Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals
Abstract
:1. Introduction
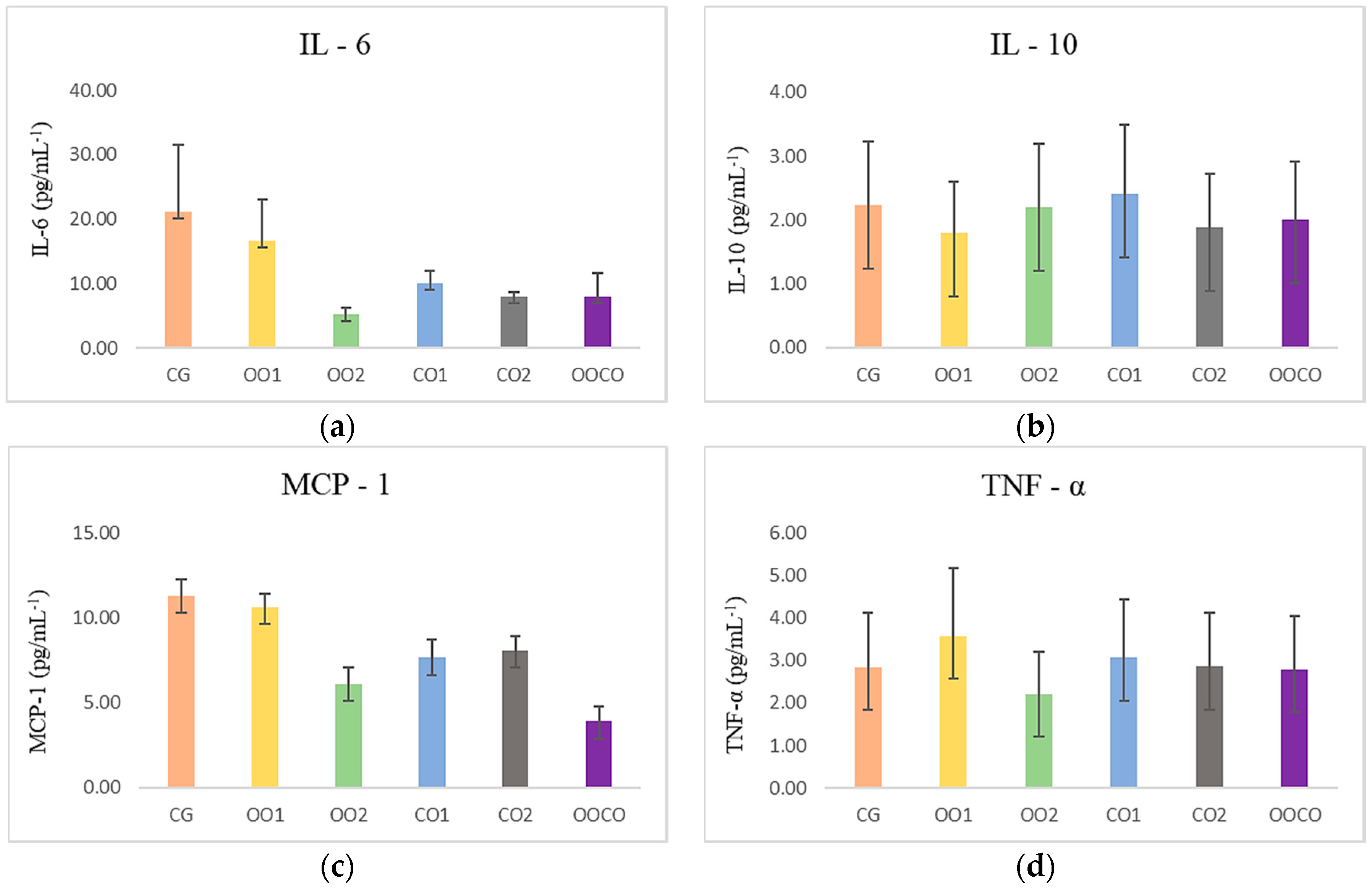
2. Results and Discussion
3. Materials and Methods
3.1. Raw Material
3.2. Quality and Identity of C. brasiliense Oil
3.2.1. Color
3.2.2. Total Carotenoids
3.2.3. Optical Properties
3.2.4. Thermal Analyses: Thermogravimetry/Derived Thermogravimetry (TGA/DTG)
3.2.5. Oxidative Stability
3.2.6. Profile of Fatty Acids
3.3. Experimental Design
3.3.1. Biochemical Analysis
3.3.2. Body Weight, Visceral Fat, and Liver Weight
3.3.3. Histopathological Analysis
3.3.4. Quantification of Cytokines
3.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dyer, J.M.; Stymne, S.; Green, A.G.; Carlsson, A.S. High-value oils from plants. Plant. J. 2008, 54, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Codex Alimentarius. Standard For Named Vegetable Oils Codex Stan 210-1999 Adopted. J. Chem. Inf. Model. 1990, 1–13. [Google Scholar]
- Idris, C.A.C.; Sundram, K.; Razis, A.F.A. Effect of Consumption Heated Oils with or without Dietary Cholesterol on the Development of Atherosclerosis. Nutrients 2018, 10, 1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Sharma, A.; Upadhyaya, K. Vegetable oil: Nutritional and industrial perspective. Curr. Genom. 2016, 17, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.D.; Conceição, J.N.; Oliveira, I.P.; Lescano, C.H.; Muzzi, R.M.; Omar Filho, P.; Caires, A.R. Oxidative stability of baru (Dipteryx alata Vogel) oil monitored by fluorescence and absorption spectroscopy. J. Spectrosc. 2015, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, P.S.; Candido, C.J.; Jaques, J.A.; Nunes, Â.A.; Caires, A.R.; Michels, F.S. Oxidative stability of sesame and flaxseed oils and their effects on morphometric and biochemical parameters in an animal model. J. Sci. Food Agric. 2017, 97, 3359–3364. [Google Scholar] [CrossRef]
- Ben-Dror, K.; Birk, R. Oleic acid ameliorates palmitic acid-induced ER stress and inflammation markers in naive and cerulein-treated exocrine pancreas cells. Biosci. Rep. 2019, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [Green Version]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.D.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; Di Stefano, V.; Di Bella, G.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Fidalgo, S.; Sánchez De Ibargüen, L.; Cárdeno, A.; Alarcón De La Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune. 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Roccisano, D.; Henneberg, M. Soy Consumption and Obesity. Food Sci. Nutr. 2012, 3, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: Potential role for the liver. PLoS ONE 2015, 10, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Roll, M.M.; Miranda-Vilela, A.L.; Longo, J.P.F.; Agostini-Costa, T.S.; Grisolia, C.K. The pequi pulp oil (Caryocar brasiliense Camb.) provides protection against aging-related anemia, inflammation and oxidative stress in Swiss mice, especially in females. Genet. Mol. Biol. 2018, 41, 858–869. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Vilela, A.L.; Pereira, L.C.S.; Gonçalves, C.A.; Grisolia, C.K. Pequi fruit (Caryocar brasiliense Camb.) pulp oil reduces exercise-induced inflammatory markers and blood pressure of male and female runners. Nutr. Res. 2009, 29, 850–858. [Google Scholar] [CrossRef]
- Torres, L.R.O.; Shinagawa, F.B.; Araújo, E.S.; Oropeza, M.V.C.; Macedo, L.F.L.; Almeida-Muradian, L.B.; De Lima, H.C.; Mancini-Filho, J. Physicochemical and antioxidant properties of the pequi (Caryocar brasiliense Camb.) almond oil obtained by handmade and cold-pressed processes. Int. Food Res. J. 2016, 23, 1541–1551. [Google Scholar]
- Traesel, G.K.; De Araújo, F.H.S.; Castro, L.H.A.; Lima, F.F.; Menegati, S.E.L.T.; Justi, P.N.; Kassuya, C.A.L.; Cardoso, C.A.L.C.; Argandoña, E.J.S.; Oesterreich, S.A. Safety assessment of oil from pequi (Caryocar brasiliense Camb.): Evaluation of the potential genotoxic and clastogenic effects. J. M. Food 2017, 20, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Silva, N.R.R.D.; Naves, M.M.V. Potential of Whole Pequi (Caryocar spp.) Fruit—Pulp, Almond, Oil, and Shell—as a Medicinal Food. J. Med. Food 2019, 22, 952–962. [Google Scholar] [CrossRef]
- Hildebrand, C.R.; Silva, A.C.M.B.A.; Thomaz, D.M.C.; Arakaki, D.G.; Inada, A.C.; Marcelino, G.; Gonzaga, C.S.A.M.; Figueiredo, P.S.; Silva, G.T.; Fernandes, M.R.; et al. Animal models in the evaluation of inflammation caused by carbohydrate-rich diets: Brief scientific literature review. IJDR 2017, 7, 15609–15615. [Google Scholar]
- Aremu, M.O.; Ibrahim, H.; Andrew, C. Comparative studies on the lipid composition of blood Plum (Haematostaphis barteri) pulp and seed oils. Open Biochem. J. 2017, 11, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sena, D.M.; Rodrigues, F.F.G.; Freire, P.T.C.; De Lima, S.G.; Coutinho, H.D.M.; Juan, C.L.; Da Costa, J.G.M. Physicochemical and spectroscopical investigation of Pequi (Caryocar coriaceum Wittm.) pulp oil. Grasas. Y. Aceites 2010, 61, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Lescano, C.H.; Iwamoto, R.D.; Sanjinez-Argandoña, E.J.; Kassuya, C.A.L. Diuretic and anti-inflammatory activities of the microencapsulated Acrocomia aculeata (Arecaceae) oil on Wistar rats. J. Med. Food 2015, 18, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.F.G.; Feitosa, M.K.S.B.; Costa, M.S.C.; Tintino, S.R.T.; Rodrigues, F.F.G.; Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M.; Sousa, E.O.S. Characterization, antibacterial activity and antibiotic modifying action of the Caryocar coriaceum Wittm. pulp and almond fixed oil. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Bello, M.O.; Akindele, T.L.; Adeoye, D.O.; Oladimeji, A. Effect of roasting temperature on the physicochemical properties of Jatropha curcas Kernel oil extracted with cold hexane and hot water. Int. J. Basic Appl. Sci. 2011, 11, 9–14. [Google Scholar] [CrossRef]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. A multivariate analysis of the composition and properties of extra virgin olive oils produced from different cultivars grown in Iran. J. AOAC Int. 2017, 100, 1804–1813. [Google Scholar] [CrossRef]
- Marcelino, G.; Donadon, J.R.; Caires, A.R.; Michels, F.S.; Oliveira, L.C.; Cortes, M.R. Characterization and oxidative stability of oils and bioactive compounds of the fruits of Byrsonima cydoniifolia A. Juss. at different ripening stages. J. Sci. Food Agric. 2019, 99, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, X.; Yu, X.; Huyan, Z.; Wang, X. Rapid and simultaneous determination of the iodine value and saponification number of edible oils by FTIR spectroscopy. Eur. J. Lipid Sci. Technol. 2018, 120, 170–396. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Standard for Edible Fats and Oils Not Covered by Individual Standards (1999). Available online: http://www.fao.org/docrep/004/y2774e/y2774e03.htm (accessed on 22 July 2020).
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profile in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DAD-APCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Da Cruz Rodrigues, A.M.; De Freitas, R.A.; De Almeida Meirelles, A.J.; Darnet, S.H.; Da Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Ayala, J.V.; Silva, S.M.; Ceriani, R.; Verhé, R.; Meirelles, A.J. Thermal degradation kinetics of carotenoids in palm oil. J. Am. Oil Chem. Soc. 2013, 90, 191–198. [Google Scholar] [CrossRef]
- Oliveira, I.P.; Correa, W.A.; Neves, P.V.; Silva, P.V.; Lescano, C.H.; Michels, F.S. Optical analysis of the oils obtained from Acrocomia aculeata (jacq.) lodd: Mapping absorption-emission profiles in an induced oxidation process. Photonics 2017, 4, 3. [Google Scholar] [CrossRef]
- Fan, Z.; Krahl, J. Determination of oxidation stability and degradation degree of rapeseed oil methyl ester by fluorescence spectroscopy. Fuel 2017, 195, 123–130. [Google Scholar] [CrossRef]
- Del-Río, J.C.; Evaristo, A.B.; Marques, G.; Martín-Ramos, P.; Martín-Gil, J.; Gutiérrez, A. Chemical composition and thermal behavior of the pulp and kernel oils from macauba palm (Acrocomia aculeata) fruit. Indus Crop. Prod. 2016, 84, 294–304. [Google Scholar] [CrossRef]
- Chen, B.; Mcclements, D.J.; Decker, E.A. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef]
- Lampi, A.M.; Kataja, L.; Kamal-Eldin, A.; Vieno, P. Antioxidant activities of R- and γ-tocopherols in the oxidation of rapeseed oil triacylglycerols. JAOCS 1999, 76, 749–755. [Google Scholar] [CrossRef]
- Romero, N.; Robert, P.; Masson, L.; Ortiz, J.; González, K.; Tapia, K.; Dobaganes, C. Effect of tocopherol, tocotrienol and rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem. 2007, 104, 383–389. [Google Scholar] [CrossRef]
- Vaskova, H.; Buckova, M. Thermal degradation of vegetable oils: Spectroscopic measurement and analysis. Procedia. Eng. 2015, 100, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Kraemer, H.C. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A metaanalysis. Arter. Thromb. Vasc. Biol. 1995, 15, 1917–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.L.; Litman, B.J. Determination of membrane cholesterol partition coefficient using a lipid vesicle–cyclodextrin binary system: Effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 2002, 83, 3408–3415. [Google Scholar] [CrossRef] [Green Version]
- Bos, M.B.; De Vries, J.H.; Feskens, E.J.; Van Dijk, S.J. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 591–598. [Google Scholar] [CrossRef]
- Wanders, A.J.; Brouwer, I.A.; Siebelink, E.; Katan, M.B. Effect of a High Intake of Conjugated Linoleic Acid on Lipoprotein Levels in Healthy Human Subjects. PLoS ONE 2010, 5, e9000. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Morita, S.J. Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol. Pharm Bull. 2016, 39, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Della, M.C.V.; Muñoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germanó, M.J.; Tosi, M.E.R. A Mouse Model of Diet-Induced Obesity Resembling Most Features of Human Metabolic Syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Candido, C.J.; Figueiredo, P.; Del Ciampo, R.S.; Candeloro, L.P. Protective Effect of α-Linolenic Acid on Non-Alcoholic Hepatic Steatosis and Interleukin-6 and-10 in Wistar Rats. Nutrients 2020, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, N.; Savarese, M.; Paduano, A.; Guidone, G.; De Marco, E.; Sacchi, R. Nutritional quality assessment of extra virgin olive oil from the Italian retail market: Do natural antioxidants satisfy EFSA health claims? J. Food Compos. Anal. 2015, 40, 154–162. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3t3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiani, G.; Periago-Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Valoti, M. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, 194–218. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, S.M.; Silva, P.R.; Ferrão, J.S.; Ladd, A.A.; Dagli, M.L.; Grisolia, C.K.; Hernandez-Blazquez, F.J. Chemopreventive effects of pequi oil (Caryocar brasiliense Camb.) on preneoplastic lesions in a mouse model of hepatocarcinogenesis. Eur. J. Cancer Prev. 2015, 25, 299–305. [Google Scholar] [CrossRef]
- Bersch-Ferreira, A.C.; Sampaio, G.R.; Gehringer, M.O.; Torres, E.A.F.S.; Ross-Fernandes, M.B.; Da Silva, J.T.; Torreglosa, C.R.; Kovacs, C.; Alves, R.; Magnoni, C.D.; et al. Association between plasma fatty acids and inflammatory markers in patients with and without insulin resistance and in secondary prevention of cardiovascular disease, a cross-sectional study. Nutr. J. 2018, 17, 26. [Google Scholar] [CrossRef]
- Galan, A.; Mayer, I.; Rafaj, R.B.; Bendelja, K.; Susic, V.; Ceron, J.J.; Mrljak, V. MCP-1, KC-like and IL-8 as critical mediators of pathogenesis caused by Babesia canis. PLoS ONE 2018, 13, e0190474. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.O.; Ryu, H.W.; So, Y.; Jin, C.H.; Baek, J.Y.; Park, K.H.; Byum, E.M.; Jeong, I.Y. Hepatoprotective effect of 2,3-dehydrosilybin on carbon tetrachlorideinduced liver injury in rats. Food Chem. 2013, 138, 107–115. [Google Scholar] [CrossRef]
- Ponmari, G.; Annamalai, A.; Gopalakrishnan, V.K.; Lakshmi, P.T.; Guruvayoorappan, C. NF-kB activation and proinflammatory cytokines mediated protective effect of Indigofera caerulea Roxb. on CCl4 induced liver damage in rats. Int. Immunopharmacol. 2014, 23, 672–680. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press: Urbana, IL, USA, 1990. [Google Scholar]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R.P. Statistical optimisation of cell growth and carotenoid production by Rhodotorula mucilaginosa. Braz. J. Microbiol. 2012, 43, 109–115. [Google Scholar] [CrossRef] [Green Version]
- White, P.A.; Cercato, L.M.; Batista, V.S.; Camargo, E.A.; De Lucca, W., Jr.; Oliveira, A.S. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J. Ethnopharmacol. 2016, 179, 92–100. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Ribas-Filho, J.M.; Nassif, P.A.N.; Dietz, U.A.; Henriques, G.S.; Aoki, S.; Pizzol, F.D. Avaliação morfométrica da mucosa do intestino grosso após derivação jejunoileal em ratos. Arq. Bras. Cir. Dig. 2006, 19, 140–145. [Google Scholar]
- Moraes, C.M. Avaliação das Alterações Inflamatórias e Funcionais do Pulmão no Curso da Pancreatite Aguda Experimental Induzida por Ceruleína. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2013. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, Y.; Wang, B.; Zhao, J.; Yan, X.; Huang, Y.; Du, M.; Zhu, M.J. Maternal obesity exacerbates insulitis and type I diabetes in non-obese diabetic (NOD) mice. Reproduction 2014, 148, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, R.; Parimelazhagan, T.; Shanmugam, S.; Thankarajan, S. Antidiabetic activity of Syzygium calophyllifolium in streptozotocin-nicotinamide induced type-2 diabetic rats. Biomed. Pharm. 2016, 82, 547–554. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Index | Values |
|---|---|
| Peroxide index (mEq O2 kg−1) | 13.63 ± 0.97 |
| Acidity index in oleic acid (mg KOH/g−1) | 1.27 ± 0.02 |
| Saponification index (mg KOH g−1) | 136.5 ± 0.60 |
| Refraction index at 40 °C | 1.46 ± 0.00 |
| Iodine index (g I2 100−1 g) | 76.7 ± 0.72 |
| Relative density | 0.57 ± 0.00 |
| L* | 36.6 ± 0.03 |
| C* | 22.2 ± 0.02 |
| h (deg) | 74.3 ± 0.04 |
| a* | 6.0 ± 0.02 |
| b* | 21.4 ± 0.05 |
| Carotenoids (µg/g) | 2.39 ± 0.04 |
| Fatty Acids | Values |
|---|---|
| Saturated | |
| Butyric. C4:0 | 0.04 ± 0.01 |
| Mystiric. C14:0 | 0.08 ± 0.00 |
| Palmitic. C16:0 | 37.78 ± 1.07 |
| Heptadecanoic. C17:0 | 0.05 ± 0.00 |
| Stearic. C18:0 | 1.84 ± 0.00 |
| Arachidic. C20:0 | 0.19 ± 0.02 |
| Behenic. C22:0 | 0.06 ± 0.01 |
| Lignoceric. C24:0 | 0.07 ± 0.01 |
| TOTAL | 40.04 |
| Monounsaturated | |
| Palmitoleic. C16:1 | 0.57 ± 0.01 |
| Oleic. C18:1 (𝜔-9) | 52.61 ± 1.06 |
| Cis-11- eicosenic. C20:1 | 0.19 ± 0.00 |
| TOTAL | 53.56 |
| Polyunsaturated | |
| Linoleic. C18:2 (𝜔-6) | 3.9 ± 0.15 |
| 0.40 ± 0.00 | |
| 0.04 ± 0.00 | |
| TOTAL | 4.34 |
| Parameters (mg dL−1) | CG | OO1 | OO2 | CO1 | CO2 | OOCO |
|---|---|---|---|---|---|---|
| Total cholesterol | 188.79 ± 9.18 | 177.68 ± 9.59 | 163.22 ± 10.80 | 134.61 ± 5.29 *,§ | 138.90 ± 6.01 *,§ | 149.85 ± 5.15 * |
| HDL-c | 120.93 ± 5.18 | 119.62 ± 7.48 | 118.54 ± 6.37 | 97.26 ± 4.08 | 109.33 ± 6.13 | 111.75 ± 5.74 |
| LDL-c | 43.88 ± 5.02 | 33.36 ± 6.25 | 19.65 ± 6.05 * | 14.10 ± 1.42 * | 6.58 ± 1.88 *,§ | 15.47 ± 2.60 * |
| Non-HDL-c | 69.10 ± 5.90 | 58.05 ± 6.46 | 44.68 ± 6.77 | 37.35 ± 1.88 * | 29.57 ± 1.83 *,§ | 38.08 ± 2.91 * |
| VLDL-c | 23.97 ± 4.18 | 24.70 ± 3.06 | 25.03 ± 3.31 | 23.25 ± 3.95 | 22.99 ± 2.35 | 22.62 ± 2.67 |
| Triglycerides | 188.77 ± 5.80 | 123.49 ± 4.43 | 125.16 ± 4.26 | 116.25 ± 5.27 | 114.97 ± 2.72 | 113.14 ± 3.86 |
| Glucose | 180.64 ± 21.50 | 179.12 ± 16.78 | 234.46 ± 13.19 | 216.68 ± 14.47 | 191.90 ± 10.33 | 229.29 ± 13.89 |
| Parameters | CG | OO1 | OO2 | CO1 | CO2 | OOCO |
|---|---|---|---|---|---|---|
| Initial weight (g) | 39.231 ± 1.277 | 37.692 ± 1.157 | 40.286 ± 1.197 | 39.429 ± 1.561 | 38.500 ± 1.185 | 39.333 ± 0.873 |
| Final weight (g) | 48.923 ± 1.916 | 48.615 ± 1.591 | 52.714 ± 1.535 | 51.286 ± 1.871 | 47.429 ± 1.349 | 46.333 ± 2.028 |
| Omental weight (g) | 0.073 ± 0.011 | 0.050 ± 0.009 | 0.053 ± 0.006 | 0.038 ± 0.007 * | 0.031 ± 0.006 * | 0.026 ± 0.004 * |
| Epididymal weight (g) | 1.970 ± 0.224 | 1.817 ± 0.123 | 1.715 ± 0.175 | 1.583 ± 0.177 | 1.281 ± 0.134 * | 1.055 ± 0.132 *,§ |
| Mesenteric weight (g) | 1.008 ± 0.110 | 0.970 ± 0.095 | 1.083 ± 0.164 | 0.847 ± 0.107 | 0.850 ± 0.115 | 0.528 ± 0.074 *,§,¥ |
| Retroperitoneal weight (g) | 0.693 ± 0.088 | 0.670 ± 0.065 | 0.488 ± 0.064 | 0.462 ± 0.066 | 0.363 ± 0.047 *,§ | 0.330 ± 0.036 *,§ |
| Perirenal weight (g) | 0.391 ± 0.046 | 0.368 ± 0.047 | 0.304 ± 0.050 | 0.212 ± 0.030 * | 0.209 ± 0.031 * | 0.151 ± 0.017 *,§ |
| Adiposity index (%) | 8.290 ± 0.620 | 7.888 ± 0.378 * | 6.799 ± 0.632 * | 5.986 ± 0.567 | 5.640 ± 0.515 § | 4.422 ± 0.436 *,§,¥ |
| Liver (g) | 1.70 ± 0.086 | 1.66 ± 0.070 | 1.55 ± 0.118 | 1.43 ± 0.093 | 1.33 ± 0.035 *,§ | 1.40 ± 0.046 |
| Variable | CG | OO1 | OO2 | CO1 | CO2 | OOCO |
|---|---|---|---|---|---|---|
| Steatosis (p = 0.17 A) | ||||||
| <5% | 36.36 (4) | 60.0 (6) | 80.0 (8) | 80.0 (8) | 40.0 (4) | 66.7 (6) |
| 5–33% | 18.18 (2) | 20.0 (2) | 10.0 (1) | 10.0 (1) | 40.0 (4) | 33.3 (3) |
| 33–66% | 45.45 (5) | 20.0 (2) | 10.0 (1) | 10.0 (1) | 20.0 (2) | 0.0 (0) |
| Microvesicular Steatosis (p = 0.45) | ||||||
| Absent | 81.8 (9) | 90.0 (9) | 100.0 (10) | 90.0 (9) | 100.0 (10) | 88.9 (8) |
| Present | 18.2 (2) | 10.0 (1) | 0.0 (0) | 10.0 (1) | 0.0 (0) | 11.1 (1) |
| Lobular Inflammation (p = 0.16 B) | ||||||
| Absent | 54.6 (6) | 70.0 (7) | 50.0 (5) | 50.0 (5) | 20.0 (2) | 77.8 (7) |
| <2 focus | 45.4 (5) | 30.0 (3) | 50.0 (5) | 50.0 (5) | 70.0 (7) | 22.2 (2) |
| 2–4 focuses | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 10.0 (1) | 0.0 (0) |
| Ballooning (p = 0.06 B) | ||||||
| Absent | 72.7 (8) | 80.0 (8) | 20.0 (2) | 0.0 (0) | 10.0 (1) | 0.0 (0) |
| Few cells | 27.3 (3) | 20.0 (2) | 20.0 (2) | 30.0 (3) | 30.0 (3) | 11.1 (1) |
| Many cells | 0.0 (0) | 0.0 (0) | 40.0 (6) | 70.0 (7) | 60.0 (6) | 88.9 (8) |
| Mallory’s Hyaline (p < 0.001 *,B) | ||||||
| Absent | 100.0 (11) | 80.0 (8) | 40.0 (4) | 20.0 (2) | 40.0 (4) | 11.1 (1) |
| Rare | 0.0 (0) | 20.0 (2) | 40.0 (4) | 40.0 (4) | 40.0 (4) | 66.7 (6) |
| Some | 0.0 (0) | 0.0 (0) | 20.0 (2) | 40.0 (4) | 20.0 (2) | 22.2 (2) |
| Apoptosis (p = 0.001 *) | ||||||
| Absent | 81.8 (9) | 100.0 (10) | 70.0 (7) | 20.0 (2) | 30.0 (3) | 44.4 (4) |
| Present | 18.2 (2) | 0.0 (0) | 30.0 (3) | 80.0 (8) | 70.0 (7) | 55.6 (5) |
| Glycogenated Nuclei (p = 0.07) | ||||||
| None/rare | 100.0 (11) | 90.0 (9) | 100.0 (10) | 90.0 (9) | 100.0 (10) | 88.9 (8) |
| Some | 0.0 (0) | 10.0 (1) | 0.0 (0) | 10.0 (1) | 0.0 (0) | 11.1 (1) |
| Variable | Experimental Group % (n) | |||||
|---|---|---|---|---|---|---|
| CG | OO1 | OO2 | CO1 | CO2 | OOCO | |
| Islet of Langerhans (p = 93 A) | ||||||
| No change | 45.4 (5) | 33.33 (3) | 36.4 (4) | 33.33 (3) | 45.4 (5) | 36.4 (4) |
| Discrete atrophy | 36.4 (4) | 66.7 (6) | 33.33 (3) | 33.33 (3) | 33.33 (3) | 33.33 (3) |
| Atrophy | 0.0 (0) | 0.0 (0) | 22.2 (2) | 33.3 (3) | 11.1 (1) | 0.0 (0) |
| Hypertrophy | 9.1 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 22.2 (2) |
| Not available | 9.1 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Pancreatic Acini (B) | ||||||
| No change | 100.0 (11) | 100.0 (9) | 100.0 (9) | 100.0 (9) | 100.0 (9) | 100.0 (9) |
| Inflammatory Cells (p = 0.38) | ||||||
| No change | 100.0 (11) | 100.0 (9) | 88.9 (8) | 100.0 (9) | 100.0 (9) | 100.0 (9) |
| Insulits | 0.0 (0) | 0.0 (0) | 11.1 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Silva, G.; Di Pietro Fernandes, C.; Hiane, P.A.; Freitas, K.d.C.; Figueiredo, P.S.; Inada, A.C.; Filiú, W.F.; Maldonade, I.R.; Nunes, Â.A.; Oliveira, L.C.S.d.; et al. Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals. Molecules 2020, 25, 4530. https://doi.org/10.3390/molecules25194530
Torres Silva G, Di Pietro Fernandes C, Hiane PA, Freitas KdC, Figueiredo PS, Inada AC, Filiú WF, Maldonade IR, Nunes ÂA, Oliveira LCSd, et al. Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals. Molecules. 2020; 25(19):4530. https://doi.org/10.3390/molecules25194530
Chicago/Turabian StyleTorres Silva, Gabriela, Carolina Di Pietro Fernandes, Priscila Aiko Hiane, Karine de Cássia Freitas, Priscila Silva Figueiredo, Aline Carla Inada, Wander Fernando Filiú, Iriani Rodrigues Maldonade, Ângela Alves Nunes, Lincoln Carlos Silva de Oliveira, and et al. 2020. "Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals" Molecules 25, no. 19: 4530. https://doi.org/10.3390/molecules25194530
APA StyleTorres Silva, G., Di Pietro Fernandes, C., Hiane, P. A., Freitas, K. d. C., Figueiredo, P. S., Inada, A. C., Filiú, W. F., Maldonade, I. R., Nunes, Â. A., Oliveira, L. C. S. d., Caires, A. R. L., Michels, F., Candido, C. J., Cavalheiro, L. F., Arakaki Asato, M., Rodrigues Donadon, J., Bacelar de Faria, B., Tatara, M. B., Rosa Croda, J. H., ... Guimarães, R. d. C. A. (2020). Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals. Molecules, 25(19), 4530. https://doi.org/10.3390/molecules25194530








