz and 10.5 Hz in the 1H-NMR spectrum of 57 (Scheme 8) confirmed the cis-substitution of the δ-lactone ring.
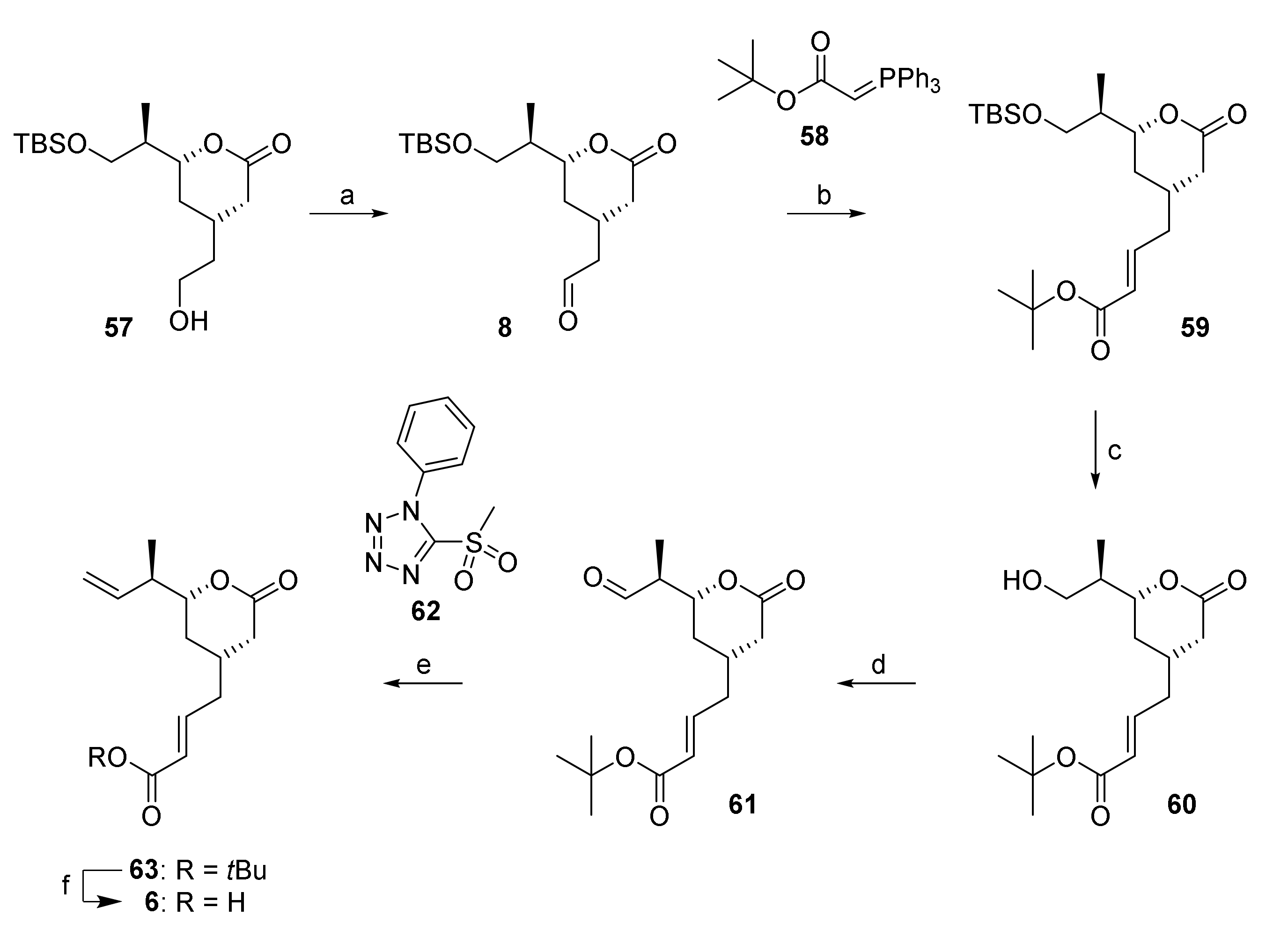
The further elaboration of lactone 57 into acid building block 6 in a first stage required a two-carbon extension at the free hydroxy end that was achieved by Swern oxidation, to yield aldehyde 8, followed by Wittig olefination with stabilized ylide 58 (Scheme 9). Ester 59 was obtained as a single isomer in 79% overall yield from 57. In contrast to the Wittig reaction with ylide 58, the HWE reaction of aldehyde 8 with the tbutyl diethylphosphonoacetate gave ester 59 in much lower and highly variable yields. After installation of the carboxy functionality, completion of the carbon framework of acid building block 6 required the elaboration of the terminal double bond at the non-carboxy end of the structure. To this end, the TBS-ether moiety in 59 was cleaved with buffered (AcOH) TBAF, to afford alcohol 60 in excellent yield (provided that the acid present was not neutralized with NaHCO3 during work-up). After Dess-Martin oxidation [31], aldehyde 61 was isolated as a crude product after simple extractive work-up, as significant racemization on silica gel precluded chromatographic purification. Surprisingly, the conversion of the carbonyl group into the terminal double bond proved to be highly challenging. Thus, Wittig methylenation with MePPh3Br/KHMDS was accompanied by epimerization of the α-stereocenter and the desired olefin was only obtained in 8% yield. A whole series of alternative methods for double bond formation were then investigated, including Tebbe [55], Takai [56], Oshima-Lombardo [57], Julia [58], as well as Rh- [59] and Cu-catalyzed [60] methylenations; of these, only the Julia olefination of 61 with the phenyltetrazol sulfone 62 gave the desired olefin 63 in a preparatively useful yield of 38% (based on alcohol 60); but the compound could only be isolated as a mixture with residual sulfone 62. The latter was readily removed after tbutyl ester cleavage with TFA, which gave the desired acid 6 in quantitative yield.
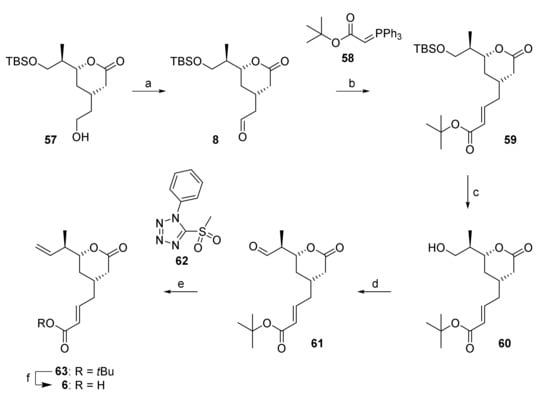
Scheme 9.
Reagents and conditions: (a) (COCl)2, DMSO, NEt3, CH2Cl2, −78 °C, 94%; (b) 58, CH2Cl2, 0 °C to rt, 84%; (c) TBAF, AcOH, CH2Cl2, 0 °C to rt, 96%; (d) DMP, CH2Cl2, 0 °C to rt; (e) NaHMDS, PT-sulfone 62, THF, −78 °C to rt, 38% (2 steps); (f) TFA/CH2Cl2 (ca. 14 equiv TFA), rt, quant. DMP, Dess-Martin periodinane (1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one); NaHMDS, sodium bis(trimethylsilyl)amide; PT, phenyltetrazole; TFA, trifluoroacetic acid.
2.4. Building Block Assembly and Attempted Ring-Closing Olefin Metathesis
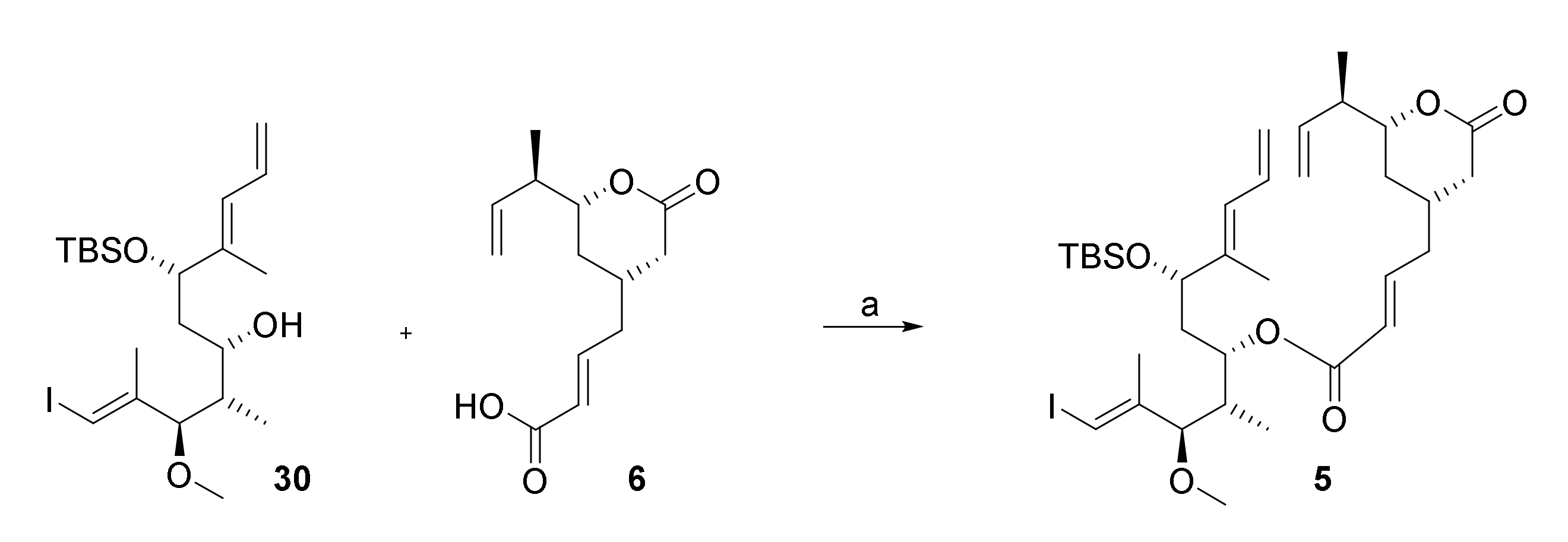
The esterification of acid 6 with alcohol 30 was investigated with a number of different coupling agents, including EDCI/DMAP; 2,4,6-trichlorobenzoyl chloride (TCBC)/NEt3/DMAP, 2-methyl-6-nitrobenzoic anhydride (MNBA)/NEt3/DMAP, and N-cyclohexyl-N′-(β-[N-methyl-morpholino]ethyl) carbodiimide p-toluenesulfonate (CME-CDI); however, except for the use of CDI-DME, none of the desired ester 5 could be isolated (Scheme 10). In general, alcohol 30 could be recovered from the reaction mixtures in high yield (>80%), while acid 6 completely decomposed. CME-CDI gave the desired ester in a yield of 14%, which, obviously, was unacceptably low at this stage of the synthesis (Scheme 10).
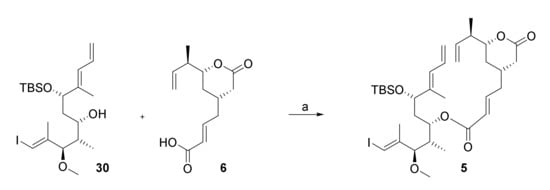
Scheme 10.
Reagents and conditions: (i) 6, CME-CDI, 0 °C, CH2Cl2, molecular sieves 4 Å; (ii) 30, 0 °C, then DMAP, rt; (iii) 0 °C, 60 h, 14% (72% 30 recovered). CME-CDI, N-cyclohexyl-N′-(β-[N-methyl-morpholino]ethyl) carbodiimide p-toluenesulfonate; DMAP, 4-(dimethylamino)pyridine.
At the same time, there was ample evidence from previous total syntheses of rhizoxins that the esterification of the C(15) hydroxy group in different rhizoxin precursors with dialkylphosphonoacetic acids was unproblematic. This observation immediately suggested that the projected RCM precursor 5 should be accessible from existing advanced intermediates that we had prepared at this point with very limited additional effort; more specifically, alcohol 30 was to be esterified with diethylphosphonoacetic acid (55) and the phosphono ester would then be submitted to HWE reaction with aldehyde 67 (Scheme 11). The latter would be obtained from alcohol intermediate 57 (cf. Scheme 9) by orthogonal protection of the primary hydroxy group, selective cleavage of the TBS-ether, and elaboration of the free hydroxy group into the desired double bond.
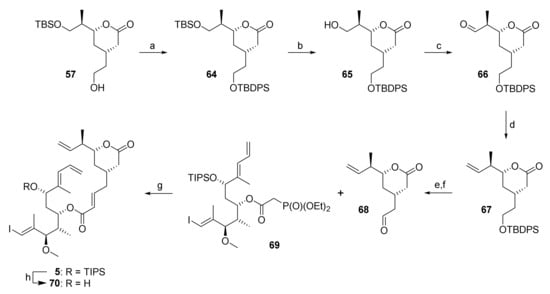
Scheme 11.
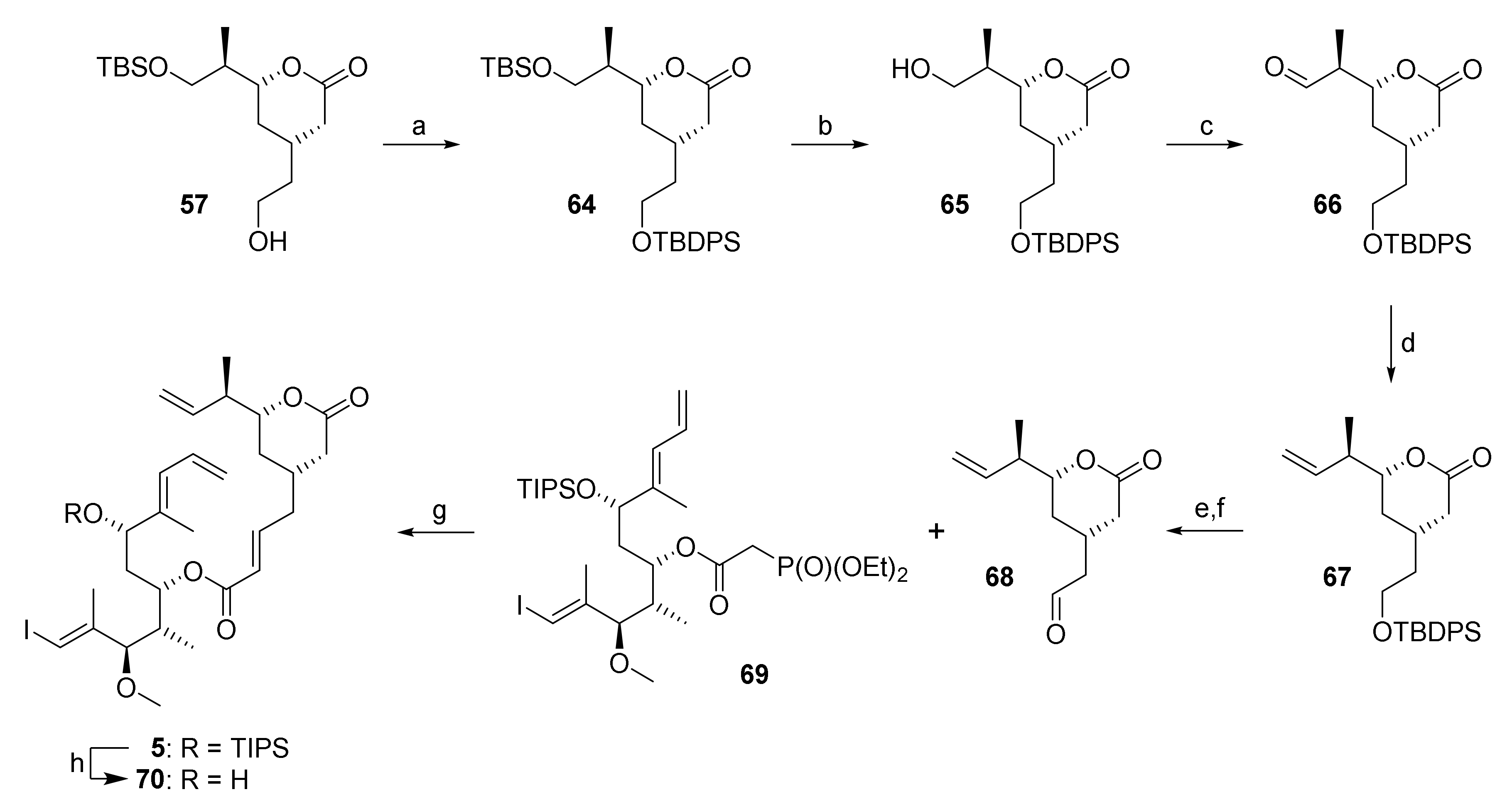
Reagents and conditions: (a) TBDPSCl, imidazole, DMF, rt, 95%; (b) NaIO4, THF/water (4:1), rt, 82%; (c) DMP, CH2Cl2, 0 °C to rt; (d) NaHMDS, PT-sulfone 62, THF, −78 °C to rt, 65% (2 steps); (e) TBAF, AcOH, THF, 0 °C to rt, 87%, (f) (COCl)2, DMSO, NEt3, CH2Cl2, −78 °C, 95%; (g) DBU, LiCl, CH3CN, 0 °C, 85%; (h) HF•py, py, THF, 0 °C to rt, 86%. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DMP, Dess–Martin periodinane (1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one); NaHMDS. sodium bis(trimethylsilyl)amide; PT, phenyltetrazole; TBAF, tetra-n-butylammonium fluoride; TBDPS, tert-butyldiphenylsilyl.
To implement this revised strategy, alcohol 57 was converted into TBDPS-ether 64 followed by selective cleavage of the TBS-ether moiety with an excess of NaIO4 in aqueous THF [61] to afford alcohol 65 in 78% overall yield (based on 57) (Scheme 11). Subsequent Dess-Martin oxidation gave aldehyde 66 in low yields if purified by column chromatography, in contrast to what had been reported for a closely related aldehyde (bearing a TIPS- in place of a TBDPS-ether group) by Pattenden and co-workers [22]. The aldehyde was thus used crude in the subsequent Julia olefination with PT-sulfone 62, which delivered the desired olefin 67 in 65% overall yield from 65. TBAF-mediated TBDPS deprotection followed by Swern oxidation then furnished aldehyde 68; the latter underwent smooth HWE olefination with phosphono ester 69 (obtained by CEM-CDI-mediated esterification of 30 with diethylphosphonoacetic acid (55)) under Masamune-Roush conditions [62] to provide the desired RCM substrate 5 in 79% overall yield from 67.
With pentaene 5 in hand, the stage was set for the assessment of the key ring-closing olefin metathesis reaction. To this end, a number of ruthenium catalysts was assessed, including the commonly used 1st and 2nd generation Grubbs and Hoveyda-Grubbs catalysts, but also the less commonly employed Grubbs 3 and Piers-Grubbs2catalysts [63], as well as the slowly initiating pyridine-containing complex (sIMes)(Cl)2Ru(CH(CH2)2-C,N-2-C5H4N) [64] (see Table S1). Unfortunately, whenever the starting material was consumed, it was converted into an array of products. While the desired macrocycle 4 was sometimes detectable by ESI-MS, it could never be isolated.
In order to alter the properties of the RCM-substrate, the TIPS protecting group was removed from 5 by treatment with HF•py, which gave alcohol 70 in high yield (86%). Notably, if the deprotection was carried out with TBAF, 5 was mainly decomposed and the yield of 70 was poor (27%). To our disappointment, however, all attempts at ring-closing olefin metathesis with 70 were also unsuccessful under a set of standard conditions (see Table S2). As for TIPS-ether 5, the material was either decomposed to numerous unidentified products or remained unchanged.
As the original, RCM-based synthetic plan could not be implemented, we needed to assess possible alternatives for ring-closure (excepting the commonly employed intramolecular HWE olefination at C(2)−C(3); vide supra) that would allow us to capitalize on the work expended at this point to the greatest extent possible. In this context, the concept of relay ring-closing olefin metathesis (RRCM) as developed by Hoye and co-workers presented itself as a plausible and attractive approach [65]. RRCM broadens the scope of RCM to substrates that are either sterically hindered or electronically deactivated by attaching a five-carbon tether with an easily accessible terminal double bond. The latter is intended to direct the metathesis catalyst to the site of choice via a favorable loss of cyclopentene. Our first choice was to attach the tether to the diene moiety of 5, as this part of the substrate was presumed to display low reactivity towards metathesis catalysts.
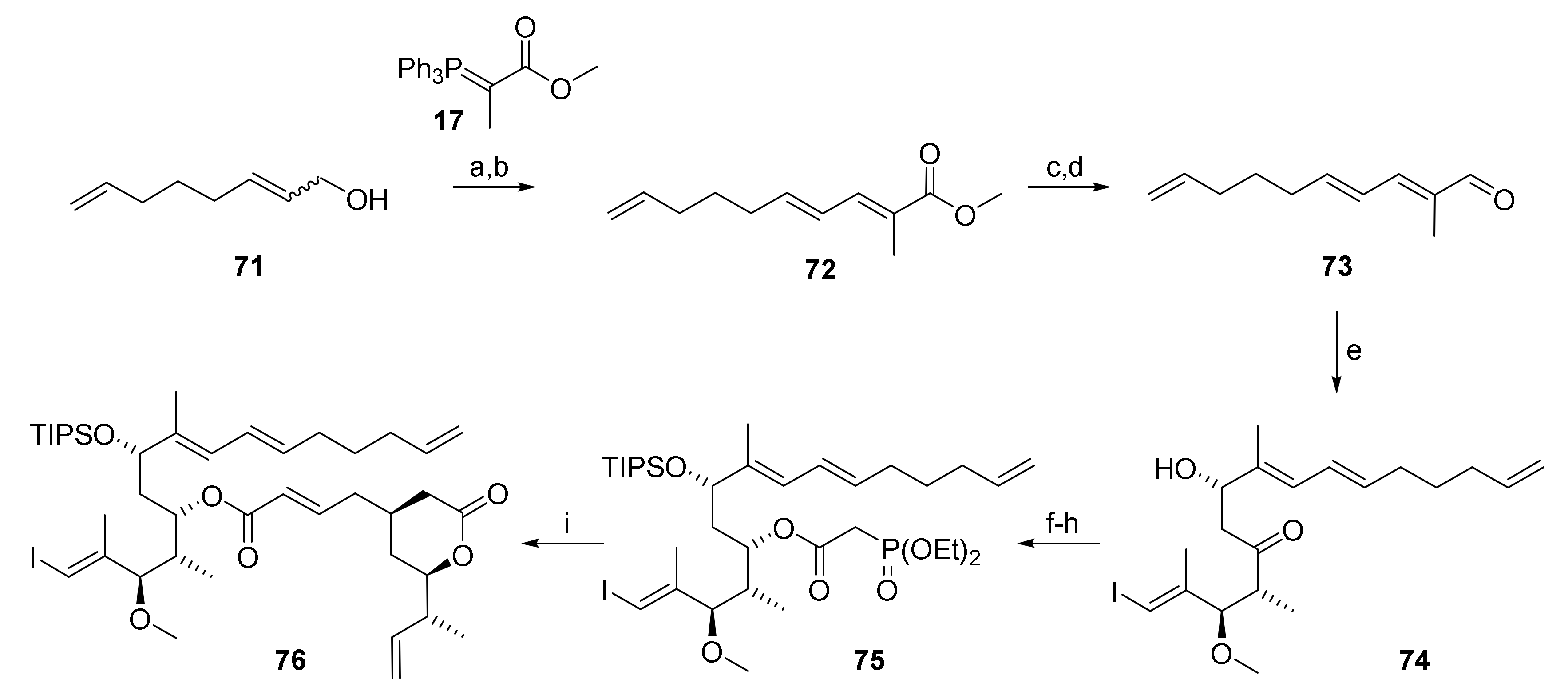
The requisite substrate, substrate 76, was prepared according to the same Paterson aldol-based strategy that had led to ene-diene 5 (vide supra) (Scheme 12). Thus, departing from a mixture of cis- and trans 2,7-octadienol (71), aldehyde 73 was obtained by PCC oxidation, Wittig olefination of the ensuing trans/trans aldehyde (obtained in 60% yield) with phosphonium ylide 17, reduction of the resulting unsaturated ester 72 with LiAlH4 and oxidation of the ensuing primary alcohol with activated MnO2. Aldehyde 73 was obtained in 37% overall yield for the 4-step sequence from 71. Subsequent Paterson aldol reaction of 73 with the boron enolate derived from ketone 27 gave β-hydroxy ketone 74 in 6% yield, although with lower selectivity (dr 7.2:1) than for reaction of 27 aldehyde 26 (dr 14.8:1, vide supra). Reduction of 74 followed by regioselective TIPS protection of the resulting diol gave 75; esterification of the latter with phosphonoacetic acid (55) and, finally, HWE reaction of the phosphono ester with aldehyde 68 uneventfully delivered relay substrate 76 in 51% overall yield under the conditions elaborated previously.
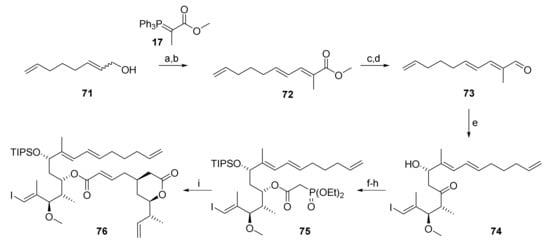
Scheme 12.
Reagents and conditions: (a) PCC, celite, CH2Cl2, 82%; (b) 17, CH2Cl2, rf, 60% trans only, plus 33% trans:cis 4:1; (c) LiAlH4, Et2O, 0 °C, 94%; (d) activated MnO2, 3 Å molecular sieves, CH2Cl2, rt, 85%; (e) (i) 27, (+)-DIPCl, NEt3, CH2Cl2, −78 °C, then 73, −78 °C to −20 °C; (ii) H2O2, phosphate buffer (pH7), MeOH, 0 °C to rt, 68%, dr 7.2:1; (f) NMe4BH(OAc)3, CH3CN/AcOH (2:1), −40 °C to rt, 86%, dr 7.8:1 (from aldol); (g) TIPSOTf, 2,6-lutidine, 3 Å sieves, CH2Cl2, −78 °C, 85%; (h) diethylphosphonoacetic acid (55), CME-CDI, DMAP, 3 Å sieves, CH2Cl2, 0 °C to rt, 82%; (i) 68, DBU, LiCl, CH3CN, 0 °C to rt, 85%. CME-CDI, N-cyclohexyl-N′-(β-[N-methyl-morpholino]ethyl) carbodiimide p-toluenesulfonate; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DIPCl, B-chlorodiisopinocampheylborane; TIPS, triisopropylsilyl.
Various conditions were then screened to effect macrocyclization. Unfortunately, however, macrocycle 4 was never obtained; instead, the tether was cleaved within seconds in an intermolecular reaction, to produce 5, which did not undergo RCM, as had been found previously. The formation of 5 by tether cleavage from 76 was observed at concentrations as low as 1.2 μM.
In addition to the conditions for macrocyclization that had been investigated in previous screenings (see Tables S1 and S2), experiments were also conducted where 76 was added slowly to a refluxing solution of the catalyst via a syringe pump, a procedure that had been reported by Porco and co-workers to suppress unproductive relay cleavage [66]. However, this method proved to be unsuccessful with 76. Likewise, microwave-assisted heating according to Robinson et al. [67] or the use of CuI as a phosphine scavenger [68], did not effect RRCM.
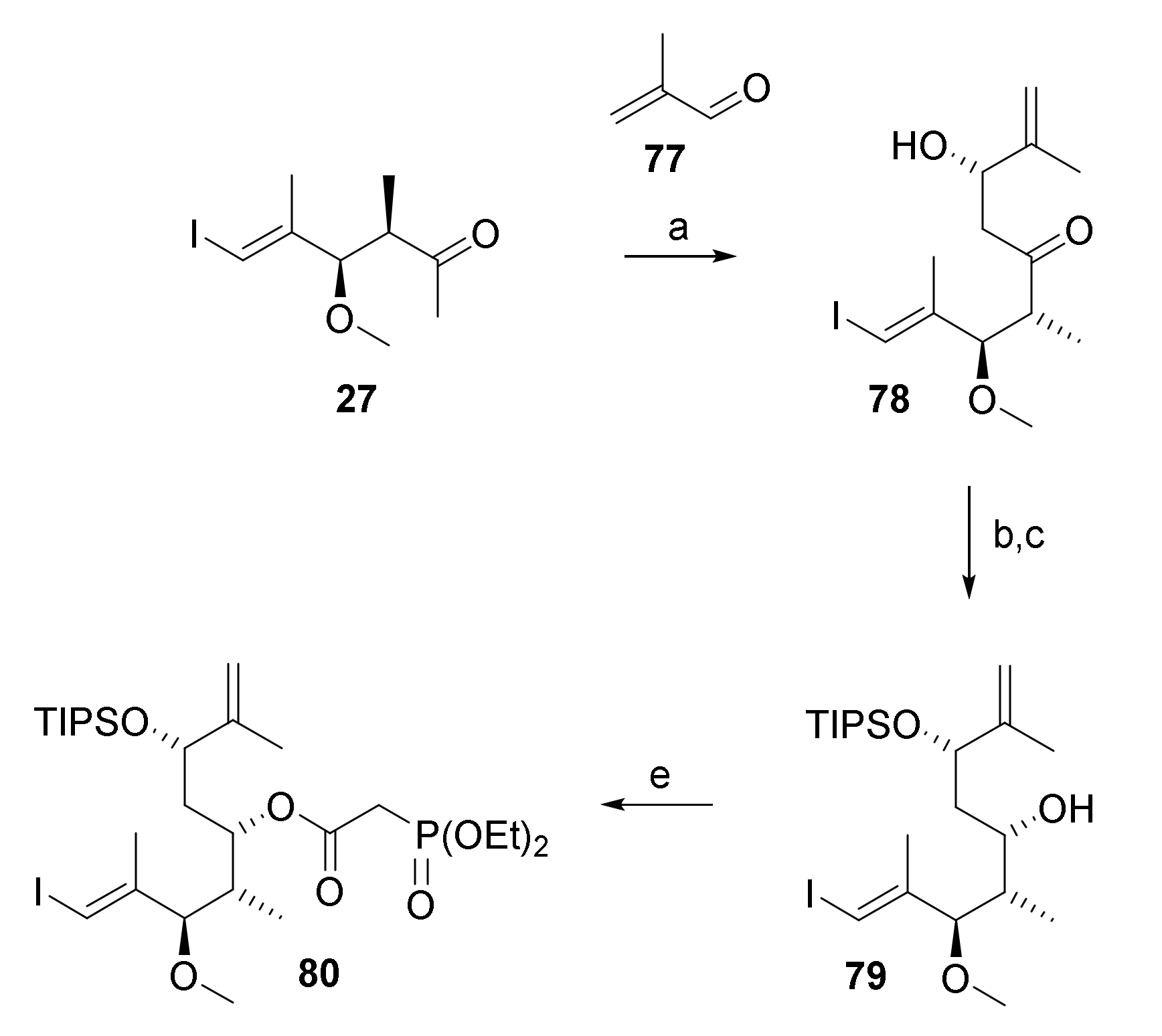
The resistance of substrate 76 towards RRCM led us to consider shifting the site of ring-closure from C(9)/C(10) to C(11)/C(12) as a possible alternative approach towards macrocycle 4. The requisite RRCM substrate was prepared based on the same overall strategy that had been followed for the synthesis of 5 and 76. Thus, phosphono ester 80 (Scheme 13) was obtained from ketone 27 via Paterson aldol reaction with methacrolein (77), stereoselective reduction of the ensuing β-hydroxy ketone 78, regioselective TIPS protection of the resulting diol product and esterification of the monoprotected diol 79 with diethylphosphono acetic acid (55) in 49% overall yield.
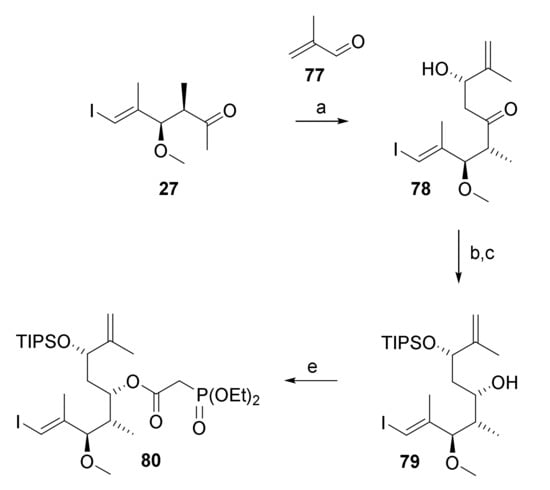
Scheme 13.
Reagents and conditions: (i) (+)-DIPCl, NEt3, CH2Cl2, −78 °C, then 77, −78 °C to −20 °C; (ii) H2O2, phosphate buffer (pH7), MeOH, 0 °C to rt, 67%, dr 19.6:1; (b) NMe4BH(OAc)3, CH3CN/AcOH (1:1), −40 °C to −18°C to rt, 95%; (c) TIPSOTf, 2,6-lutidine, 3 Å molecular sieves, CH2Cl2, −78 °C, 97%; d) diethylphosphonoacetic acid (55), CME-CDI, DMAP, 3 Å molecular sieves, CH2Cl2, 0 °C to rt, 81%. CME-CDI, N-cyclohexyl-N′-(β-[N-methyl-morpholino]ethyl) carbodiimide p-toluenesulfonate; DMAP, 4-(dimethylamino)pyridine, DIPCl, B-chlorodiisopinocampheylborane; TIPS, triisopropylsilyl.
Notably, of all aldehydes submitted to Paterson aldol reaction with ketone 27, methacrolein (77) gave the cleanest reaction and furnished the aldol product with the highest selectivity ((dr 19.6:1).
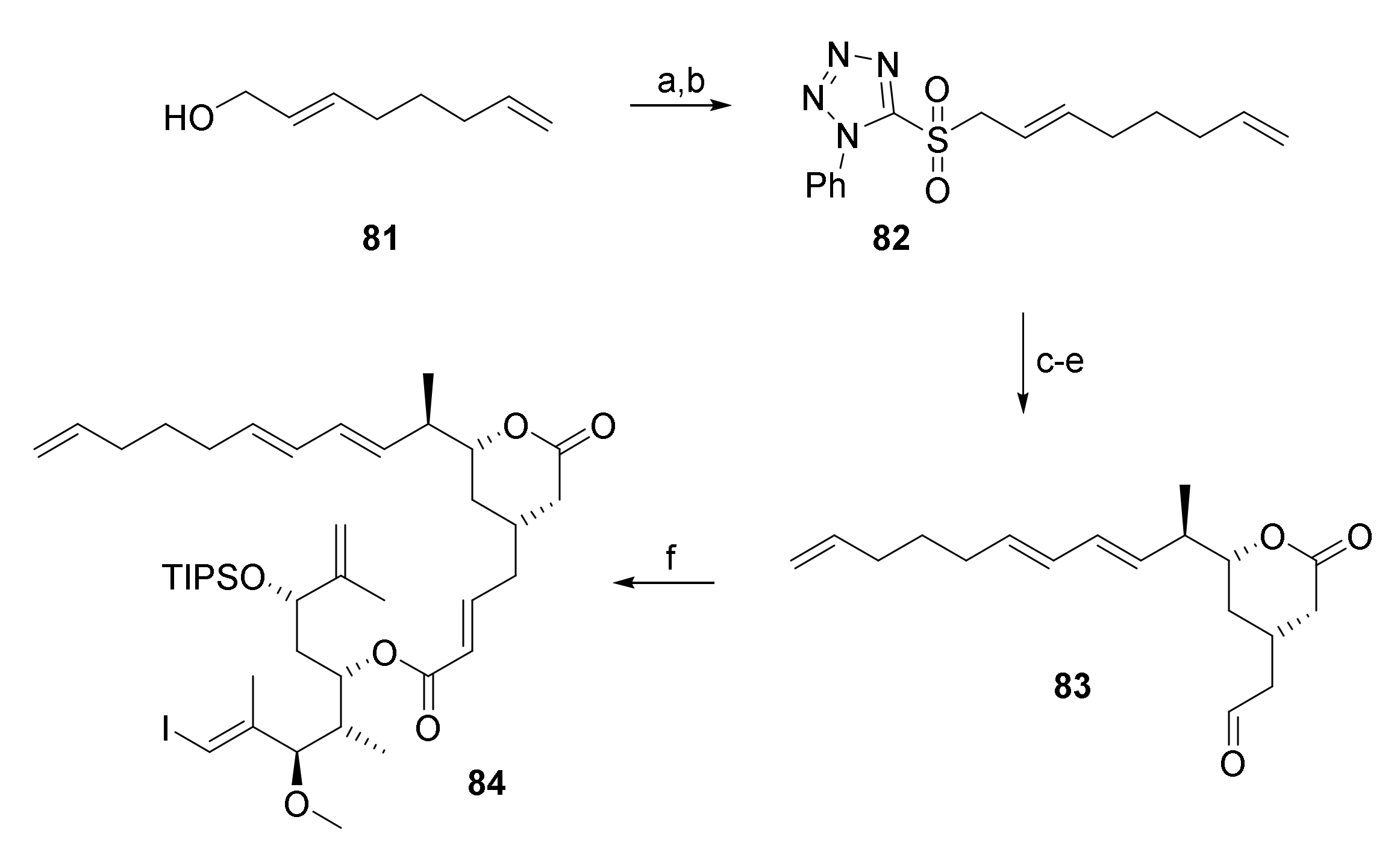
As shown in Scheme 14, aldehyde 83 was obtained from aldehyde 66 by Julia-Kocienski olefination with sulfone 82 (obtained from 2,7-octadienol (81) via Mitsunobu reaction and subsequent oxidation of the thioether with Mo7O24(NH4)6, H2O2 in 57% yield) followed by TBDPS removal and Swern oxidation of the resulting free alcohol in 30% overall yield. HWE reaction of 80 and 83 under Masamune-Roush conditions then furnished the RRCM substrate 84 in 69% yield.
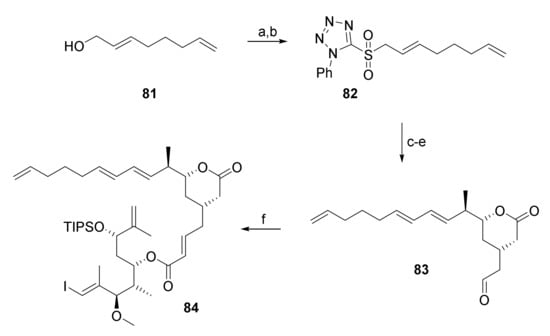
Scheme 14.
Reagents and conditions: (a) 2-Mercaptophenyltetrazole, PPh3, DEAD, THF, rt; (b) Mo7O24(NH4)6, H2O2, EtOH, 0 °C to rt, 57% (2 steps); (c) LiHMDS, aldehyde 66, 34% (E/Z > 30:1); (d) TBAF, AcOH, THF, 0 °C to rt, 89%; (e) (COCl)2, DMSO, NEt3, CH2Cl2, −78 °C, 95%; (f) 80, DBU, LiCl, CH3CN, 0 °C to rt, 69%. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DEAD, diethyl azodicarboxylate; LiHMDS, lithium bis(trimethylsilyl)amide; TBAF, tetra-n-butylammonium fluoride.
Unfortunately, treatment of 84 with Grubbs 2nd generation catalyst in toluene, which was the only system that had shown some initial promise in screening experiments, did not result in macrocyclization (employing catalyst loadings of 5 mol% or 10 mol% at temperatures of 45 °C, 60 °C, or 90 °C). As for 76, only the tether was cleaved and more and more side products appeared over time.
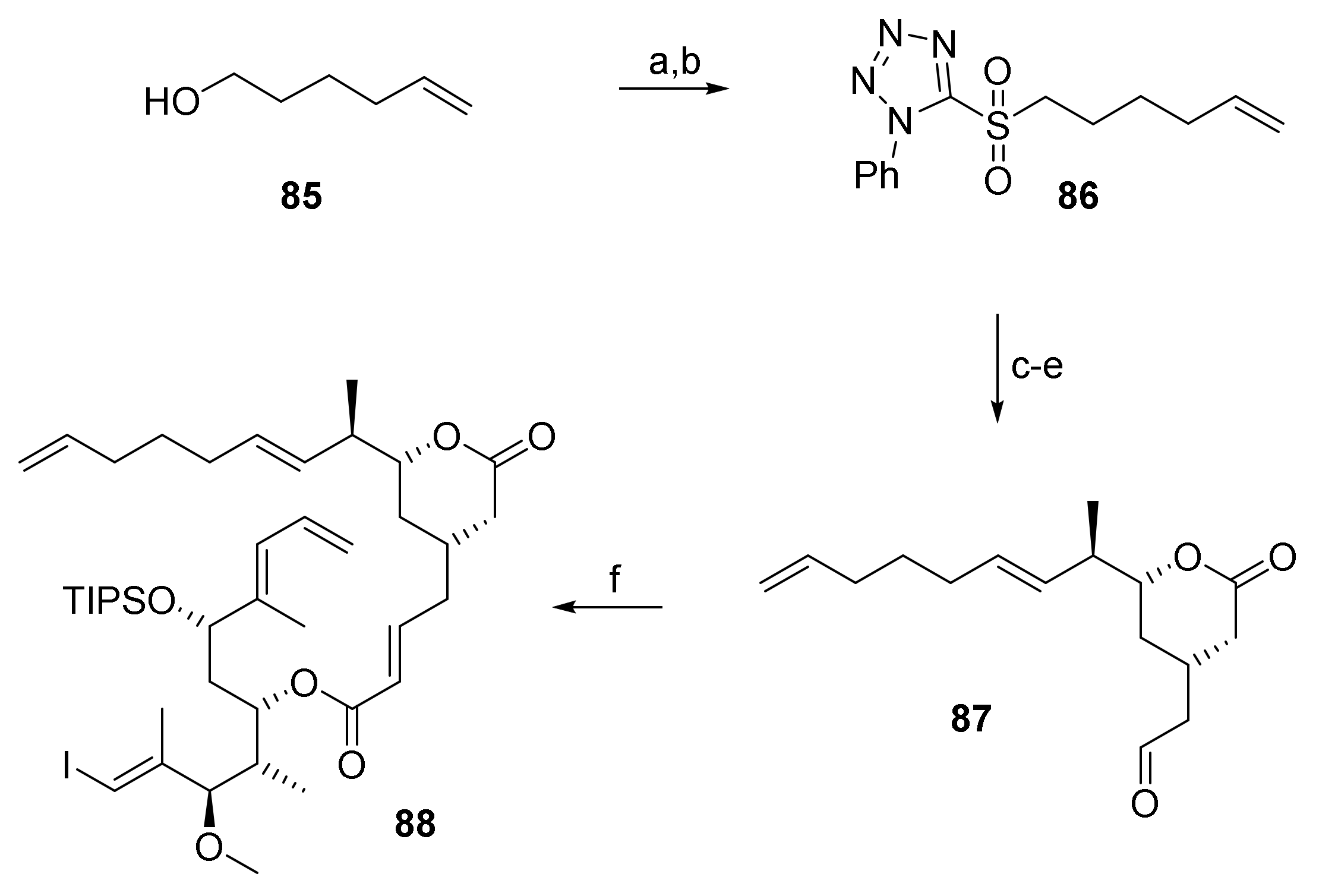
As simply shifting the position of the (projected) site of cyclization from C(9)/C(10) in 76 to C(11)/C(12) in 84 did not enable RRCM, we went back to investigate the possibility of RRCM at the originally conceived site between C(9) and C(10), but now with the tether attached to the terminal double bond of the eastern part of the original RCM substrate 5. Directing the catalyst to this double bond was felt to offer some promise, as the experiments with RRCM substrate 76 had suggested that it did not react with a ruthenium-carbene at C10. The corresponding RRCM substrate 88 was obtained by HWE of phosphono ester 69 and aldehyde 87 under Masamune-Roush conditions (Scheme 15). The latter was prepared from the known phenyltetrazole sulfone 86 [66] via Julia-Koczienski olefination, followed by TBDPS removal and Swern oxidation.
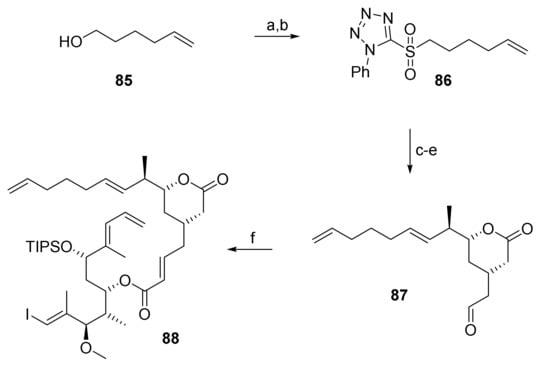
Scheme 15.
Reagents and conditions: (a) 2-Mercaptophenyltetrazole, DEAD, PPh3, THF, rt; (b) H2O2, (NH4)6Mo7O24, EtOH/THF (5:3), 0 °C to rt, 78% (2 steps); (c) NaHMDS, THF, −78 °C to −9 °C, then 66, −78 °C to 0 °C, 62%, E only; (d) TBAF, AcOH, THF, 0 °C to rt, 94%; (e) (COCl)2, DMSO, CH2Cl2, −78 °C, then NEt3, −78 °C to rt, 92%, E:Z = 9.6:1; (f) 87, DBU, LiCl, CH3CN, 0 °C to rt, 85%. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DEAD, diethyl azodicarboxylate; NaHMDS, sodium bis(trimethylsilyl)amide; TBAF, tetra-n-butylammonium fluoride.
Disappointingly, 88, as all other previous substrates, did not undergo macrocyclization to any detectable extent for a variety of solvents and catalysts.
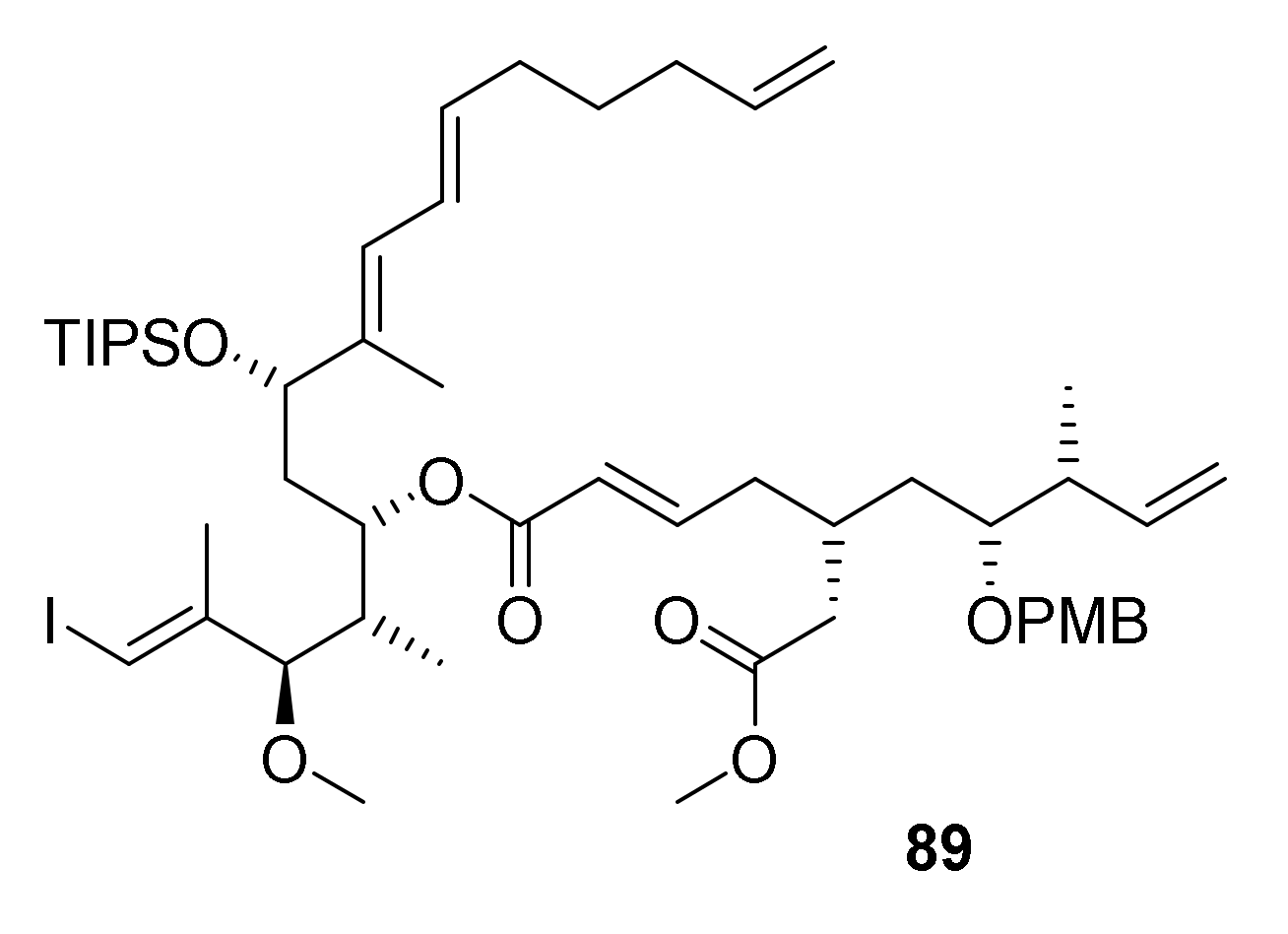
In light of the various unsuccessful attempts at making the rhizoxin core macrocycle either by RCM or RRCM, the question arose if specific structural features of the corresponding cyclization precursors might perhaps prevent the adoption of a favorable conformation for productive ring-closure. While it was not entirely clear what these features would be, we hypothesized that macrocylization could perhaps be promoted by a more flexible cyclization precursor that did not incorporate the δ-lactone ring that had been present in all RCM/RRCM substrates investigated up to this point. To address this question, we targeted the potential RRCM substrate 89 (Figure 2), which incorporates two additional, rotatable bonds when compared to 5, 76, 84, and 88.
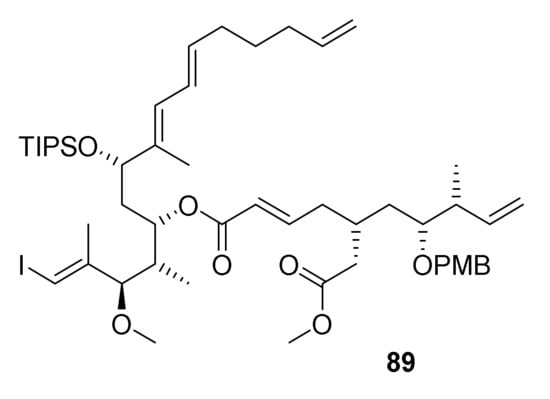
Figure 2.
Structure of the potential RRCM substrate 89.
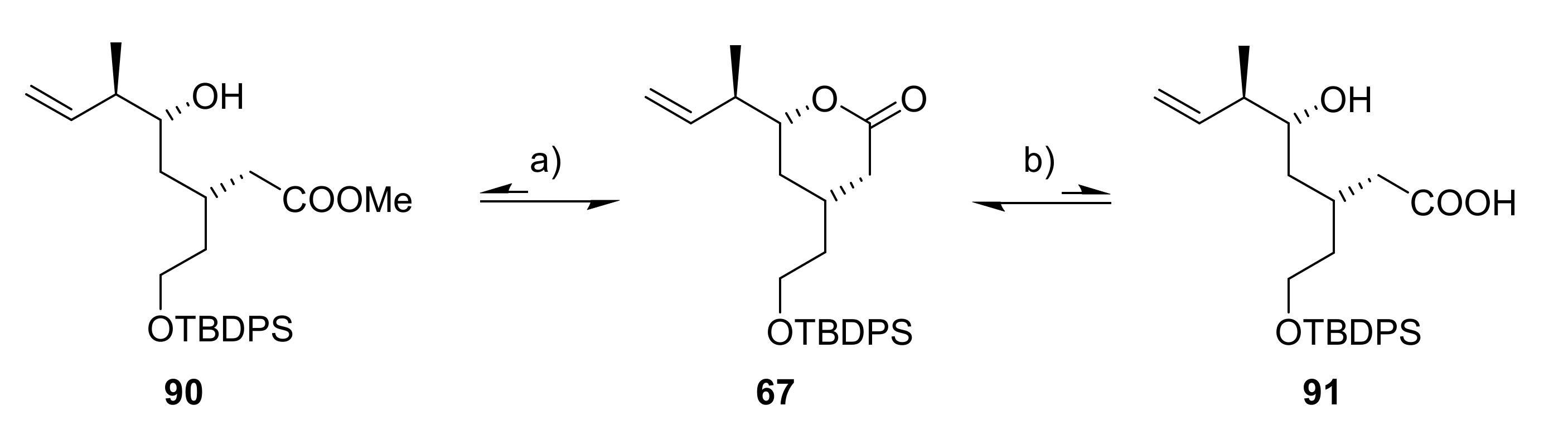
During the initial planning phase for the synthesis of 89, we assumed that ester 90 could serve as a suitable intermediate that would also be readily accessible from lactone 67 by simple treatment with NaOMe (Scheme 16).
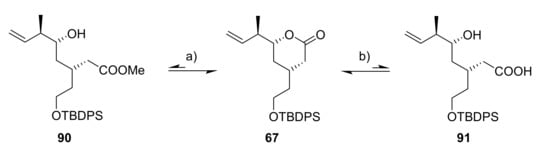
Scheme 16.
Reagents and conditions: (a) NaOMe, or NEt3, MeOH, or LiOMe; (b) (i) LiOH, THF/water (1:1), 0 °C to rt, 98%, (ii) TMSCHN2, Et2O/MeOH, rt, quant (67). TMS, trimethylsilyl.
However, while initial attempts using a large excess of NaOMe led to led to significant but still incomplete conversion to hydroxy ester 90 according to TLC, even neutral or slightly acidic conditions during attempted purification on silica gel or in deuterated chloroform were sufficient to shift the equilibrium back to the starting material 67. This was also the case for the trapped lithium carboxylate of acid 91, which immediately recyclized to δ-lactone 67 after work-up or TMS diazomethane treatment.
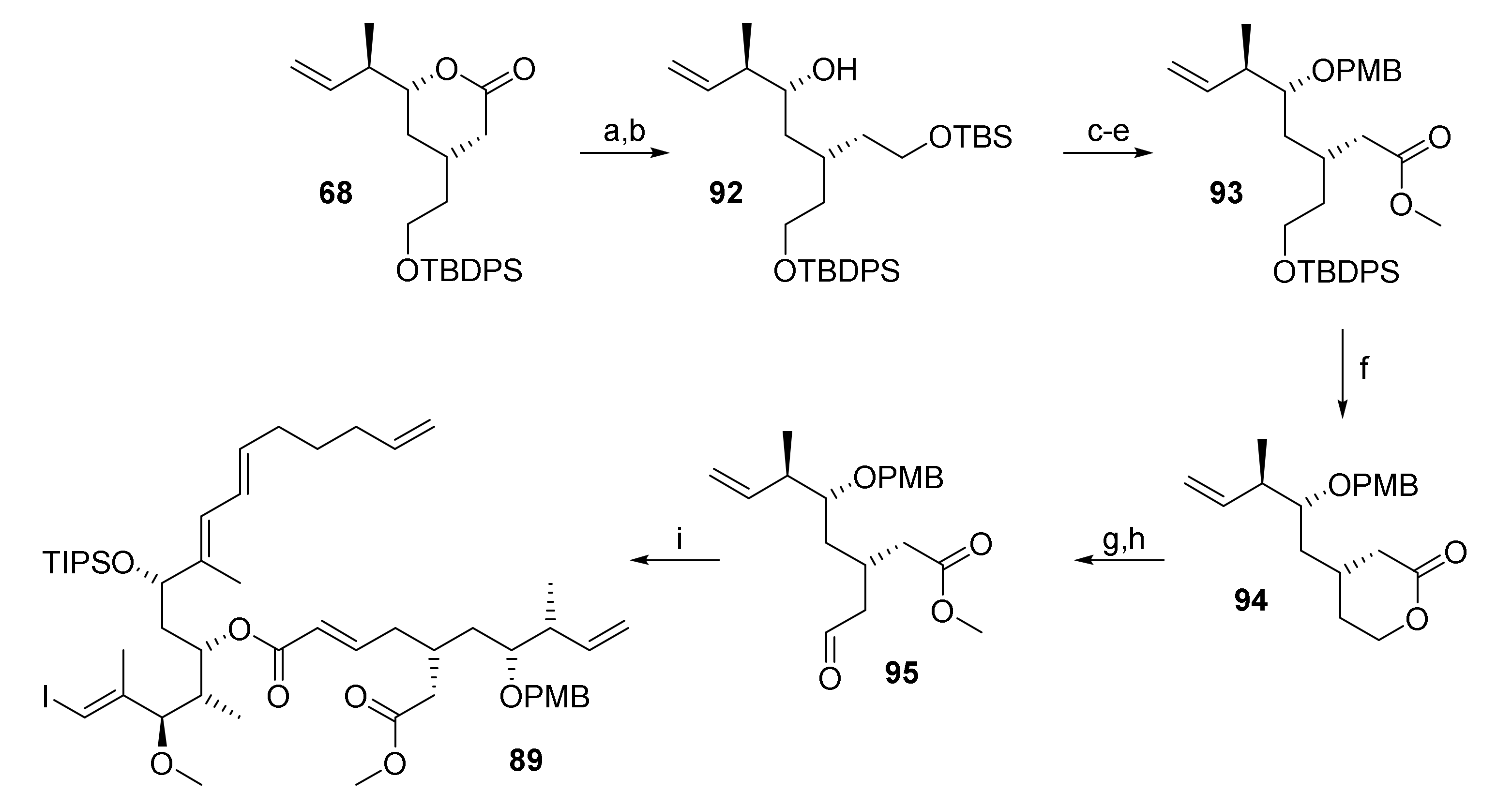
For this reason, a longer route toward 91 had to be elaborated which is summarized in Scheme 17. Thus, 67 was reduced with LiAlH4, to furnish a diol which was selectively TBS-protected at the primary hydroxy group in 98% overall yield (from 67). Lewis acid-catalyzed PMB protection followed by selective TBS deprotection furnished a primary alcohol that was elaborated into methyl ester 93 in 53% overall yield in a three-step sequence involving Swern oxidation, Pinnick oxidation of the ensuing aldehyde to the acid, and methyl ester formation with TMS-diazomethane. TBDPS deprotection with buffered TBAF furnished a 9:1 mixture of lactone 94 and the corresponding hydroxy (methyl)ester in 88% combined yield. After sequential treatment of this (separable) mixture with lithium methoxide and Dess-Martin periodinane, aldehyde 95 was isolated in 68% yield (93% brsm). Subsequent HWE reaction of 95 with phosphonate 69 under Masamune-Roush conditions furnished ester 89.
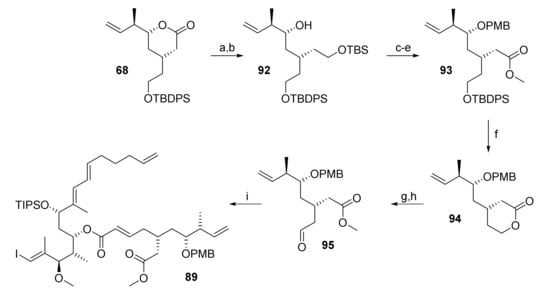
Scheme 17.
Reagents and conditions: (a) LiAlH4, Et2O, 0 °C to rt, quant; (b) TBSCl, imidazole, CH2Cl2, rt, 98% (2 steps); (c) Sc(OTf)3 (6 mol%), PMB-2,2,2-trichloroacetimidate, toluene, rt; (d) NaIO4, THF/H2O (4:1), rt, 67% (2 steps); (e) (i) (COCl)2, DMSO, CH2Cl2, −78 °C, then NEt3, −78 °C to rt, (ii) NaClO2, NaH2PO4, 2-methyl-2-butene, tBuOH, THF, rt, (iii) TMSCHN2, toluene/MeOH, 0 °C, 79% (3 steps); (f) TBAF, AcOH, THF, rt, 79%; g) LiOMe, MeOH, rt; (h) DMP, CH2Cl2, 0 °C to rt, 68% (2 steps) (93% brsm); (i) 75, LiCl, DBU, THF/MeCN (3:1), 0 °C to rt, 78%. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DMP, Dess–Martin periodinane (1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one); PMB, para-methoxy benzyl; TBS, tert-butyldimethylsilyl; TMS, trimethylsilyl.
Unfortunately, as for other RRCM substrates, Grubbs 2nd and Hoveyda-Grubbs 2nd generation catalysts in dichloroethane led to efficient cleavage of the relay tether, but no macrocyclization was observed based on MS and TLC analysis of reaction mixtures. In refluxing toluene, both catalysts led to a complex mixture of products as evidenced by HPLC and NMR analysis.
2.5. Macrocyclization by Ring-Closing Alkyne Metathesis (RCAM)
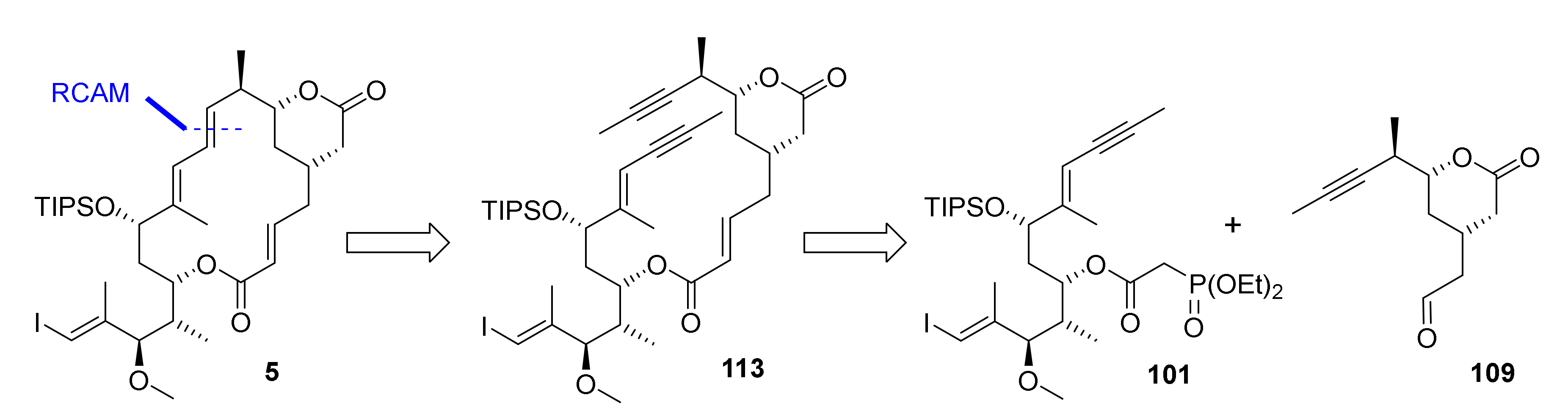
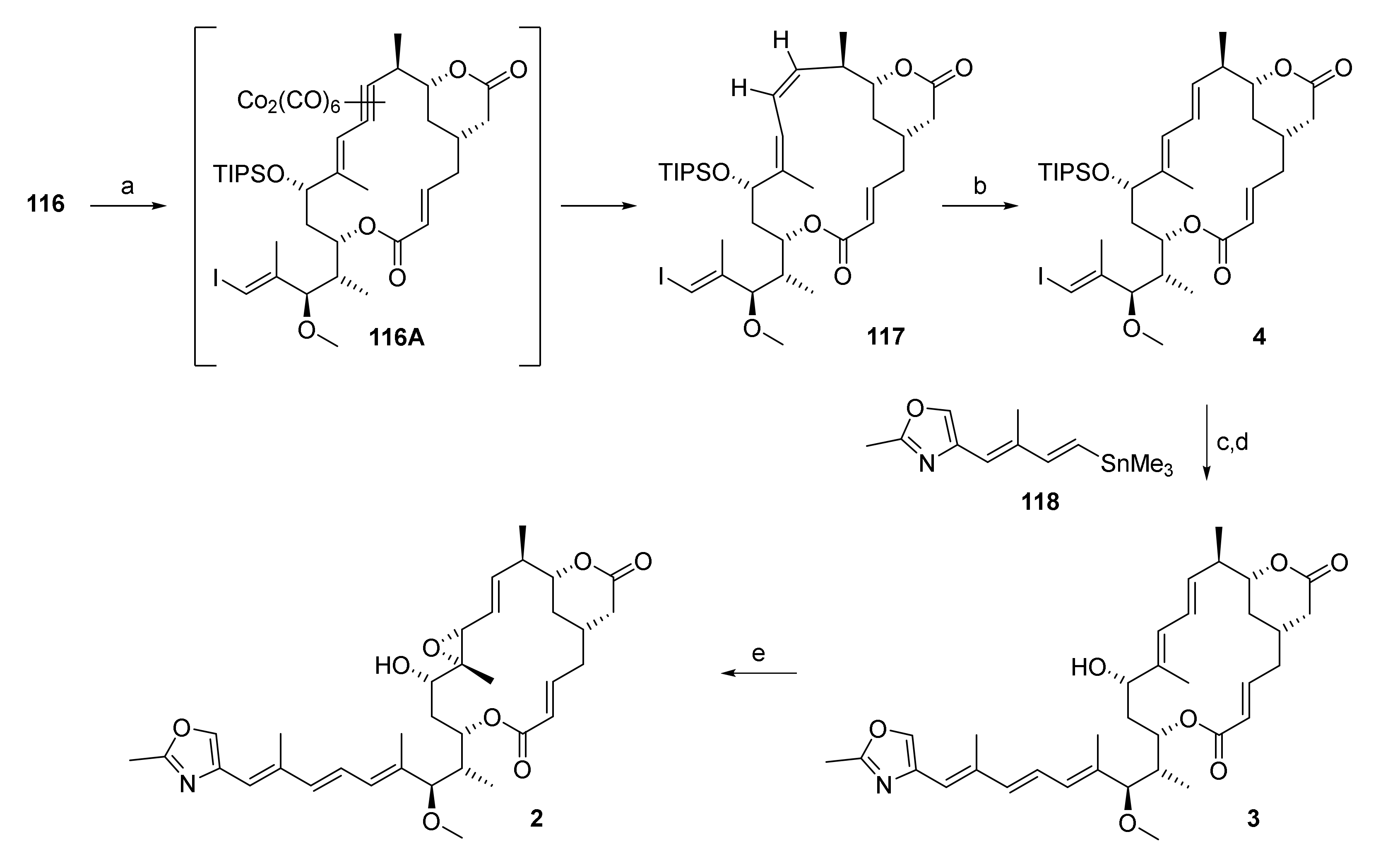
At the point where our numerous attempts at synthesizing the macrocyclic portion of rhizoxin F (2) by means of RCM/RRCM had all met with failure, we felt that a radical change in strategy was necessary. Triggered by Fürstner’s groundbreaking work on RCAM in the field of natural product synthesis (for early examples, see in [69,70,71,72,73,74,75,76,77,78]; for more recent examples, see in [23,79,80,81], we reasoned that this method would be a promising alternative to RCM. (For a review on the use of RCAM in natural product synthesis, see in [82].) Thus, macrocyclization was planned to be achieved by RCAM at C(9)/C(10), i.e., at the same site that was initially foreseen for RCM, leading to diyne 113 as the ultimate cyclisation precursor (Scheme 18). Obviously, the triple bond in the RCAM product would have to be converted into a trans double bond, but methods for the trans-selective reduction of alkynes to olefins had been described (see, for example, in [83,84,85]).
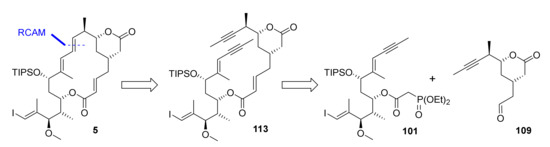
Scheme 18.
Global retrosynthesis of 5 via 113.
Diyne 113 was to be obtained from phosphono ester 101 and aldehyde 109 in analogy to the synthesis of the various RCM/RRCM substrates. This approach offered the advantage that it would allow us to capitalize on the chemistry developed in the course of the synthesis of the corresponding RCM/RRCM macrocyclization precursors. The largest part of the chemistry described in the following has already been reported in [20], but is included here to provide a coherent and complete narrative of the work that finally led to the successful synthesis of rhizoxin F.
2.5.1. Synthesis of Building Blocks
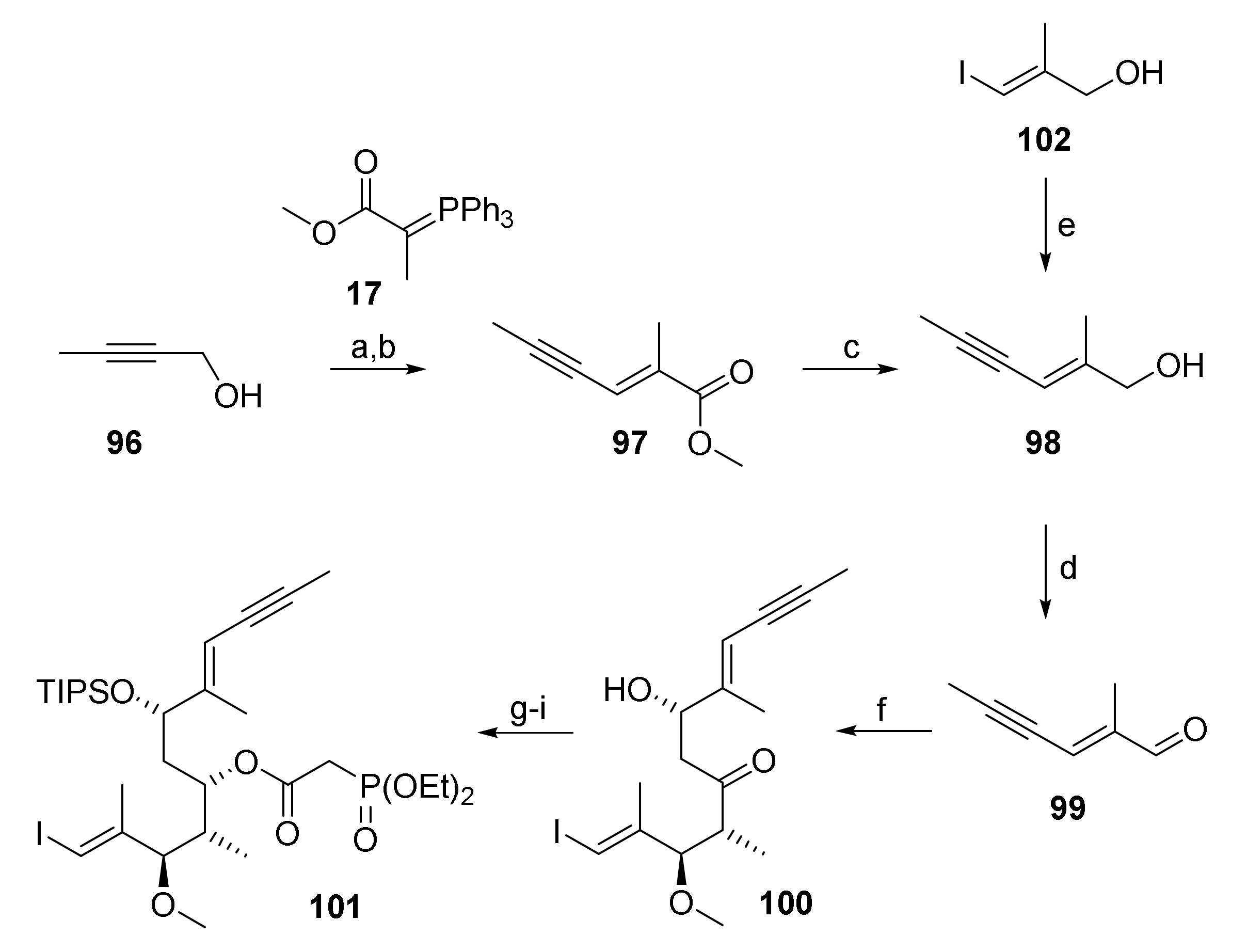
As illustrated in Scheme 19, the synthesis of phosphono ester 101 relied on the same type of aldol chemistry that had been successfully exploited in the synthesis of the different RCM/RRCM substrates. In this context, a short route was devised towards the requisite aldehyde 99, making use of vinyl iodide 102, which was already at hand from our previous synthesis of ketone 27. Thus, 102 was submitted to Sonogashira cross-coupling with propyne to yield ene-yne 98, which was readily oxidized to the desired aldehyde 99 (Scheme 19).
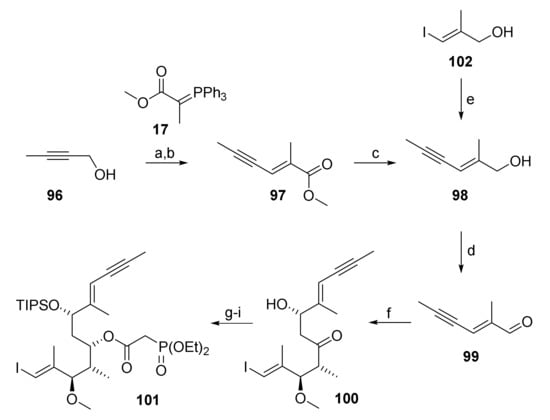
Scheme 19.
Reagents and conditions: (a) Activated MnO2, 3 Å molecular sieves, CH2Cl2, rt, 82%; (b) 17, CH2Cl2, rf, 45%; (c) LiAlH4, Et2O, 0 °C; (d) activated MnO2, 3 Å molecular sieves, CH2Cl2, rt, 82%; (e) propyne, DIPEA, CuI (20 mol%), PdCl2(PPh3)2 (15 mol%), THF, −78 °C to rt, 63%; (f) (i) 27, (+)-DIPCl, NEt3, CH2Cl2, −78 °C, then 99, −78 °C to −18 °C; (ii) H2O2, phosphate buffer (pH7), MeOH, 0 °C to rt, ca. 73%, dr n.d.; (g) NMe4BH(OAc)3, CH3CN/AcOH (2:1.5), −40 °C to rt, 90%; (h) TIPSOTf, 2,6-lutidine, 3 Å molecular sieves, CH2Cl2, −78 °C, 97%; (i) diethylphosphonoacetic acid (55), CME-CDI, DMAP, 3 Å molecular sieves, CH2Cl2, 0 °C to rt, 82%. DIPCl, B-Chlorodiisopinocampheylborane; DIPEA, N,N-diisopropylethylamine (Hünig’s base); TIPS, triisopropylsilyl.
However, the Sonogashira coupling proved to be very tedious; not only were vinyl iodide 102 and the coupling product 98 inseparable on silica gel (TLC), thus creating the need for reaction monitoring by RP-HPLC and requiring full conversion of the starting material, but the coupling also relied on the use of expensive propyne and a precious catalyst. Furthermore, it was found that the optimized conditions in small-scale experiments were not amenable to scale up (>1 g), which was a key requirement at this early stage of the synthesis. For these reasons an alternative route towards 99 was sought. In the event, oxidation of 2-butyn-1-ol (96) with activated MnO2 and subsequent Wittig olefination of the resulting propargylic aldehyde with phosphonium ylide 17 furnished ester 97, which was smoothly reduced to allylic alcohol 98 (Scheme 19). In contrast to gaseous propyne, 2-butyn-1-ol (96) is an easy-to-handle and relatively cheap liquid. Furthermore, phosphonium ylide 77 could be conveniently synthesized in large quantities (>120 g). The synthesis of 99 from 96 could thus be scaled up without difficulties.
With aldehyde 99 in hand, the synthesis of phosphono ester 101 could be addressed, relying on previously established protocols. Thus, Paterson aldol reaction of 99 with ketone 27 followed by directed reduction of β-hydroxy ketone 100 according to Evans [35], regioselective TIPS protection of the ensuing diol, and esterification with diethylphosphonacetic acid (55) furnished 101 in 52% overall yield as a single isomer.
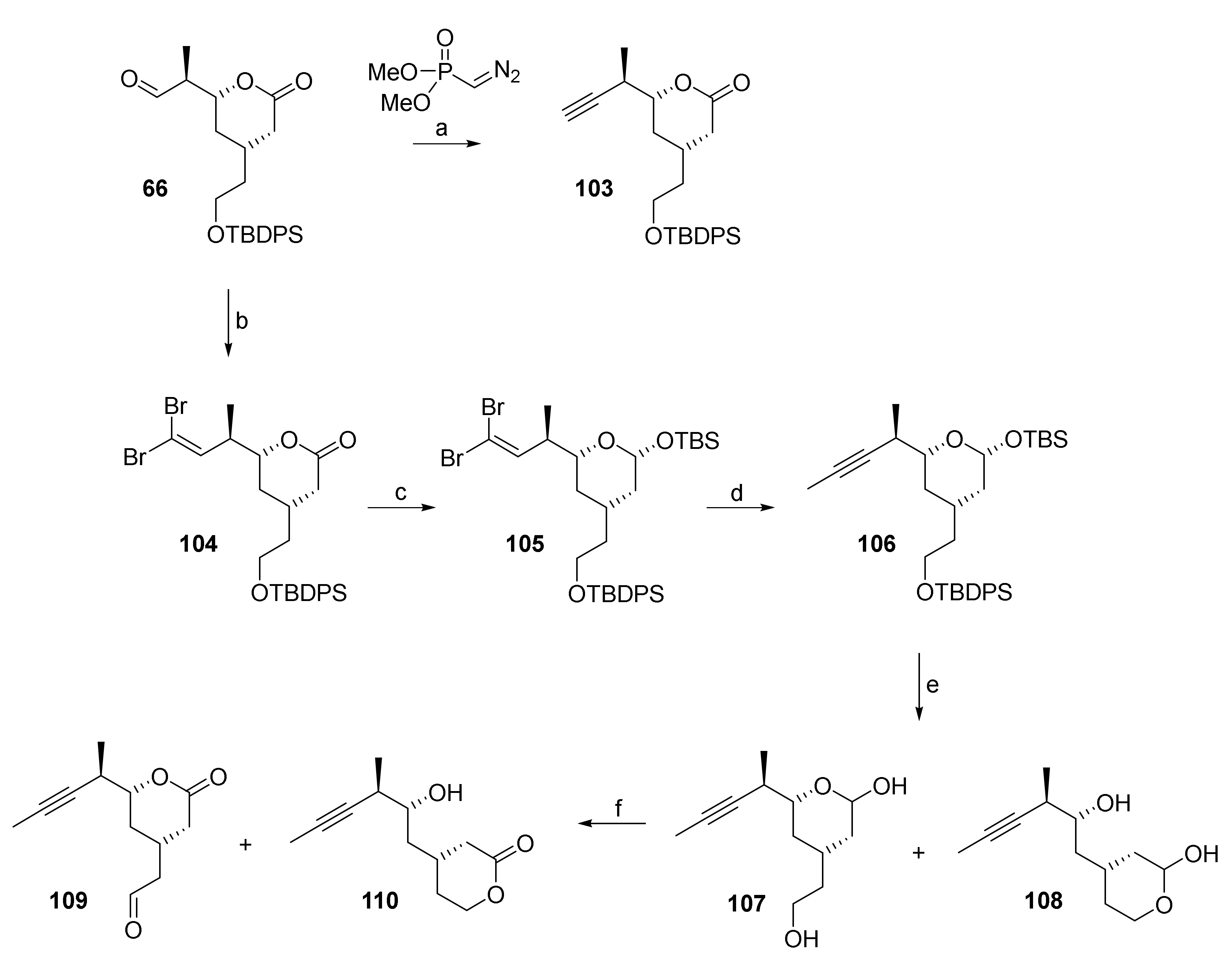
The synthesis of aldehyde 109 started from crude aldehyde 66 (obtained by Dess-Martin oxidation from primary alcohol 65, cf. Scheme 11). In an initial experiment on small scale, 66 could be directly converted into terminal alkyne 103 by Seyferth-Gilbert alkynylation [86] (Scheme 20). However, 103 was obtained only in moderate yield (43%) and no efforts were spent on optimizing this transformation. Instead, we decided to assess the feasibility of the alkynylation of 66 via a Corey-Fuchs reaction as an alternative approach. In the event, dibromo olefin 104 could be prepared from 66 in good yield (80%) but, disappointingly, the conversion of the dibromo alkene moiety into a triple bond failed completely and the treatment of 104 with nBuLi led to complete decomposition of starting material. However, the installation of the triple bond via the dibromo alkene moiety became possible after reduction of the lactone group to the corresponding hemiacetal with DIBAL and protection of the latter with a TBS group, to furnish mixed acetal 105 as a single isomer in excellent yield (87%) (Scheme 20). Treatment of 105 with nBuLi and trapping of the intermediate acetylide with MeI gave alkyne 106 in 63% yield over the four step sequence from aldehyde 66.
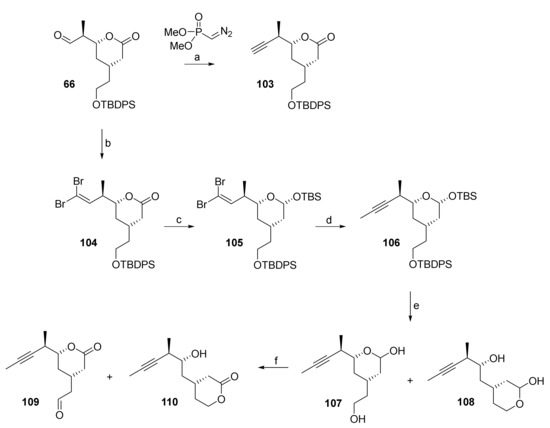
Scheme 20.
Reagents and conditions: (a) KOtBu, Seyferth-Gilbert reagent (dimethyl (diazomethyl)phosphonate), THF, −78 °C, 43%; (b) CBr4, PPh3, CH2Cl2, −78 °C, 80% (2 steps, based on 65); (c) (i) DIBAL, CH2Cl2, −78 °C, dr 1:1, (ii) TBSCl, imidazole, CH2Cl2, rt, 87% (2 steps) (single isomer); d) nBuLi, MeI, THF, −78 °C to rt, 90%; (e) TBAF, AcOH, THF, 0 °C to rt, quant. (107/108, 2:1); (f) TEMPO, BAIB, Yb(OTf)3 (cat.), CH2Cl2, 0 °C to rt, 109: 62%, 110: 13%. BAIB, bis(acetoxy)iodobenzene; DIBAL, diisobutylaluminum hydride; TBAF, tetra-n-butylammonium fluoride; TBS, tert-butyldimethylsilyl; TEMPO, 2,2,6,6-tetramethylpiperidine-1-oxyl.
Simultaneous cleavage of both silyl ethers with TBAF/AcOH produced an inseparable mixture of lactols 107 and 108 (each as a pair of diastereoisomers; 2:1 ratio in favor of the desired regioisomer 107), which was treated with bisacetoxy iodobenzene (BAIB) (2.2 equiv) in the presence of catalytic amounts of TEMPO (19 mol%) and Yb(OTf)3 (4 mol%) (this system is similar to the iodosylbenzene/TEMPO/Yb(OTf)3 system reported by Vatèle [87]) to produce the desired building block 109 as a single isomer in 62% yield (based on 106) along with the corresponding hydroxy lactone 110 (13%).
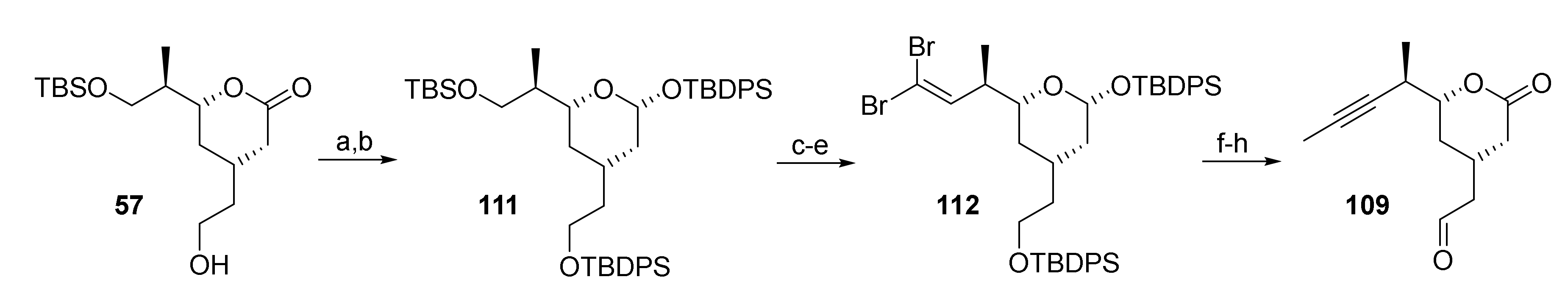
The above synthesis of aldehyde 109 from alcohol 57 involved an avoidable protection/deprotection sequence (cf. Scheme 11); a slightly shorter route to 109 was thus implemented in the scale-up process that relied on lactone reduction at the stage of alcohol 57 (Scheme 21).
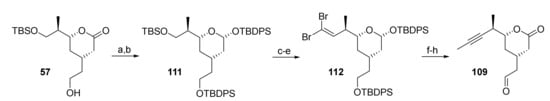
Scheme 21.
Reagents and conditions: (a) DIBAL, CH2Cl2, −78 °C, dr 1:1; (b) TBDPSCl, imidazole, CH2Cl2, rt, 90% (2 steps) (single isomer); (c) NaIO4, THF/water (4:1), rt, 87%; (d) DMP, CH2Cl2, 0 °C to rt, 88% or (COCl)2, DMSO, CH2Cl2, −78 °C, then NEt3, −78 °C to rt, 96%; (e) CBr4, PPh3, CH2Cl2, −78 °C, 99%; (f) nBuLi, THF, −78 °C to rt, then MeI, rt, 94%; (g) TBAF, AcOH, THF, 0 °C to rt; (h) TPAP (cat.), NMO, CH2Cl2, rt, 50% (2 steps). DIBAL, diisobutylaluminum hydride; DMP, Dess–Martin periodinane (1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one); NMO, 4-methylmorpholine N-oxide; TBAF, tetra-n-butylammonium fluoride; TPAP, tetrapropyl-ammonium perruthenate.
The resulting 1:1 mixture of hemiacetals was then converted into tris-silyl derivative 111, which was obtained as a single isomer in 90% yield from 57. This finding is in line with Leahy’s observation that the all-equatorial isomer of the lactol is formed preferentially after equilibration under basic conditions [88]. Selective cleavage of the TBS-ether with NaIO4 [61] followed by either Dess-Martin or Swern oxidation gave an aldehyde (in 88% or 96% yield, respectively) that could be converted into dibromo olefin 112 in 99% yield. The latter was elaborated into aldehyde 109 by treatment with nBuLi/MeI, followed by TBDPS removal and TPAP oxidation. In contrast to the previously used TEMPO-mediated oxidation of 107/108, TPAP/NMO gave aldehyde 109 as a single compound in 50% yield over 2 steps reproducibly. The former method proved to be rather unreliable for scale-up, with yields decreasing down to 20%. Aldehyde 109 could also be obtained from 107/108 by Dess-Martin oxidation, but the material was very difficult to purify by column chromatography.
2.5.2. Building Block Assembly and Completion of the Synthesis of Rhizoxin F (2)
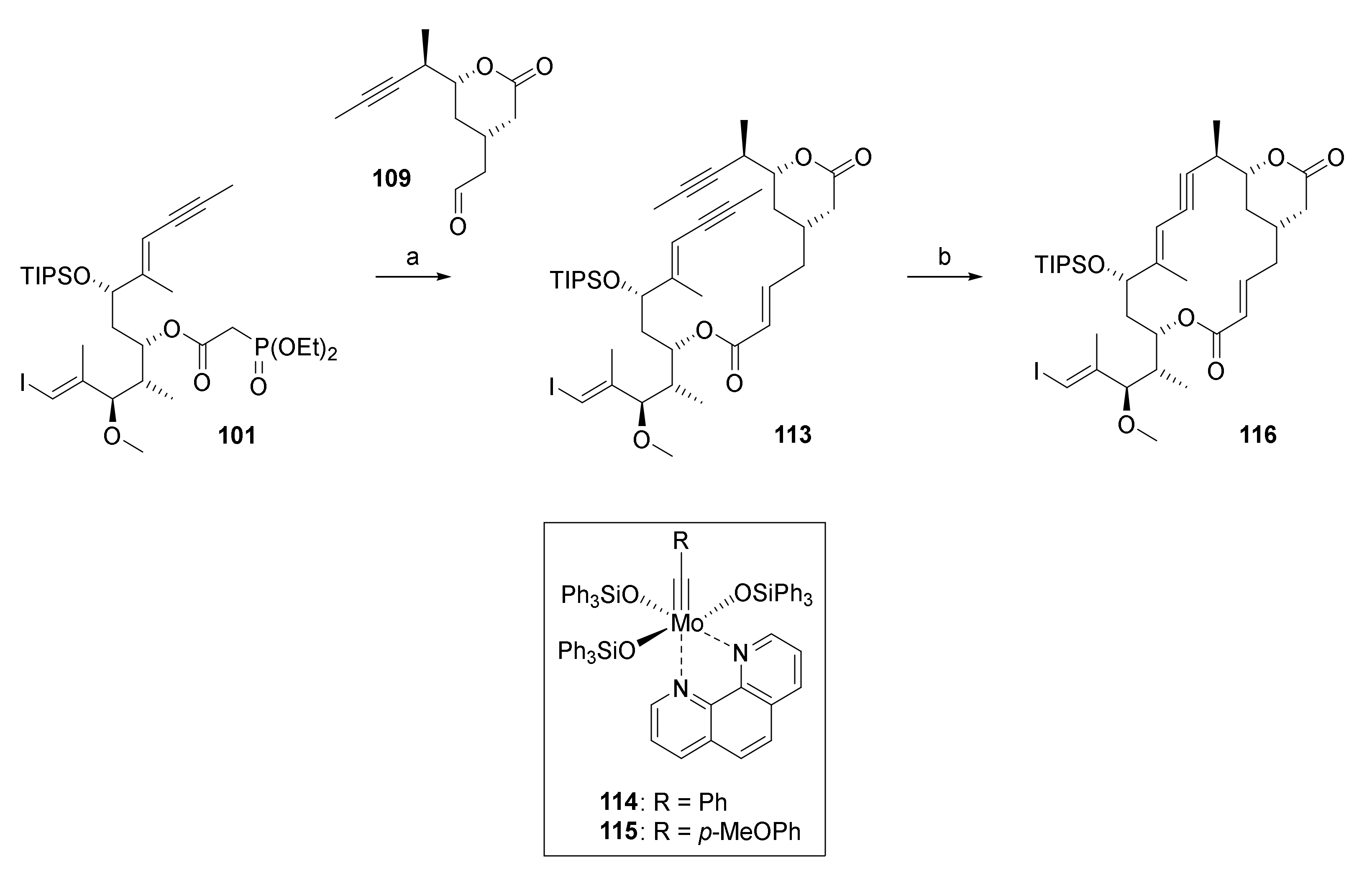
The HWE reaction between aldehyde 109 and phosphono ester 101 proceeded smoothly to deliver diyne 113 in 81% yield (Scheme 22).
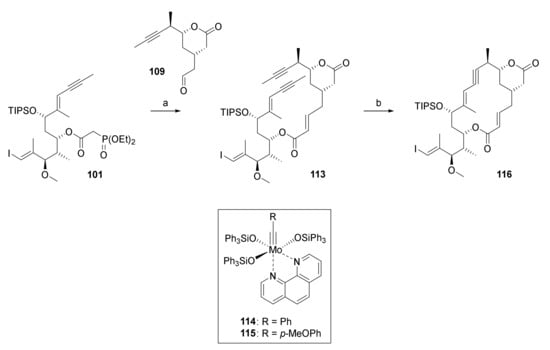
Scheme 22.
Reagents and conditions: (a) DBU, LiCl, CH3CN, 0 °C to rt, 81%; (b) Mo-complex 114 (35 mol%) or 115 (15 mol%), MnCl2, toluene, 5 Å molecular sieves, 125 °C; 114: 69%, 115: 56%/31%%. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene.
Gratifyingly, and very much to our relief, heating 113 with the active catalysts obtained by in situ decomplexation of Mo-complexes 114 or 115 (complexes 114 and 115 were generous gifts from Prof. Alois Fürstner, Max Planck Institute for Carbon Research, Mühlheim/Ruhr, Germany) with MnCl2 to 125 °C in toluene for 2.45 h (for 114) or 27 h (for 115) gave the desired macrocycle 116 in 69% and 56%/31% yield (The two yields were obtained in two independent experiments on a 700 mg scale of 113 that were performed under ostensibly identical conditions. The reasons for the significant discrepancy in yields are unknown at this point.), respectively (Scheme 22). Heating of the reaction to at least 120 °C was necessary to achieve a practical rate of cyclization; at the same time, this finding also highlights the exceptional thermal stability of active catalysts derived from complexes 114 and 115 in the reaction mixture. While the yields obtained in the macrocyclization reaction as such may not be considered spectacular, given our prior extensive unsuccessful efforts at RCM, they were an impressive demonstration of the power of RCAM is an efficient method for macrocyclization.
With the hurdle of the ring-closure finally cleared, we now had to address the E-selective alkyne reduction of the newly formed triple bond. While the possibility of generating Z or E olefins from alkynes selectively, for example, by hydrosilylation/desilylation, is usually regarded as one of the benefits of RCAM, this transformation turned out to be a major challenge for 116. In spite of extensive efforts, both the yield and selectivity of hydrosilylation and protodesilylation steps were unsatisfactory and no conditions could be identified to affect clean conversion of the ene-yne portion in 116 into the desired E,E diene moiety.
However, in the context of the above experiments, we had obtained a small amount of the C(9)/C(10) Z isomer of 5, which we found could be cleanly isomerized to 5 by refluxing with thiophenol and AIBN in benzene [89]. This observation suggested that an alternative approach towards the conversion of 116 into 4 could be the Z-selective reduction of the triple bond followed by double bond isomerization. As a consequence, experiments were conducted aiming to access C(9)/C(10)-Z-5 (i.e., 117, Scheme 23) selectively; this included the examination of Red-Al-, Birch-, SmI2-, and hydrazine-promoted alkyne reductions, and a plethora of hydrogenations (transfer methods or application of elemental H2) and hydrometallations (with boron, tin, silicon), which are not to be discussed here in detail. Suffice it to say, that none of these methods delivered the desired diene 117.
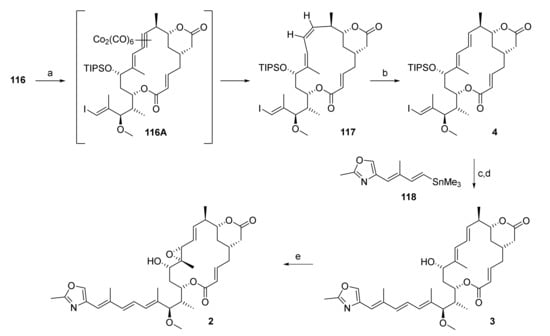
Scheme 23.
Reagents and conditions: (a) (i) Co2(CO)8, CH2Cl2, rt; (ii) N-ethylpiperidinium hypophosphite, benzene, reflux, 74% over 3 cycles, Z only; (b) AIBN, PhSH, benzene, reflux, 88%, E/Z = 20:1; (c) 3, PdCl2(MeCN)2, DMF, rt, 68%; (d) HF·py, py, THF, 0 °C to rt, 54%; (e) tBuOOH, VO(acac)2, benzene, 0 °C to rt, 65%, 29% after preparative HPLC. acac, acetylacetonato; AIBN, azobisisobutyronitrile; py, pyridine.
While alkyne 116 could not be reduced to the corresponding Z olefin directly, it could be converted cleanly into its biscobalthexacarbonyl complex 116A (Scheme 23) by simple treatment with Co2(CO)8 [90,91]. We considered this a promising finding, as Isobe and co-workers had reported the reductive decomplexation of endocyclic biscobalthexacarbonyl complexes with rhodium on charcoal [92,93] or Wilkinson catalyst [94] under elevated hydrogen pressure to give olefins. They later discovered that the reductive decomplexation could also be brought about not only under radical conditions using nBu3SnH [94], but also with the less toxic sodium hypophosphite monohydrate [95].
In our case, reductive decomplexation with nBu3SnH was not compatible with the vinyl iodide moiety and the use of hydrogen at elevated pressure with Wilkinson’s catalyst or rhodium on charcoal was considered somewhat impracticable. Thus, the decomplexation of 116A with sodium hypophosphite was deemed to be the most viable approach towards diene 117. Unfortunately, Isobe’s original protocol led to transesterification of the δ-lactone moiety with the solvent 2-ethoxy ethanol (cellosolve). At the same time, partial reduction of the complex was observed, thus suggesting that 117 might indeed be accessible from 116A if the decomplexation was carried out in a different solvent. When the reaction was performed in DMSO or CH3CN/H2O (6:1), no reduction took place. In order to allow for screening also in more lipophilic solvents, the tetrabutylammonium salt of hypophosphorous acid was then prepared, which proved to be soluble in CH2Cl2 and THF. Treatment of 116A with this salt in CH2Cl2 or THF afforded a mixture of diene 117 and ene-yne 116 (ca. 50% conversion).
The ultimate choice of reducing agent was N-ethylpiperidinium hypophosphite, which was commercially available and which had been employed as a substitute for nBu3SnH in the radical deoxygenation of alcohols [96]. N-Ethylpiperidinium hypophosphite proved to be more soluble than tetrabutylammonium hypophosphite; the most soluble reductant investigated was diphenylphosphine oxide (DPPO) [97], but this only led to decomposition of 116A. In analogy to Isobe’s protocol with nBu3SnH [94], benzene was the solvent of choice for the reduction of 116A to 117 with N-ethylpiperidinium hypophosphite. After some fine-tuning of conditions, the reaction reliably afforded C(9)/C(10) Z olefin 117 in a highly selective fashion. In order to achieve full consumption of alkyne 116, the two-step sequence of complex formation and reductive decomplexation had to be performed three times, as 116 was inseparable from olefin 117 by silica gel chromatography. This procedure initially provided 117 in 47% yield; while this yield was moderate, it was still considered very satisfactory, given the fact that an array of well-established methods had failed in reducing ene-yne 116. Gratifyingly, it was discovered in subsequent experiments (by serendipity) that the yield for the conversion of 116 into 117 could be increased substantially, up to 74%, if an aqueous workup after the hypophosphite reduction was omitted.
Radical isomerization of 117 with AIBN and thiophenol then proceeded smoothly furnished the desired E,E-diene 4 in 88% yield (Scheme 23). Subsequent Stille coupling of 4 with the known vinyl stannane 118 [29], followed by HF-promoted silyl ether cleavage, gave rhizoxin D (3) in 37% overall yield. For reasons unknown, this yield is lower than those reported in the literature for the same two-step sequence (50% [29] and 76% [98]); in our hands, all intermediates with the C(15) side chain attached were prone to decomposition, in agreement with reports by Hertweck [99] and Stella [100]. Finally, directed epoxidation of rhizoxin D (3) according to Ohno and co-workers [19] proceeded in 65% yield, thus completing the first synthesis of rhizoxin F (2).
3. Materials and Methods
Detailed protocols for the synthesis of new compounds and the associated analytical data can be found in the Supplementary Material.
4. Conclusions
The total synthesis of the bacterial macrolide rhizoxin F (2) (also known as WF1360F) was accomplished based on macrocyclization by ring-closing alkyne metathesis (RCAM) at the C(9)–C(10) site, followed by a highly selective radical-based reduction/isomerization sequence to install the macrocyclic (E,E)-diene unit. The synthesis comprised 24 steps for the longest linear sequence from 1,4-butanediol (44) and delivered the target structure in ~1.5% yield overall yield. Notably, in spite of extensive efforts, all attempts to access macrocycle 4 by ring-closing olefin metathesis (RCM) met with complete failure. Our findings not only further highlight the general potential of RCAM in natural product synthesis, which by now is well established through the work of Fürstner and others; but it also shows that RCAM can serve as a valuable alternative when macrocyclization cannot be effected by RCM. We have also developed a new alkyne semireduction protocol, which reliably furnished diene 4 in good yields and with exquisite selectivity. This protocol provides an additional option for the elaboration of macrocyclic alkynes obtained by RCAM into the corresponding olefins. Finally, although this is not discussed here, macrocyclic vinyl iodide 4 has been smoothly converted into a number of side chain-modified analogs of 2; in addition, our overall strategy for the synthesis of 2 was also adaptable to the synthesis of a (side chain-modified) lactam analog.
Supplementary Materials
The following are available online. synthesis protocols and analytical data for all new compounds, Table S1: RCM screening for substrate 5, Table S2: RCM screening for substrate 70.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. Conceptualization, K.-H.A., C.M.N., and M.L.; methodology, K.-H.A., C.M.N., and M.L.; formal analysis, C.M.N., M.L., and K.-H.A.; investigation, C.M.N. and M.L.; data curation, C.M.N., and M.L.; writing, K.-H.A.; supervision, K.-H.A.; funding acquisition, K.-H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swiss National Science Foundation (SNF projects 200021_126511 and 200020_143269).
Acknowledgments
We thank Kurt Hauenstein, ETH Zürich, for excellent technical assistance; Bernhard Pfeiffer, ETH Zürich, for NMR support; and Louis Bertschi, ETH Zürich and the entire ETHZ-LOC MS-Service for HRMS spectra acquisition. We are very grateful to Alois Fürstner, Max Planck Institute for Carbon Research, Mülheim/Ruhr, Germany, for a generous gift of Mo-complexes 114 and 115. We also want to thank one of the reviewers for the very careful reading of the manuscript. The comments of this reviewer have led to a significant improvement of our manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Iwasaki, S.; Kobayashi, H.; Furukawa, J.; Namikoshi, M.; Okuda, S. Studies on macrocyclic lactone antibiotics. vii. structure of a phytotoxin "Rhizoxin" produced by Rhizopus Chinensis. J. Antibiot. 1984, 37, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Namikoshi, M.; Kobayashi, H.; Furukawa, J.; Okuda, S. Studies on macrocyclic lactone antibiotics. viii. Absolute structures of rhizoxin and a related compound. J. Antibiot. 1986, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Matsumoto, S.; Iwasaki, S.; Yahara, I. Molecular basis for determining the sensitivity of eucaryotes to the antimitotic drug rhizoxin. Mol. Gen. Genet. 1990, 222, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Koga-Ban, Y.; Niki, T.; Nagamura, Y.; Sasaki, T.; Minobe, Y. cDNA Sequences of Three Kinds of β-tubulins from Rice. DNA Res. 1995, 2, 21–26. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. A Gene Cluster Encoding Rhizoxin Biosynthesis in “Burkholderia rhizoxina”, the Bacterial Endosymbiont of the Fungus Rhizopus microspores. ChemBioChem 2007, 8, 41–45. [Google Scholar] [CrossRef]
- Scherlach, K.; Partida-Martinez, L.P.; Dahse, H.-M.; Hertweck, C. Antimitotic Rhizoxin Derivatives from a Cultured Bacterial Endosymbiont of the Rice Pathogenic Fungus Rhizopus microsporus. J. Am. Chem. Soc. 2006, 128, 11529–11536. [Google Scholar] [CrossRef]
- Prota, A.; Bargsten, K.; Díaz, J.F.; Marsh, M.; Cuevas, C.; Liniger, M.; Neuhaus, C.; Andreu, J.M.; Altmann, K.-H.; Steinmetz, M.O. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 13817–13821. [Google Scholar] [CrossRef]
- Iwasaki, S.; Namikoshi, M.; Kobayashi, H.; Furukawa, J.; Okuda, S. Studies on macrocyclic lactone antibiotics. IX: Novel macrolides from the fungus Rhizopus chinensis: Precursors of rhizoxin. Chem. Pharm. Bull. 1986, 34, 1387–1390. [Google Scholar] [CrossRef][Green Version]
- Kiyoto, S.; Kawai, Y.; Kawakita, T.; Kino, E.; Okuhara, M.; Uchida, I.; Tanaka, H.; Hashimoto, M.; Terano, H.; Kohsaka, M.; et al. A new antitumor complex, Wf-1360, Wf-1360A, B, C, D, E and F. J. Antibiot. 1986, 39, 762–772. [Google Scholar] [CrossRef]
- Brendel, N.; Partida-Martinez, L.P.; Scherlach, K.; Hertweck, C. A cryptic PKS-NRPS gene locus in the plant commensal Pseudomonas fluorescens Pf-5 codes for the biosynthesis of an antimitotic rhizoxin complex. Org. Biomol. Chem. 2007, 5, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Henkels, M.D.; Shaffer, B.T.; Valeriote, F.A.; Gross, H. Isolation and identification of rhizoxin analogs from Pseudomonas fluorescens Pf-5 by using a genomic mining strategy. Appl. Environ. Microbiol. 2008, 74, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Henkels, M.D.; Rangel, L.I.; Olcott, M.H.; Walker, F.L.; Bond, K.L.; Kidarsa, T.A.; Hesse, C.N.; Sneh, B.; Stockwell, V.O.; et al. Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf-5 to Drosophila melanogaster. Environ. Microbiol. 2016, 18, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.S.; Prasad, V.; Roach, M.C.; Takahashi, M.; Iwasaki, S.; Luduena, R.F. Interaction of rhizoxin with bovine brain tubulin. Cancer Res. 1990, 50, 4277–4280. [Google Scholar]
- Takahashi, M.; Iwasaki, S.; Kobayashi, H.; Okuda, S.; Murai, T.; Sato, Y. Rhizoxin binding to tubulin at the maytansine-binding site. Biochim. Biophys. Acta 1987, 926, 215–223. [Google Scholar] [CrossRef]
- Hendriks, H.R.; Plowman, J.; Berger, D.P.; Paull, K.D.; Fiebig, H.H.; Fodstad, Ø.; van der Meulen, H.C.D.; Henrar, R.E.C.; Pinedo, H.M.; Schwartsmann, G. Preclinical antitumour activity and animal toxicology studies of rhizoxin, a novel tubulin-interacting agent. Ann. Oncol. 1992, 3, 755–763. [Google Scholar] [CrossRef]
- Tsuruo, T.; Ohara, T.; Iida, H.; Tsukagoshi, S.; Sato, Z.; Matsuda, I.; Iwasaki, S.; Okuda, S.; Shimizu, F.; Sasagawa, K.; et al. Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines. Cancer Res. 1986, 46, 381–385. [Google Scholar]
- Hanauske, A.-R.; Catimel, G.; Aamdal, S.; Huinink, W.T.B.; Paridaens, R.; Pavlidis, N.; Kaye, S.B.; Velde, A.T.; Wanders, J.; Verweij, J. Phase II clinical trials with rhizoxin in breast cancer and melanoma. Br. J. Cancer 1996, 73, 397–399. [Google Scholar] [CrossRef]
- Nakada, M.; Kobayashi, S.; Shibasaki, M.; Iwasaki, S.; Ohno, M. The first total synthesis of the antitumor macrolide, rhizoxin. Tetrahedron Lett. 1993, 34, 1039–1042. [Google Scholar] [CrossRef]
- Neuhaus, C.M.; Liniger, M.; Stieger, M.; Altmann, K.-H. Total synthesis of the tubulin inhibitor WF-1360F based on macrocycle formation through ring-closing alkyne metathesis. Angew. Chem. Int. Ed. 2012, 52, 5866–5870. [Google Scholar] [CrossRef]
- Hong, J.; White, J.D. The chemistry and biology of rhizoxins, novel antitumor macrolides from Rhizopus chinensis. Tetrahedron 2004, 60, 5653–5681. [Google Scholar] [CrossRef]
- Mitchell, I.S.; Pattenden, G.; Stonehouse, J. A total synthesis of the antitumour macrolide rhizoxin. Org. Biomol. Chem. 2005, 3, 4412–4431. [Google Scholar] [CrossRef] [PubMed]
- Karier, P.; Ungeheuer, F.; Ahlers, A.; Anderl, F.; Wille, C.; Fürstner, A. Metathesis at an Implausible Site: A Formal Total Synthesis of Rhizoxin D. Angew. Chem. Int. Ed. 2018, 58, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Mukayama, T.; Naraska, K.; Banno, K. A New Aldol Type Reaction. Chem. Lett. 1973, 2, 1011–1014. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Banno, K.; Narasaka, K. New Cross-Aldol reactions. Reactions of Silyl Enol Ethers with Carbonyl Compounds Activated by Titanium Tetrachloride. J. Am. Chem. Soc. 1974, 96, 7503–7509. [Google Scholar] [CrossRef]
- Paterson, I.; Goodman, M. Aldol reactions of methylketones using chiral boron reagents: A reversal in aldehyde enantioface selectivity. Tetrahedron Lett. 1989, 30, 997–1000. [Google Scholar] [CrossRef]
- Hosomi, A.; Sakurai, H. Syntheses of γ,δ-unsaturated alcohols from allylsilanes and carbonyl compounds in the presence of titanium tetrachloride. Tetrahedron Lett. 1976, 16, 1295–1298. [Google Scholar] [CrossRef]
- Keck, G.E.; Tarbet, K.H.; Geraci, L.S. Catalytic Asymmetric Allylation of Aldehydes. J. Am. Chem. Soc. 1993, 115, 8467–8468. [Google Scholar] [CrossRef]
- White, J.D.; Blakemore, P.R.; Green, N.J.; Hauser, E.B.; Holoboski, M.A.; Keown, L.E.; Nylund Kolz, C.S.; Phillips, B.W. Total Synthesis of Rhizoxin D, a Potent Antimitotic Agent from the Fungus Rhizopus chinensis. J. Org. Chem. 2002, 67, 7750–7760. [Google Scholar] [CrossRef]
- Menche, D.; Hassfeld, J.; Li, J.; Rudolph, S. Total synthesis of archazolid A. J. Am. Chem. Soc. 2007, 129, 6100–6101. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A Useful 12-I-5 Triacetoxyperiodinane (the Dess-Martin Periodinane) for the Selective Oxydation of Primary or Secondary Alcohols and a Variety of Related 12-I-5 Species. J. Am. Chem. Soc. 1991, 113, 7277–7278. [Google Scholar] [CrossRef]
- House, H.O.; Rasmussen, G.H. Stereoselective Synthesis of α-Substituted α,β-Unsaturated Esters. J. Org. Chem. 1961, 26, 11–4278. [Google Scholar] [CrossRef]
- Nahm, S.; Weinreb, S.M. N-methoxy-n-methylamides as effective acylating agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Cazeau, P.; Duboudin, F.; Moulines, F.; Babot, F.; Dunogues, J. A new practical synthesis of silyl enol ethers: II. From α,β-unsaturated aldehydes and ketones. Tetrahedron 1987, 43, 2089–2093. [Google Scholar] [CrossRef]
- Evans, D.A.; Chapman, K.T.; Carreira, E.M. Directed Reduction of β-Hydroxy Ketones Employing Tetramethylammonium Triacetoxyborohydride. J. Am. Chem. Soc. 1988, 110, 3560–3578. [Google Scholar] [CrossRef]
- Sugiyama, H.; Yokokawa, F.; Shioiri, T. Total synthesis of mycothiazole, a polyketide heterocycle from marine sponges. Tetrahedron 2003, 59, 6579–6593. [Google Scholar] [CrossRef]
- Poon, K.W.C.; Dudley, G.B. Mix-and-Heat Benzylation of Alcohols Using a Bench-Stable Pyridinium Salt. J. Org. Chem. 2006, 71, 3923–3927. [Google Scholar] [CrossRef]
- Iversen, T.; Bundle, D.R. Benzyl trichloroacetimidate, a versatile reagent for acid-catalysed benzylation of hydroxy-groups. Chem. Commun. 1981, 1240–1241. [Google Scholar] [CrossRef]
- Negishi, E.-I. Palladium- or Nickel-Catalyzed Cross Coupling. A New Selective Method for Carbon-Carbon Bond Formation. Acc. Chem. Res. 1982, 15, 340–348. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Knappke, C.E.; Von Wangelin, A.J. 35 years of palladium-catalyzed cross-coupling with Grignard reagents: How far have we come? Chem. Soc. Rev. 2011, 40, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Mouri, M.; Gao, Q.; Maruyama, T.; Furuta, K.; Yamamoto, H. Catalytic Asymmetric Allylation Using a Chiral (Acyloxy)borane Complex as a Versatile Lewis Acid Catalyst. J. Am. Chem. Soc. 1993, 115, 11490–11495. [Google Scholar] [CrossRef]
- Williams, D.R.; Meyer, K.G. Total Synthesis of (+)-Amphidinolide K. J. Am. Chem. Soc. 2001, 123, 765–766. [Google Scholar] [CrossRef]
- Schlosser, M. Superbases for organic synthesis. Pure Appl. Chem. 1988, 60, 1627–1634. [Google Scholar] [CrossRef]
- Prandi, C.; Venturello, P. α,β-Unsaturated Acetals as Precursors of α-Substituted Ethoxy Dienes. Useful Reagents for Nucleophilic Acylation. J. Org. Chem. 1994, 59, 5458–5462. [Google Scholar] [CrossRef]
- Moreno-Dorado, F.J.; Guerra, F.M.; Manzo, F.L.; Aladro, F.J.; Jorge, Z.D.; Massanet, G.M. CeCl3/NaClO: A safe and efficient reagent for the allylic chlorination of terminal olefins. Tetrahedron Lett. 2003, 44, 6691–6693. [Google Scholar] [CrossRef]
- Smith, A.B.; Sfouggatakis, C.; Risatti, C.A.; Sperry, J.B.; Zhu, W.; Doughty, V.A.; Tomioka, T.; Gotchev, D.B.; Bennett, C.S.; Sakamoto, S.; et al. Spongipyran synthetic studies. Evolution of a scalable total synthesis of (+)-spongistatin 1. Tetrahedron 2009, 65, 6489–6509. [Google Scholar] [CrossRef]
- Weigand, S.; Brückner, R. Direct Preparation of Allylstannanes from Allyl Alcohols: Convenient Synthesis of β-Substituted Allylstannanes and of Stereodefined γ-Substituted Allylstannanes. Synthesis 1996, 475–482. [Google Scholar] [CrossRef]
- Takano, S.; Inomata, K.; Samizu, K.; Tomita, S.; Yanase, M.; Suzuki, M.; Iwabuchi, Y.; Sugihara, T.; Ogasawara, K. A Convenient One-flask Synthesis of α-Methylenealdehydes from Primary Alcohols. Chem. Lett. 1989, 1283–1284. [Google Scholar] [CrossRef]
- Naruta, Y.; Nishigaichi, Y.; Maruyama, K. Extremely Facile and Stereoselective Preparation of Allylstannanes with Use of Ultrasound. Chem. Lett. 1986, 1857–1860. [Google Scholar] [CrossRef]
- Corey, E.J.; Imwinkelried, R.; Pikul, S.; Xiang, Y.B. Practical enantioselective Diels-Alder and aldol reactions using a new chiral controller system. J. Am. Chem. Soc. 1989, 111, 5493–5495. [Google Scholar] [CrossRef]
- Williams, D.R.; Meyer, K.G.; Shamim, K.; Patnaik, S. Diastereoselectivity in asymmetric allylations: The role of vicinal chirality in the allyl nucleophile for SE2′ reactions with aldehydes. Can. J. Chem. 2004, 82, 120–130. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Regan, A.C. Synthesis of a C1–C9 fragment of rhizoxin. Tetrahedron Lett. 2000, 41, 7619–7622. [Google Scholar] [CrossRef]
- Tebbe, F.N.; Parshall, G.W.; Reddy, G.S. Olefin Homologation with Titanium Methylene Compounds. J. Am. Chem. Soc. 1978, 100, 3611–3613. [Google Scholar] [CrossRef]
- Okazoe, T.; Hibino, J.-I.; Takai, K.; Nozaki, H. Chemoselective methylenation with a methylenedianion synthon. Tetrahedron Lett. 1985, 26, 5581–5584. [Google Scholar] [CrossRef]
- Takai, K.; Nitta, K.; Utimoto, K. Simple and selective method for aldehydes (RCHO). fwdarw.(E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system. J. Am. Chem. Soc. 1986, 108, 7408–7410. [Google Scholar] [CrossRef]
- Aissa, C. Improved Julia−Kocienski Conditions for the Methylenation of Aldehydes and Ketones. J. Org. Chem. 2006, 71, 360–363. [Google Scholar] [CrossRef]
- Lebel, H.; Paquet, V. Rhodium-Catalyzed Methylenation of Aldehydes. J. Am. Chem. Soc. 2004, 126, 320–328. [Google Scholar] [CrossRef]
- Lebel, H.; Parmentier, M. Copper-Catalyzed Methylenation Reaction: Total Synthesis of (+)-Desoxygaliellalactone. Org. Lett. 2007, 9, 3563–3566. [Google Scholar] [CrossRef]
- Li, J.; Menche, D. Selective Deprotection of Silyl Ethers with Sodium Periodate. Synthesis 2009, 1904–1908. [Google Scholar] [CrossRef]
- Blanchette, M.A.; Choy, W.; Davis, J.T.; Essenfeld, A.P.; Masamune, S.; Roush, W.R.; Sakai, T. Horner-Wadsworth-Emmons reaction: Use of lithium chloride and an amine for base-sensitive compounds. Tetrahedron Lett. 1984, 25, 2183–2186. [Google Scholar] [CrossRef]
- Schrodi, Y.; Pederson, R.L. Evolution and Applications of Second-Generation Ruthenium Olefin Metathesis Catalysts. Aldrichim. Acta 2007, 40, 45–52. [Google Scholar]
- Ung, T.; Hejl, A.; Grubbs, R.H.; Schrodi, Y. Latent Ruthenium Olefin Metathesis Catalysts That Contain an N-Heterocyclic Carbene Ligand. Organometallics 2004, 23, 5399–5401. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Tennakoon, M.A.; Wang, J.; Zhao, H. Relay Ring-Closing Metathesis (RRCM): A Strategy for Directing Metal Movement Throughout Olefin Metathesis Sequences. J. Am. Chem. Soc. 2004, 126, 10210–10211. [Google Scholar] [CrossRef]
- Wang, X.; Bowman, E.J.; Bowman, B.J.; Porco, J.A., Jr. Total Synthesis of the Salicylate Enamide Macrolide Oximidine III: Application of Relay Ring-Closing Metathesis. Angew. Chem. Int. Ed. 2004, 43, 3601–3604. [Google Scholar] [CrossRef]
- Robinson, A.J.; Elaridi, J.; Van Lierop, B.J.; Mujcinovic, S.; Jackson, W.R. Microwave-assisted RCM for the synthesis of carbocyclic peptides. J. Pept. Sci. 2007, 13, 280–285. [Google Scholar] [CrossRef]
- Voigtritter, K.; Ghorai, S.; Lipshutz, B.H. Rate Enhanced Olefin Cross-Metathesis Reactions: The Copper Iodide Effect. J. Org. Chem. 2011, 76, 4697–4702. [Google Scholar] [CrossRef]
- Fürstner, A.; Mathes, C.; Lehmann, C.W. Mo[N(t-Bu)(Ar)]3 Complexes As Catalyst Precursors: In Situ Activation and Application to Metathesis Reactions of Alkynes and Diynes. J. Am. Chem. Soc. 1999, 121, 9453–9454. [Google Scholar] [CrossRef]
- Brewitz, L.; Llaveria, J.; Yada, A.; Fürstner, A. Formal Total Synthesis of the Algal Toxin (−)-Polycavernoside A. Chem. Eur. J. 2013, 19, 4532–4537. [Google Scholar] [CrossRef]
- Chaładaj, W.; Corbet, M.; Fürstner, A. Total Synthesis of Neurymenolide A Based on a Gold-Catalyzed Synthesis of 4-Hydroxy-2-pyrones. Angew. Chem. Int. Ed. 2012, 51, 6929–6933. [Google Scholar] [CrossRef] [PubMed]
- Willwacher, J.; Kausch-Busies, N.; Fürstner, A. Divergent Total Synthesis of the Antimitotic Agent Leiodermatolide. Angew. Chem. Int. Ed. 2012, 51, 12041–12046. [Google Scholar] [CrossRef] [PubMed]
- Lehr, K.; Fürstner, A. An efficient route to the musk odorant (R,Z)-5-muscenone via base-metal-catalysis. Tetrahedron 2012, 68, 7695–7700. [Google Scholar] [CrossRef]
- Hickmann, V.; Kondoh, A.; Gabor, B.; Alcarazo, M.; Fürstner, A. Catalysis-Based and Protecting-Group-Free Total Syntheses of the Marine Oxylipins Hybridalactone and the Ecklonialactones A, B, and C. J. Am. Chem. Soc. 2011, 133, 13471–13480. [Google Scholar] [CrossRef]
- Benson, S.; Collin, M.-P.; Arlt, A.; Gabor, B.; Goddard, R.; Fürstner, A. Second-Generation Total Synthesis of Spirastrellolide F Methyl Ester: The Alkyne Route. Angew. Chem. Int. Ed. 2011, 50, 8739–8744. [Google Scholar] [CrossRef]
- Lehr, K.; Mariz, R.; Leseurre, L.; Gabor, B.; Fürstner, A. Total Synthesis of Tulearin C. Angew. Chem. Int. Ed. 2011. [Google Scholar] [CrossRef]
- Micoine, K.; Fürstner, A. Concise Total Synthesis of the Potent Translation and Cell Migration Inhibitor Lactimidomycin. J. Am. Chem. Soc. 2010, 132, 14064–14066. [Google Scholar] [CrossRef]
- Hickmann, V.; Alcarazo, M.; Fürstner, A. Protecting-Group-Free and Catalysis-Based Total Synthesis of the Ecklonialactones. J. Am. Chem. Soc. 2010, 132, 11042–11044. [Google Scholar] [CrossRef]
- Schaubach, S.; Gebauer, K.; Ungeheuer, F.; Hoffmeister, L.; Ilg, M.K.; Wirtz, C.; Fürstner, A. A Two-Component Alkyne Metathesis Catalyst System with an Improved Substrate Scope and Functional Group Tolerance: Development and Applications to Natural Product Synthesis. Chem. Eur. J. 2016, 22, 8494–8507. [Google Scholar] [CrossRef]
- Kwon, Y.; Schulthoff, S.; Dao, Q.M.; Wirtz, C.; Fürstner, A. Total Synthesis of Disciformycin A and B: Unusually Exigent Targets of Biological Significance. Chem. Eur. J. 2018, 24, 109–114. [Google Scholar] [CrossRef]
- Mata, G.; Woelfl, B.; Fürstner, A. Synthesis and Molecular Editing of Callyspongiolide, Part 1: The Alkyne Metathesis/trans-Reduction Strategy. Chem. Eur. J. 2019, 25, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.W. Ring-closing alkyne metathesis in natural product synthesis. In Metathesis in Natural Product Synthesis; Cossy, J., Arseniyadis, S., Meyer, C., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2010; pp. 205–223. [Google Scholar]
- Trost, B.M.; Ball, Z.T. Alkyne Hydrosilylation Catalyzed by a Cationic Ruthenium Complex: Efficient and General Trans Addition. J. Am. Chem. Soc. 2005, 127, 17644–17655. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, F.; Radkowski, K.; Seidel, G.; Fürstner, A. (E)-Cycloalkenes and (E,E)-cycloalkadienes by ring closing diyne- or enyne-yne metathesis/semi-reduction. Tetrahedron 2004, 60, 7315–7324. [Google Scholar] [CrossRef]
- Kleinbeck, F.; Carreira, E.M. Total Synthesis of Bafilomycin A1. Angew. Chem. Int. Ed. 2009, 121, 586–589. [Google Scholar] [CrossRef][Green Version]
- Gilbert, J.C.; Weerasooriya, U. Diazoethenes: Their Attempted Synthesis from Aldehydes and Aromatic Ketones by Way of the Horner-Emmons Modification of the Wittig Reaction. A Facile Synthesis of Alkynes1. J. Org. Chem. 1982, 47, 1837–1845. [Google Scholar] [CrossRef]
- Vatèle, J.-M. Yb(OTf)3-Catalyzed Oxidation of Alcohols with Iodosylbenzene Mediated by TEMPO. Synlett 2006, 2055–2058. [Google Scholar] [CrossRef]
- Lafontaine, J.A.; Provencal, D.P.; Gardelli, C.; Leahy, J.W. Enantioselective Total Synthesis of the Antitumor Macrolide Rhizoxin D. J. Org. Chem. 2003, 68, 4215–4234. [Google Scholar] [CrossRef]
- Hama, N.; Matsuda, T.; Sato, T.; Chida, N. Total Synthesis of (−)-Agelastatin A: The Application of a Sequential Sigmatropic Rearrangement. Org. Lett. 2009, 11, 2687–2690. [Google Scholar] [CrossRef]
- Sternberg, H.W.; Greenfield, H.; Friedel, R.H.; Wotiz, J.H.; Markby, R.; Wender, I. A new type of metallo-organic complex derived fromdicobalt octacarbonyl and acetylenes. J. Am. Chem. Soc. 1954, 76, 1457–1458. [Google Scholar] [CrossRef]
- Greenfield, H.; Sternberg, H.W.; Friedel, R.H.; Wotiz, J.H.; Markby, R.; Wender, I. Acetylenic Dicobalt Hexacarbonyls. Organometallic Compounds Derived from Alkynes and Dicobalt Octacarbonyl. J. Am. Chem. Soc. 1956, 78, 120–124. [Google Scholar] [CrossRef]
- Yenjai, C.; Isobe, M. One-step recyclization of sugar acetylenes to form medium ether rings via dicobalthexacarbonyl complexes. Tetrahedron 1998, 54, 2509–2520. [Google Scholar] [CrossRef]
- Isobe, M.; Yenjai, C.; Tanaka, S. Medium Size Ether Ring Formation of C-Alkynylated Sugars via Dicobalthexacarbonyl Complexes. Synlett 1994, 916–918. [Google Scholar] [CrossRef]
- Hosokawa, S.; Isobe, M. Reductive decomplexation of biscobalthexacarbonyl acetylenes into olefins. Tetrahedron Lett. 1998, 39, 2609–2612. [Google Scholar] [CrossRef]
- Takai, S.; Ploypradith, P.; Hamajima, A.; Kira, K.; Isobe, M. Sodium Hypophosphite Decomplexation of Acetylenebiscobalthexacarbonyls to cis-Olefins. Synlett 2002, 588–592. [Google Scholar] [CrossRef]
- Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio End Group Removal from RAFT-Synthesized Polymers by Radical-Induced Reduction. Macromolecules 2007, 40, 4446–4455. [Google Scholar] [CrossRef]
- Jang, D.O.; Cho, D.H.; Kim, J. Diphenylphosphine Oxide: An Alternative to Organotin Hydrides in the Radical Deoxygenation of Alcohols. Synth. Commun. 1998, 28, 3559–3565. [Google Scholar] [CrossRef]
- Kende, A.S.; Blass, B.E.; Henry, J.R. Enantioselective total synthesis of didesepoxyrhizoxin. Tetrahedron Lett. 1995, 36, 4741–4744. [Google Scholar] [CrossRef]
- Kusebauch, B.; Scherlach, K.; Kirchner, H.; Dahse, H.-M.; Hertweck, C. Antiproliferative Effects of Ester- and Amide-Functionalized Rhizoxin Derivatives. Chemmedchem. 2011, 6, 1998–2001. [Google Scholar] [CrossRef]
- Stella, V.J.; Umprayn, K.; Waugh, W.N. Development of parenteral formulations of experimental cytotoxic agents. I. Rhizoxin (NSC-332598). Int. J. Pharm. 1988, 43, 191–199. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).