Abstract
Novel organocatalytic systems based on the recently developed (S)-proline derivative (2S)-[5-(benzylthio)-4-phenyl-(1,2,4-triazol)-3-yl]-pyrrolidine supported on mesoporous silica were prepared and their efficiency was assessed in the asymmetric aldol reaction. These materials were fully characterized by FT-IR, MS, XRD, and SEM microscopy, gathering relevant information regarding composition, morphology, and organocatalyst distribution in the doped silica. Careful optimization of the reaction conditions required for their application as catalysts in asymmetric aldol reactions between ketones and aldehydes afforded the anticipated aldol products with excellent yields and moderate diastereo- and enantioselectivities. The recommended experimental protocol is simple, fast, and efficient providing the enantioenriched aldol product, usually without the need of a special work-up or purification protocol. This approach constitutes a remarkable improvement in the field of heterogeneous (S)-proline-based organocatalysis; in particular, the solid-phase silica-bonded catalytic systems described herein allow for a substantial reduction in solvent usage. Furthermore, the supported system described here can be recovered, reactivated, and reused several times with limited loss in catalytic efficiency relative to freshly synthesized organocatalysts.
1. Introduction
In the last two decades organocatalysis has developed into an important strategy in asymmetric organic synthesis because of its enormous potential in the efficient preparation of chiral molecules [1,2,3,4,5]. In this regard, immobilization and recycling of asymmetric organic catalysts is of significant current interest [6]. Of particular relevance is research involving immobilization and recycling of organic catalysts such as proline and proline derivatives [7,8]. In order to provide useful catalytic materials to be applied in stereoselective transformations, one important challenge with catalyst immobilization is to retain the activity and stereoselectivity of the immobilized catalysts. Furthermore, the separation and recovery of the immobilized catalysts should be readily achieved by a simple operation such as filtration.
In this context, polymeric materials are widely used as useful supports in heterogeneous organic synthesis and catalysis [9]. For example, several small peptides have been immobilized on insoluble supports such as silica in order to get easily recyclable catalytic materials [10]. Thus, immobilization of organocatalysts on insoluble supports allows recovery and recycling of organic catalysts, thus providing more sustainable synthetic protocols [11].
In this context, several representative organocatalysts present two or more functional groups that act in cooperative or bifunctional strategies [12,13,14,15,16,17,18]. Several research groups have used natural and unnatural amino acids and peptides [19,20,21,22,23,24,25,26,27,28,29,30,31], chiral ureas and thioureas [32,33,34,35,36,37,38,39,40], and chiral amides [41,42,43,44,45] as building blocks or templates in organocatalyst design. In this context, a significant number of (S)-proline derivatives have been developed while searching for an improvement in the catalytic efficiency and stereoselectivity [46,47,48,49,50,51,52,53].
The present work was inspired by recent studies describing novel mesoporous and nanoporous materials based on modified silicas, PEG-resins, zeolites, and MOFs that function as solid supports for the corresponding organocatalytic modules and are capable to catalyze organic reactions such as the Henry reaction, Negishi coupling, and the aldol reaction [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Remarkably, mesoporous and nanoporous silicas [58,62,67,69,70,71] have been widely used as hosts for organocatalytic acids or bases, which by comparison with other solid supported-materials are more affordable, versatile and more resilient in a variety of synthetic organic transformations [72,73].
On the other hand, our research group recently reported the preparation of three novel and efficient (S)-proline-derived organocatalysts containing a 1,2,4-triazolyl-5-thione moiety, that proved highly efficient in asymmetric aldol reactions [74]. Relevantly, this organocatalytic system does not require of the presence of any additive to accomplish the desired transformation affording an eco-friendly protocol that avoids extra purification procedures to obtain the corresponding aldol products. Furthermore, one can take advantage of the 1,2,4-triazole moiety as a hydrogen bridge inducer to potentially anchor electrophilic aldehydes used in the aldol reaction (see ref. [74] for more details about the reaction’s mechanism and the synthesis of the catalyst).
Herein we report the development of four novel supported catalysts that incorporate such (S)-proline-based organocatalysts on mesoporous commercial silica. Significantly, the resulting heterogeneous organocatalysts proved highly efficient providing the desired aldol products without the need of laborious purification procedures. Furthermore, the silica-supported organocatalysts can be conveniently recycled and reused in a green aldol reaction protocol.
2. Results and Discussion
Nowadays the chemical literature describes two main methodologies employed to covalently bind organic catalysts to silica. The first one is called “co-condensation method” [75,76,77], which consists of the initial synthesis of the required precursors, is followed by a second step involving the coupling or incorporation on the solid support. The second methodology is denominated “post-synthetic grafting”, which focuses on the step-by-step construction of the catalyst on the silica surface [78,79,80,81]. As it turned out, the present development required a hybrid synthetic approach starting from commercial silica and involving both co-condensation and post-synthetic grafting starting from commercial silica, as illustrated in the preparation of compound (S)-5 (Scheme 1).
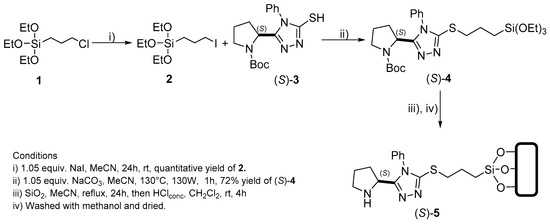
Scheme 1.
Synthetic route for the preparation of supported organocatalyst (S)-5.
Thus, seeking to increase the reactivity of the halide as leaving group for SN2 reactions, a Finkelstein-type reaction was carried out on (3-chloropropyl)triethoxysilane 1 to obtain the corresponding iodide derivative 2 in quantitative yield [82]. Siloxyiodide 2 was subjected to an SN2 substitution reaction with thiol (S)-3 (synthetized according to the previously described procedure [74]) under basic conditions to obtain thioether (S)-4 in 72% yield. Subsequently 1.0 mmol of thioether (S)-4 was exposed to 2.64 g of commercial silica and heated to reflux for 24 h. The silica-supported derivative (S)-5 was filtered and treated with five drops of conc. HCl to remove the N-tert-butoxy carbonyl (N-Boc) protecting group. Lastly, the resulting suspension of the silica-supported catalyst was filtered, and impurities were removed under Soxhlet extraction (see general procedure 7). Finally, the solid product (S)-5 was filtered, dried, and isolated. According to gravimetric analysis, 18% (0.18 mmol, 44.22 mg) of (S)-4 was incorporated to 2.64 g of silica; this amount represents 1.67% w/w of the organocatalyst on the support.
On the other hand, an alternative, step-by-step strategy for the incorporation of the organocatalyst to the silica´s surface was explored. Scheme 2 shows the corresponding procedure: triethoxide silane 1 was reacted with silica, under microwave activation (MW) for 1.5 h in the presence of 0.1 equiv. of pyridine and toluene as solvent [79]. The crude product 6 was filtered and washed with hexane, EtOAc, acetone, water and MeOH before it was dried under vacuum to afford pure silica-supported chloride 6 in quantitative yield. Siloxy-chloride 6 was subjected to a Finkelstein reaction to exchange chloride for iodide to afford derivative 7 in quantitative yield. Subsequently, siloxy-iodide 7 was suspended in acetonitrile and treated with thiol (S)-3 in the presence of base and under MW irradiation to afford silica-supported, N-Boc-protected organocatalyst (S)-8. Finally, removal of the N-Boc group was achieved with conc. HCl to give (S)-5 in 96% yield.
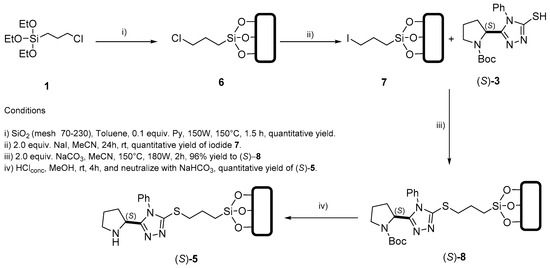
Scheme 2.
Optimized synthetic route for the preparation of silica-supported organocatalyst (S)-5.
Once the supported organocatalyst (S)-5 was available in sufficient quantity, its load on the silica support was determined by TGA and gravimetric analysis (see Supplementary Materials), finding that heterocycle (S)-3 had been incorporated in a ratio of 114.6 mg (0.4641 mmol) per gram of silica, which represents a 4.34% w/w ratio. This load is significantly higher than the one observed according to the synthetic route described in Scheme 1. For this reason, the approach described in Scheme 2 was employed in the preparation of additional silica-supported organocatalysts. In terms of composition, morphology, and organocatalyst’s distribution in different batches of material, the reproducibility of the synthetic protocol was rather good, as evidenced by the appearance of representative micrographs. In the case of particle size, conventional heating results in reduced fragmentation of the samples, relative to preparation under microwave heating; nevertheless, the difference is not significant.
Scheme 3 depicts the synthetic route followed to incorporate a 1,2,3-triazole group as a connecting block within the organocatalytic system. According to previous reports, the triazole segment could act as a good hydrogen bond acceptor at the transition state of the aldol reaction increasing the rigidity of such transition state and in this way possibly increasing the stereoselectivity of the organocatalytic system [83]. In the present system, the triazole fragment was used as spacer and connector between the silica support and the prolinol group that is involved in enamine formation, since it was anticipated that this would enable hydrogen bond interactions to anchor electrophilic substrates during the aldol reaction [83,84,85,86,87].
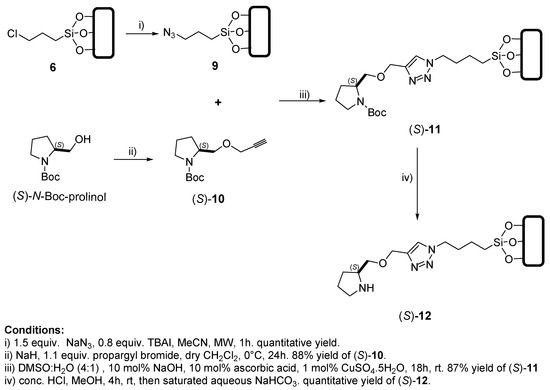
Scheme 3.
Convergent synthetic route followed for the preparation of (S)-12 using the “in situ generation” approach.
Firstly, chloride 6 was subjected to an SN2 reaction to introduce an azide group in derivative 9. This substitution reaction proceeded in quantitative yield. On the other hand, N-Boc-(S)-prolinol, which was prepared according to previous literature reports [88], was used as starting material to generate, by means of NaH treatment followed by propargyl bromide addition, the corresponding propargyl ether derivative (S)-10 in 88% yield [89,90]. Subsequently, compounds 9 and (S)-10 reacted under the conditions of a Huisgen type-reaction [79,84,91], followed by a deprotection process in acidic media. Following neutralization with NaHCO3, a silica-anchored organocatalyst (S)-12 was obtained in 87% yield (Scheme 3).
Organocatalysts (2S,4R,1′S)-18 and (2S,4R,1′R)-18 incorporate an amide functional group in the (S)-proline-framework. This structural feature has proved rather successful in other organocatalysts derived from (S)-proline [42,43,44]. Furthermore, the fragment of (R)- or (S)-phenylethylamine was introduced to examine the potential effect of an additional center of chirality in the organocatalytic system. The synthetic route is outlined in Scheme 4. Firstly, trans-(2S,4R)-hydroxyproline was N-protected with benzyl chloroformate to provide the substrate (2S,4R)-13, which was activated with ethyl chloroformate before treatment with (R)- or (S)-phenylethylamine [(R)-14 or (S)-14]. Then the coupling products were treated with propargyl bromide to afford the propargylic ethers (2S,4R,1′S)-16 and (2S,4R,1′R)-16 in 67% overall yield. These terminal alkynes were subjected to a Huisgen-type protocol [79,84,91] with azide 9 and deprotected under basic conditions to afford (2S,4R,1′R)-18 or (2S,4R,1′S)-18 in 77% of yield in both cases.
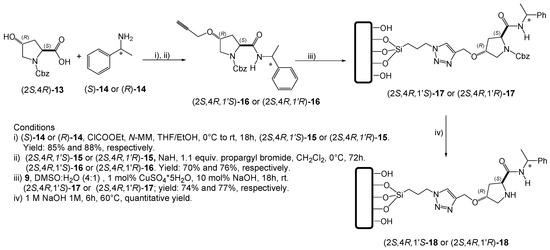
Scheme 4.
Synthetic route followed to generate (2S,4R,1′S)-18 and (2S,4R,1′R)-18.
Figure 1 presents the four silica-supported organocatalysts developed in this work to carry out asymmetric aldol-type reactions.
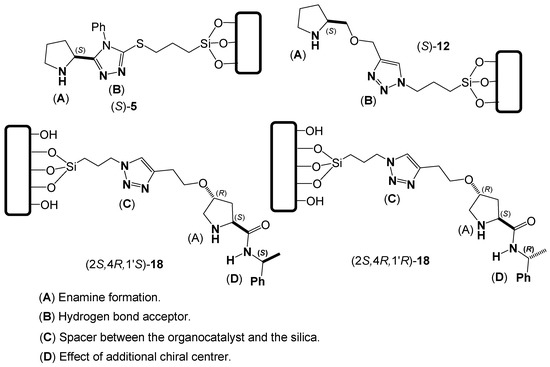
Figure 1.
Novel silica-supported organocatalysts synthesized in this work.
Evaluation of Organocatalysts (S)-5, (S)-12, (2S,4R,1′S)-18 and (2S,4R,1′R)-18
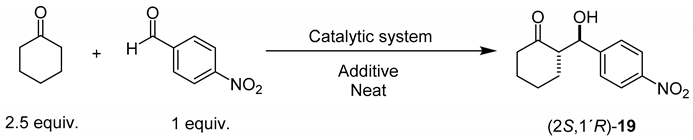
With the four novel silica-supported organocatalysts at hand, their catalytic efficiency in the enantioselective aldol reaction between cyclohexanone and 4-nitrobenzaldehyde was assessed. Table 1 summarizes the outcome of the reactions that were carried out initially. First, and according to previous work [74] it was assumed that best results would be observed under neat conditions or in the presence of water. However, as it can be appreciated in entries 1 and 2, although reaction yields were quite high, the observed stereoselectivities turned out to be low. Therefore, the potential beneficial effect of various additives that are commonly used as adjuvant to increase the strength of non-covalent interactions in the transition state of the catalytic cycle [92,93,94,95] was examined (entries 3–6 in Table 1).

Table 1.
Asymmetric aldol reaction between cyclohexanone and 4-nitrobenzaldehyde in the presence of organocatalysts (S)-5, (S)-12, (2S,4R,1′S)-18 and (2S,4R,1′R)-18.
Best results, in terms of yield (99%) and enantioselectivity (92% ee) were recorded with silica-supported organocatalyst (S)-5, employing an amount of silica equivalent to 10 mol% of catalyst and 10 mol% of p-nitrobenzoic acid as additive, under solvent-free conditions (Table 1, entry 6). Nevertheless, the diastereoselectivity was only moderate.
In the case of organocatalyst (S)-12, although the reaction yield is comparable to those observed with (S)-5, the catalyst has the capacity to give moderate-to-high diastereoselectivity in the presence of water as additive (see entry 10 in Table 1). Similar stereoselectivity was observed with diastereomeric organocatalysts (2S,4R,1′S)-18 and (2S,4R,1′R)-18. This observation suggests that the α-phenylethyl group is not sufficiently close to the reacting site to provoke noticeable stereoinduction.
Once the best reaction conditions had been established (Table 1, entry 6), substrate screening was performed in aldol-type reactions. Several aromatic aldehydes were tested as electrophiles obtaining excellent yields in all cases and moderate diastereo- and enantio-selectivities (Table 2).

Table 2.
Scope of the asymmetric aldol reaction between cyclohexanone and aromatic aldehydes catalyzed by (S)-5.
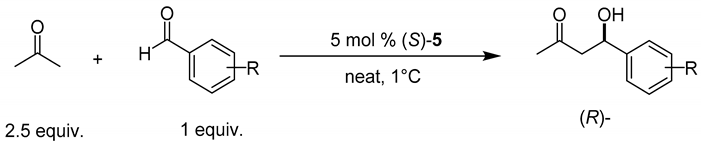
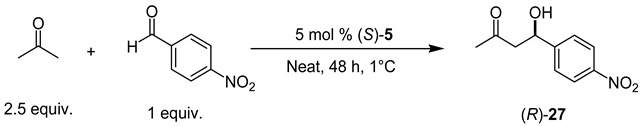
As seen in Table 2, rather high yields are recorded in all cases, although the observed diastereomeric and enantiomeric values are moderate. With these results on hand it was decided to extend the reaction scope using acetone as enamine precursor. It is well known that it is difficult to control the selectivity of enantioselective aldol reaction employing acetone [96,97,98,99,100,101] owing to its high symmetry. Initially, an optimization of the reaction conditions to obtain (R)-27 was carried out finding that best results are obtained under solvent-free reaction conditions, employing (S)-5 as organocatalyst and at a temperature of 1 °C (Table 3).

Table 3.
Asymmetric aldol reaction between acetone and aromatic aldehydes employing (S)-5 as catalyst.
Table 3 shows the results achieved in the asymmetric aldol reaction employing acetone as enamine precursor and several aromatic aldehydes as electrophiles. In a control experiment, the reaction does not proceed in the absence of organocatalyst. By contrast, excellent reaction yields were recorded when catalyzed by (S)-5, although the enantioselectivity changed from low to moderate. In entries 4, 6, 9 and 10, a purification process was necessary to isolate the pure aldol products because of minor impurities; nevertheless, in the rest of the cases, no purification was necessary. The product was simply filtered and dried, which represents a great advantage in terms of sustainability of the process, because of the minimal amount of solvent required to purify the products [102,103,104,105].
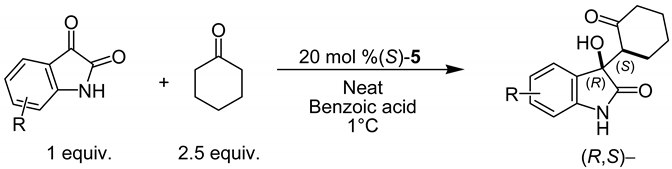
A silica-supported organocatalyst (S)-5 was also evaluated in the aldol reaction between the enamine derived from cyclohexanone and relevant isatins. Table 4 summarizes the results that exhibit excellent yields and moderate diastereo- and enantio-selectivities. It is worthy of mention that recovery of the catalyst required a reactivation process (see Material and Methods section).

Table 4.
Asymmetric aldol reaction between isatin derivatives and aromatic aldehydes catalyzed by (S)-5.
One important advantage when using immobilized catalysts and supported organocatalytic systems is that the catalyst can be recovered and reused. The reuse of organocatalyst (S)-5, which is covalently bonded to the supporting material-silica in the present study, is no exception. Indeed, recovery and reuse of the organocatalyst was feasible up to 15 catalytic cycles in the aldol reaction between the enamine derived from acetone and 4-nitrobenzaldehyde, as can be appreciated in Table 5. Although the stereoselectivity was slightly diminished with each cycle, the high reaction yield was maintained.

Table 5.
Asymmetric aldol reaction between acetone and aromatic aldehydes employing silica-supported (S)-5 as organocatalyst.
The decrease in catalytic efficiency suggests that the silica support possibly suffers a contamination provoked by an impurity that could be generated during each reaction process. Indeed, comparison of XRD and SEM images of fresh and reused catalyst (S)-5, shows that both materials present an irregular shape with a smooth surface characteristic of mesoporous amorphous solids (see Figure 2, A1 to C2). Although the particle size corresponds to a mesoporous solid, the micrographic analysis exhibits significant fragmentation of the material apparently provoked by microwave irradiation. Furthermore, comparison of recovered catalyst with commercial silica shows that the former material does not present the characteristic roughness exhibited in commercial silica. In Figure 2, C2 shows the formation of some clusters that can be formed by the accumulation of additive used in each reaction. It is also worthwhile to point out that TGA analysis of a reused catalyst showed that rather than decreasing, the amount of organic material increased (see Supplementary Materials).
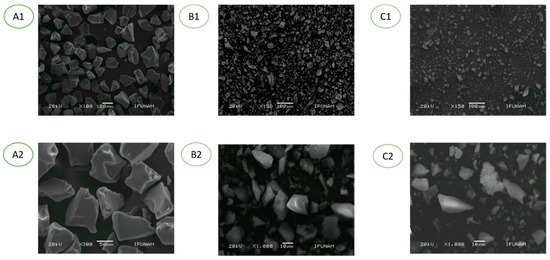
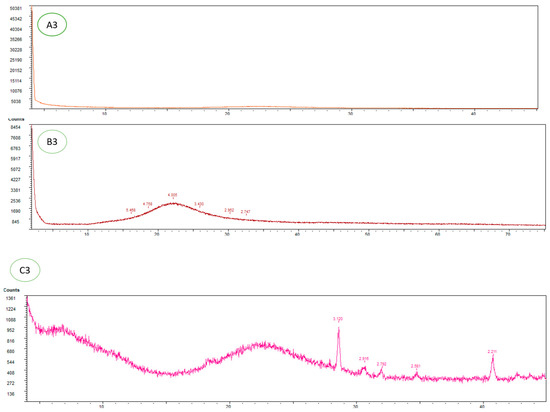
Figure 2.
SEM and XRD imaging: A1–A3 commercial silica. B1–B3 Silica-supported organocatalyst (S)-5. C1–C3 Silica-supported organocatalyst (S)-5 after 10 catalytic cycles.
As for an XRD analysis, commercial silica Figure 2(A3) exhibits the anticipated diffractogram characteristic of mesoporous material; that is, no peaks are visible. By contrast, in the case of (S)-5 Figure 2(B3) a broad signal at ca. 22.2° is recorded, with absence of other peaks confirming that our material conserves its mesoporous structure and suggest that our organocatalyst is well-distributed. Finally, for the recycled (S)-5 material Figure 2(C3), one encounters an intense diffraction pattern that apparently is caused by the presence of additive (p-nitrobenzoic acid), arising from condensation with active Si-OH groups. Additionally, in order to expand the study of the composition and distribution of the organocatalyst on silica, an FT-IR analysis exhibited some characteristics bands (3500, broad signal from 3300 to 3000 cm−1 and 2200 to 1900 cm−1). These signals appear to confirm the presence of the organocatalyst in the material (S)-5 (see Supplementary Materials, page S72). Indeed, TGA analysis shows that the amount of organic material present on the silica-supported catalyst (Figure 2(C3)) is greater than that found in freshly prepared (S)-5 (Figure 2(B3)). Furthermore, substantial weight loss takes place in the temperature range between 200 and 450 °C, which is in line with leaking of organic material, suggesting that the additive (p-nitrobenzoic acid) had incorporated to the silica surface. Indeed, the amount of organic material on the silica-supported material (Figure 2(C3)) is greater than that found in freshly prepared (S)-5 (Figure 2(B3)). Therefore, the weight loss that takes place in the temperature range between 200 and 450 °C is in line with the leaking of organic compounds [106,107].
3. Materials and Methods
3.1. Starting Materials and Their Analysis
Unless otherwise indicated, all reagents were purchased from Sigma-Aldrich and used without further purification. The progress of reactions was routinely monitored by TLC on silica gel 60 (precoated F254 Merck plates) under UV lamp (254 nm) irradiation to visualize starting materials and products. Flash chromatography was performed using silica gel (230−400 mesh).
NMR spectra were acquired on JEOL ECA 500 and Bruker 400 Avance III HD spectrometers. For the acquisition of 1H NMR and 13C NMR spectra, CDCl3 (7.26 and 77.0 ppm, respectively) or DMSO-d6 (2.50 and 39.5 ppm, respectively) were used as internal references. Chemical shifts (δ) are reported in parts per million (ppm). Multiplicity was abbreviated as follows: s = singlet, bs = broad singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, quint = quintet, sext = sextet, hept = heptet, m = multiplet. High resolution mass spectra were recorded on an HPLC 1100 coupled to MSD-TOF Agilent series HR-MSTOF mass spectrometer model 1969 A.
The HPLC analysis was performed in a Dionex HPLC Ultimate 3000 with a UV/Visible detector, and with diode array, at 210 and 254 nm, chiral columns such as AD-H for compounds (R,S)-38, (R,S)-39, (R,S)-40, AS-H for compounds (R)-27-37 and OD-H for (R,S)-26 compound, were employed. IR spectra were acquired on an ATR-IR Varian-640 and are expressed in wave number (cm−1). Optical rotations were measured on an Anton Paar MCP-100 Polarimeter with reagent grade solvents. Melting points were taken on a Büchi B-540 apparatus and are uncorrected. Microwave-assisted procedures were performed on the Biotage-Initiator microwave system. The micrographic images were taken in a JEOL SEM 5600LV apparatus with an acceleration voltage of 20 kV.
XRD analyses were carried out in a Bruker Advance eco. Goniometer: 280 mm. Axis movement: theta/theta. Detector: one-dimensional “lynx eye”. Generator: 1 kW, Samples are measured with: 25 mA, 30 KV. Radiation: Cu (Kalpha1 + Kalpha2) (1.5416 A). Sample rotation speed: 20 r.p.m. Counting time: 5 s. Step size: 0.02. Degree2-theta measurement interval: 5–55 degrees, and 5–70 degrees. Measurement software: DIFFRAC.MESUREMENT. Analysis Software: DIFFRAC.EVA.
3.2. Procedures and General Methods
3.2.1. General Procedure 1: Finkelstein Reaction
In a 50-mL round bottom flask provided with a magnetic bar, the corresponding halide derivative (0.264 g, 1 equiv.), NaI (0.300 g, 2 equiv.) and 10 mL of MeCN or acetone were added. The resulting mixture was stirred for 24 h at room temperature, the solvent was evaporated, and the residue washed with diethyl ether (3 × 10 mL) to give the corresponding iodide product.
3.2.2. General Procedure 2: Propargylation Reaction, in the Synthesis of (S)-10, (2S,4R,1′-S)-16 and (2S,4R,1′-R)-16
In a 50-mL round bottom flask provided with a magnetic bar, the corresponding alcohol (1 mmol, 1 equiv.), propargyl bromide (1.1 mmol, 1.1 equiv.), NaH (2 mmol, 2 equiv.) and 5 mL of dry CH2Cl2 were added. The resulting suspension was stirred for 72 h at ambient temperature, the solvent was removed at reduced pressure and the solid residue was washed with diethyl ether (3 × 10 mL). Final purification of the organic phase was achieved on a chromatographic column using hexane-EtOAc eluent [89,90].
3.2.3. General Procedure 3: Huisgen 1,3-dipolar Cycloaddition-Type Reaction (12h), in the Synthesis of (S)-11, (2S,4R,1′-S)-17 and (2S,4R,1′-R)-17
In the following order, in a 25-mL round bottom flask provided with a magnetic bar, 0.1 equiv. of NaOH, 0.1 equiv. of ascorbic acid and 5 mL of DMSO:H2O (4:1) were added. The resulting mixture was stirred for two minutes before the addition of the corresponding azide derivative (1 equiv.) and CuSO4·5 H2O (0.1 mol). The reaction mixture was stirred for two minutes and then the propargyl ether derivative was added. The resulting mixture was stirred for 18 h at ambient temperature. The solid that was obtained was filtered and washed with small portions of water, methanol, ethyl acetate, and hexanes. Finally, the residue was dried in a muffle for 24 h at 65 °C [89,90,91].
3.2.4. General Procedure 4: Coupling Reaction, in the Synthesis of (2S,4R,1′S)-15 or (2S,4R,1′R)-15
In a 100-mL round bottom flask, previously purged with argon and provided with a magnetic stirring bar, trans-4-hydroxy-L-N-Cbz-proline (1 equiv. 1.0 g, 3.77 mmol) and triethylamine (1.05 equiv., 0.55 mL, 3.96 mmol) were added. Then, 16 mL of dry THF was added and the reaction flask was submerged in an ice bath before the dropwise addition of ethyl chloroformate (1.05 equiv. 0.37 mL, 3.96 mmol). Subsequently, the reaction mixture was allowed to react under vigorous stirring for 15 min at 0 °C. The insoluble Et3N·HCl salt that precipitated was filtered off and washed with THF (2 × 6 mL) and the combined filtrate was slowly added to a solution of (R)-14 or (S)-14 (1.05 equiv. 0.50 mL, 3.96 mmol) in 14 mL of EtOH at 0 °C. The reaction mixture was stirred for 30 min at 0 °C and then at room temperature for 12 additional hours. The solvents were removed under reduced pressure and the resulting colorless oil was dissolved in 30 mL of EtOAc and washed as follows: 5 mL of 1 M HCl, 5 mL of saturated aqueous solution of NaHCO3, and brine (2 × 5 mL). The crude product was dried over anhydrous Na2SO4, concentrated, and purified by flash column chromatography with EtOAc-hexanes (1:1) as eluent to afford pure (2S,4R,1′S)-15 or (2S,4R,1′R)-15.
3.2.5. General Procedure 5: N-Boc Catalyst Deprotection Under Acidic Conditions
In a round flask provided with a magnetic stirring bar and MeOH (3 mL), (S)-4 or (S)-11 was suspended and the resulting suspension was cooled to 0 °C before the addition of six drops of concentrated HCl (approx. 0.6 mL). The reaction mixture was left standing at room temperature for 4 h, and the product was filtered under vacuum and washed with water. Finally, general procedure 7 was followed until a constant weight of the product was achieved.
3.2.6. General Procedure 6: N-Cbz Catalyst Deprotection Under Basic Conditions
An amount of 1 M NaOH (3 mL) and (2S,4R,1′S)-17 or (2S,4R,1′R)-17 was added to a round flask provided with a magnetic stirring bar and the resulting mixture was heated to 60 °C for 4 h. The crude product was filtered under vacuum and washed with 1 M HCl and water and finally general procedure 7 was followed.
3.2.7. General Procedure 7: Activation of Catalysts (S)-5, (S)-12, (2S,4R,1′S)-18 and (2S,4R,1′R)-18.
Previous to the first use of the corresponding catalyst, it was treated as follows: each silica-supported organocatalyst was placed in a Soxhlet distillation apparatus and washed under continuous refluxing mode with a mixture MeOH:H2O:EtOAc (2:1:1) for 14 h. The recovered material was placed on a heating plate for 18 h at 115 °C and finally filtered.
General Procedure 7a: Reactivation of Used Silica-Supported Organocatalysts
The recovered silica-supported organocatalyst was washed with methanol and dried in a muffle at 85 °C for 24 h. Once this operation was finished, the catalyst was then ready to be reused.
3.2.8. General Procedure 8: Aldol Reaction
In a round bottom flask provided with a magnetic stirrer, 2.5 equiv. (0.612 mmol) of the corresponding ketone, the corresponding amount of silica-supported organocatalyst (equivalent to 5 or 10 mol%) and the corresponding aldehyde (0.24 mmol, 37 mg, 1 equiv.) were deposited. In case of the use of additive, this was added in the same proportion as the catalyst. The resulting mixture was stirred at the indicated temperature (see Table 1, Table 2, Table 3 and Table 4) until complete reaction. The solid catalyst was recovered by filtration and the filtrate was evaporated at reduced pressure. The purity of samples was evaluated using TLC, and the assessment of the aldol product was established by chromatography.
3.2.9. Procedure for Functionalization of Siloxy-OH Groups on Silica; Obtention of 6
In a 20-mL Biotage+ initiator microwave system vial, provided with a magnetic stirrer, 2.64 g of commercial silica (previously activated to 250 °C) was placed before the addition of 0.1 equiv. of pyridine, 1.2 mmol (1.0 equiv.) of 3-chloro-triethoxide silane and 10 mL of toluene. The reactor vial was closed and placed in the microwave oven at 150 °C, 150 Watts for 1.5 h, with the cooler mode activated. The resulting white suspension was filtered and washed with EtOAc (3 × 25 mL) to remove the excess of 3-chloro-triethoxide silane, and finally dried. Yield: quantitative. With this procedure it was possible to functionalize 2.64 g of silica with 1.15 mmol of 3-chloro-triethoxide silane.
3.3. Procedure for SN2 Type Reaction. Preparation of (S)-8
In a 20-mL Biotage+ initiator microwave vial, provided with a magnetic stirrer, 2.70 g of 7 was placed, before the addition of 1.1 mmol (0.381 g, 1.0 equiv.) of (S)-3, 2.4 mmol (0.212 g, 2 equiv.) of NaCO3 and 10 mL of MeCN. The resulting suspension was stirred for 10 min, the vial was then closed and placed in the microwave oven at 150 °C, 180 Watts for 2.0 h, with the cooler mode activated. The resulting lightly yellow suspension was filtered and washed with EtOAc (3 × 25 mL) and methanol (3 × 25 mL) to remove the excess of 7. Yield: 96% of the dried product. With this procedure it was possible to functionalize 2.64 g of silica with 1.12 mmol of 7.
H and 13C NMR Spectra, Mass Spectra and Physical Properties
(S)-tert-Butyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidine-1-carboxylate, (S)-3, was synthetized according to the procedure described in reference 11 starting from (S)-proline. White solid, mp 210–212 °C. Yield: 1.21 g (78%) for 3 steps. [α]D25 = +13.5°, c = 0.33, CHCl3. 1H NMR (500.16 MHz, DMSO-d6, 120 °C) δ 13.33 (s, 1H, NH), 7.62–7.26 (m, 5Har), 4.45 (d, 1H, J = 4 Hz, CH), 3.25 (s, 1H, CHH), 3.15 (s, 1H, CHH), 2.07–1.70 (m, 4H, 2 × CH2, 1.32 (s, 9H, 3 × CH3) ppm. 13C NMR (125.76 MHz, DMSO-d6, 120 °C) δ 169.3, 154.0, 134.4, 129.9, 128.9, 128.7, 79.7, 53.5, 46.8, 31.8, 28.9, 23.3 ppm. IR (neat) νmax 3212, 2969, 2972, 2879, 1769, 1707, 1658, 1521, 1408, 1401, 1272, 1198, 1127, 774 cm−1. HRMS (MS-TOF) calcd. for C17H23N4O2S (M)+ 347.1536, found 347.1533.
Triethoxy(3-iodopropyl)silane, 2. General procedure 1 was followed to obtain silane 2 in quantitative yield. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 3.79 (q, J = 7.0 Hz, 6 H, 3 × CH2), 1.75–2.00 (m, 2H, CH2), 3.19 (t, J = 7.1 Hz, 2H, CH2), 1.19 (t, J = 7.0 Hz, 9H, 3 × CH3), 0.63–0.77 (m, 2H, CH2). 13C NMR (125 MHz, CDCl3) δ 58.3, 27.5, 18.2, 12.2, 10.5 ppm.
(S)-tert-Butyl-2-((prop-2-yn-1-yloxy)methyl)pyrrolidine-1-carboxylate, (2S,4R)-10. General procedure 2 was followed to obtain (2S,4R)-10 as a white wax, 1.28 g (88% yield). [α]D25 = +25.9°, c = 0.33, CHCl3. 1H NMR (400.0 MHz, CDCl3) δ 4.16 (s, 2H, CH2), 3.94 (s, CH), 3.67(dd, 1H, J1 = 3.45 Hz, J2 = 9.04 CH), 3.55–3.24 (m, 3H), 2.03–1.61 (m, 5H), 1.48 (s, 9H, 3 × CH3) ppm; 13C NMR (125.76 MHz, CDCl3) δ 154.6, 79.9, 79.3, 74.3, 70.6, 58.4, 56.3, 46.7, 28.5, 23.8, 22.9 ppm. HRMS (MS-TOF) calcd. for C13H22NO3 (M)+ 240.1594, found 240.1598.
(2S,4R,1’S)-Benzyl-4-hydroxy-2-(((S)-1-phenylethyl)carbamoyl)pyrrolidine-1-carboxylate, (2S,4R,1′S)-15. General procedure 4 was followed to obtain (2S,4R,1′S)-15 as a white foam. Yield: 1.18 g (85%). [α]D25 = +7.2, c = 0.33, CHCl3. 1H NMR (500.0 MHz, CDCl3) δ 7.48–7.01 (m, 10H, CHar), 6.74 (d, 1H, NH), 5.21–5.03 (m, 1H, CH), 5.03–4.86 (dd, 2H, J1 = J2 = 4.98, CH2), 4.49–4.33 (m, 1H, CH), 4.26 (m, 1H, OH), 4.19–3.94 (m, 1H, CH), 3.68–3.35 (m, 2H, CH2), 2.26–1.82 (m, 2H, CH2), 1.52–1.20 (m, 3H, CH3) ppm. 13C NMR (125.76 MHz, CDCl3) δ 171.2, 156.0, 143.3, 136.7, 128.7, 128.2, 127.9, 127.2, 126.08, (d) 68.4, (d) 67.5, (d) 59.5, (d) 55.2, 39.7, 38.5, 22.0. HRMS (MS-TOF): calcd. for C21H25N2O4 (M)+ 369.1736, found: 369.182041.
(2S,4R,1’R)-Benzyl-4-hydroxy-2-(((R)-1-phenylethyl)carbamoyl)pyrrolidine-1-carboxylate, (2S,4R,1′R)-15. General procedure 4 was followed to obtain (2S,4R, 1′R)-15 as a white foam, 1.22 g, yield: 88%. [α]D25 = −2.5, c = 0.33, CHCl3. 1H NMR (500.0 MHz, CDCl3) δ 7.39–7.07 (m, 10H, CHar), 6.27–6.10 (s, 1H, NH), 5.23–5.03 (m, 2H, CH2), 5.03–4.92 (p, 1H, J = 10 Hz, CH), 4.55–4.45 (bs, 1H, OH), 3.81–3.45 (m, 2H, 2 × CH), 2.67–1.64 (m, 4H, 2 × CH2), 1.52–1.17 (m, 3H, CH3) ppm. 13C NMR (125.76 MHz, CDCl3) δ 170.3, 155.7, 143.4, 136.2, 128.8, 128.3, 128.0, 127.2, 126.0, (d) 68.3, (d) 60.5, (d) 54.5, (d) 49.2, 38.7, 38.5, 22.1. HRMS (MS-TOF): calcd. for C21H25N2O4 (M)+ 369.1736, found: 369.183627.
(2S,4R)-2-Benzyl-(((S)-1-phenylethyl)carbamoyl)-4-(prop-2-yn-1-yloxy)pyrrolidine-1-carboxylate, (2S,4R,1′S)-16. General procedure 2 was followed to obtain (2S,4R,1′S)-16 as a pale-yellow foam, 0.425 g, yield: 70%. [α]D25 = +11.7, c = 0.33, CHCl3. 1H NMR (500.0 MHz, CDCl3) δ 7.49–7.05 (m, 10H, CHar), 6.51 (s, 1H, NH), 5.17 (d, 1H, J = 13 Hz, CHH), 5.08 (d, 1H, J = 12.6 Hz, CHH), 5.01 (p, 1H, J = 6.72 Hz, CH), 4.49–4.32 (m, 1H, CH), 4.32–4.17 (m, 1H, CH), 4.16–3.97 (m, 2H, CH2), 3.82–3.56 (m, 1H, CH), 3.48 (dd, 1H, J1 = 12.6, J2 = 5.36 Hz, CH), 2.55–2.25 (m, 2H, CH2), 2.19–2.01 (bs, 1H, CH), 1.80–1.67 (m, 3H, CH3) ppm. 13C NMR (125.76 MHz, CDCl3). δ 170.2, 156.3, 143.5, 136.4, 128.6, 128.2, 128.0, 127.1, 126.0, 79.5, 75.1, 67.5, 59.2, 56.6, 51.6, 49.1, 33.8, 22.5. HRMS (MS-TOF) calcd. for C24H27N2O4 (M)+ 407.1892, found: 407.205297
(2S,4R)-2-Benzyl-(((R)-1-phenylethyl)carbamoyl)-4-(prop-2-yn-1-yloxy)pyrrolidine-1-carboxylate (2S,4R,1’R)-16. General procedure 2 was followed to obtain (2S,4R,1’R)-16 as a pale-yellow foam. 0.450 g, yield: 76%. [α]D25 = −6.8, c = 0.33, CHCl3. 1H NMR (500.0 MHz, CDCl3) δ 7.50–7.05 (m, 10H, CHar), 6.75 (s, 1H, NH), 5.1 (bs, 1H, CH), 5.06–4.91 (m, 2H, CH2), 4.44–4.31 (m, 1H, CH), 4.31–4.18 (m, 1H, CH), 4.11–3.96 (m, 2H, CH2), 3.85–3.61 (m, 1H, CH), 3.61–3.50 (m, 1H, CH), 2.53–2.38 (m, 1H, CH), 2.35–2.20 (m, 1H, CHH) 2.17–2.00 (m, 1H, CHH), 1.50–1.13 (m, 3H, CH3) ppm. 13C NMR (125.76 MHz, CDCl3) δ 170.6, 155.8, 143.3, 136.4, 128.7, 128.6, 128.2, 127.9, 127.2, 126.1, 79.6, 75.3, 67.4, 59.4, 56.5, 52.1, 48.9, 34.3, 22.1 ppm. HRMS (MS-TOF) calcd. for C24H27N2O4 (M)+ 407.1892, found: 407.196976.
4. Conclusions
In this work, four novel silica-supported organocatalysts were synthesized and characterized: (S)-5, (S)-12, (2S,4R,1′S)-18 and (2S,4R,1′R)-18. These catalytic systems turned out to be efficient and versatile organocatalysts for the asymmetric aldol reaction, affording the desired aldol products in excellent yields and moderate enantio- and diastereo-selectivities usually without the need of an acidic or basic additive. In addition, these mesoporous materials could be reused and recycled with only a minor loss in their catalytic activity even after 15 or more cycles. The novel organocatalysts provide a significant advantage over other homogeneous and heterogeneous organocatalysts because they make viable sustainable aldol condensations with a drastic reduction of solvent usage.
Supplementary Materials
The following are available online. HPLC Analytical Data for aldol products, NMR Spectra for new and previous characterized compounds and aldol products, Gravimetric, TGA and DSG Analysis Data, and FR-IR.
Author Contributions
Conceptualization, E.J. and O.S.-A.; methodology, O.S.-A.; investigation, E.J. and O.S.-A. K.A.R.-S., E.C.V.-O.; resources, E.J.; writing—original draft preparation, E.J. and O.S.-A.; writing—review and editing, E.J. and O.S.-A.; project administration, E.J.; funding acquisition, E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT and Fund SEP-CINVESTAV via grants 220945 and 126, respectively. O. Sánchez-Antonio thanks CONACYT for scholarship number 397321. The APC was funded by MDPI.
Acknowledgments
We are grateful to T. Cortez-Picasso and V. M. González-Díaz for their assistance in the acquisition of NMR spectra. We thank M. A. Leyva-Ramírez for his support in the acquisition and processing of XRD data, to Geiser Cuellar for his assistance in the acquisition of MS-TOF spectra. In addition, we are grateful to Instituto de Física, UNAM, Laboratorio Central de Microscopía, J. G. Morales Morales and M. Aguilar Franco, for the images of SEM and TEM microscopy. We are also grateful to A. Obregón-Zuñiga, UANL, for his support in the acquisition and processing of TGA and DSC data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- List, B.; Lerner, L.A.; Barbas, C.F., III. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. The golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Grondal, C.; Huettl, M.R.M. Asymmetric Organocatalytic Domino Reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Trost, B.M.; Brindle, C.S. The direct catalytic asymmetric aldol reaction. Chem. Soc. Rev. 2010, 39, 1600–1632. [Google Scholar] [CrossRef]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-Supported Organic Catalysts. Chem. Rev. 2003, 103, 3401–3429. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Supported proline and proline-derivatives as recyclable organocatalysts. Chem. Soc. Rev. 2008, 37, 1666–1688. [Google Scholar] [CrossRef]
- Trindade, A.F.; Gois, P.M.P.; Afonso, C.A.M. Recyclable Stereoselective Catalysts. Chem. Rev. 2009, 109, 418–514. [Google Scholar] [CrossRef]
- Clapham, B.; Reger, T.S.; Janda, K.D. Polymer-supported catalysis in synthetic organic chemistry. Tetrahedron 2001, 57, 4637–4662. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L. Merrifield Resin Supported Dipeptides: Efficient and Recyclable Organocatalysts for Asymmetric Aldol Reactions under Neat Reaction Conditions. Synthesis 2008, 2008, 2065–2072. [Google Scholar] [CrossRef]
- Hernández, J.G.; Juaristi, E. Recent efforts directed to the development of more sustainable asymmetric organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar] [CrossRef] [PubMed]
- Thölke, S.; Zhu, H.; Jansen, D.; Octa-Smolin, F.; Thiele, M.; Kaupmees, K.; Leito, I.; Grimme, S.; Niemeyer, J. Cooperative Organocatalysis: A Systematic Investigation of Covalently Linked Organophosphoric Acids for the Stereoselective Transfer Hydrogenation of Quinolines. Eur. J. Org. Chem. 2019, 2019, 5190–5195. [Google Scholar] [CrossRef]
- Liu, N.; Xie, Y.-F.; Wang, C.; Li, S.-J.; Wei, D.; Li, M.; Dai, B. Cooperative Multifunctional Organocatalysts for Ambient Conversion of Carbon Dioxide into Cyclic Carbonates. ACS Catal. 2018, 8, 9945–9957. [Google Scholar] [CrossRef]
- Bukhryakov, K.V.; Desyatkin, V.G.; Rodionov, V.O. Cooperative organocatalysis of Mukaiyama-type aldol reactions by thioureas and nitro compounds. Chem. Commun. 2016, 52, 7576–7579. [Google Scholar] [CrossRef] [PubMed]
- Raja Mitra, R.; Niemeyer, J. Dual Brønsted-acid Organocatalysis: Cooperative Asymmetric Catalysis with Combined Phosphoric and Carboxylic Acids. ChemCatChem 2018, 10, 1221–1234. [Google Scholar] [CrossRef]
- Steiner, D.D.; Mase, N.; Barbas, C.F., III. Direct Asymmetric α-Fluorination of Aldehydes. Angew. Chem. Int. Ed. 2005, 44, 3706–3710. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.-T.; Harris, P.W.R.; Barker, D.; Brimble, M.A. Use of (S)-5-(2-Methylpyrrolidin-2-yl)-1H-tetrazole as a Novel and Enantioselective Organocatalyst for the Aldol Reaction. Eur. J. Org. Chem. 2008, 2008, 164–170. [Google Scholar] [CrossRef]
- Cruz-Hernández, C.; Landeros, J.M.; Juaristi, E. Multifunctional phosphoramide-(S)-prolinamide derivatives as efficient organocatalysts in asymmetric aldol and Michael reactions. New J. Chem. 2019, 43, 5455–5465. [Google Scholar] [CrossRef]
- Xu, L.W.; Lu, Y. Primary amino acids: Privileged catalysts in enantioselective organocatalysis. Org. Biomol. Chem. 2008, 6, 2047–2053. [Google Scholar] [CrossRef]
- Gerasimchuk, V.V.; Kucherenko, A.S.; Fakhrutdinov, A.N.; Medvedev, M.G.; Nelyubina, Y.V.; Zlotin, S.G. Towards Sustainable Amino Acid Derived Organocatalysts for Asymmetric syn-Aldol Reactions. Eur. J. Org. Chem. 2017, 2017, 2540–2544. [Google Scholar] [CrossRef]
- Kang, Q.K.; Selvakumar, S.; Maruoka, K. Asymmetric Synthesis of α-Amino Acids by Organocatalytic Biomimetic Transamination. Org. Lett. 2019, 21, 2294–2297. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Arrieta, A.; Arrastia, I.; Cossío, F.P. Organocatalysts Derived from Unnatural α-Amino Acids: Scope and Applications. Chem. Asian J. 2019, 14, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.; Wennemers, H. Effect of β3-Amino Acids on the Performance of the Peptidic Catalyst H-d Pro-Pro-Glu-NH2. Helv. Chim. Acta 2019, 102, e1900070. [Google Scholar] [CrossRef]
- Schnitzer, T.; Budinská, A.; Wennemers, H. Organocatalysed conjugate addition reactions of aldehydes to nitroolefins with anti-selectivity. Nat. Catal. 2020, 3, 143–147. [Google Scholar] [CrossRef]
- Fingerhut, A.; Grau, D.; Tsogoeva, S.B. Peptides as Asymmetric Organocatalysts. In Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 1; Royal Society of Chemistry: London, UK, 2015; Chapter 13; pp. 309–353. [Google Scholar] [CrossRef]
- Wiesner, M.; Revell, J.D.; Tonazzi, S.; Wennemers, H. Peptide catalyzed asymmetric conjugate addition reactions of aldehydes to nitroethylene—A convenient entry into γ2-amino acids. J. Am. Chem. Soc. 2008, 130, 5610–5611. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.A.C.; Mennen, S.M.; Xu, Y.; Miller, S.J. Asymmetric catalysis mediated by synthetic peptides. Chem. Rev. 2007, 107, 5759–5812. [Google Scholar] [CrossRef]
- Machuca, E.; Juaristi, E. Organocatalytic activity of α,α-dipeptide derivatives of (S)-proline in the asymmetric aldol reaction in absence of solvent. Evidence for non-covalent π-π Interactions in the transition state. Tetrahedron Lett. 2015, 56, 1144–1148. [Google Scholar] [CrossRef]
- Machuca, E.; Rojas, Y.; Juaristi, E. Synthesis and Evaluation of (S)-Proline-Containing α,β-Dipeptides as Organocatalysts in Solvent-Free Asymmetric Aldol Reactions Under Ball-Milling Conditions. Asian J. Org. Chem. 2015, 4, 46–53. [Google Scholar] [CrossRef]
- Hernández, J.G.; Juaristi, E. Asymmetric aldol reaction organocatalyzed by (S)-proline-containing dipeptides: Improved stereoinduction under solvent-free conditions. J. Org. Chem. 2011, 76, 1464–1467. [Google Scholar] [CrossRef]
- Jiménez-González, E.; Gabriela Ávila-Ortiz, C.; González-Olvera, R.; Vargas-Caporali, J.; Dewynter, G.; Juaristi, E. Solution-phase synthesis of novel seven-membered cyclic dipeptides containing α- and β-amino acids. Tetrahedron 2012, 68, 9842–9852. [Google Scholar] [CrossRef]
- Koutoulogenis, G.; Kaplaneris, N.; Kokotos, C.G. (Thio)urea-mediated synthesis of functionalized six-membered rings with multiple chiral centers. Beilstein J. Org. Chem. 2016, 12, 462–495. [Google Scholar] [CrossRef] [PubMed]
- Revelou, P.; Kokotos, C.G.; Moutevelis-Minakakis, P. Novel prolinamide-ureas as organocatalysts for the asymmetric aldol reaction. Tetrahedron 2012, 68, 8732–8738. [Google Scholar] [CrossRef]
- Bera, M.; Ghosh, T.K.; Akhuli, B.; Ghosh, P. Tris-ureas as versatile and highly efficient organocatalysts for Michael addition reactions of nitro-olefins: Mechanistic insight from in-situ diagnostics. J. Mol. Catal. A Chem. 2015, 408, 287–295. [Google Scholar] [CrossRef]
- Cruz-Hernández, C.; Martínez-Martínez, E.; Hernández-González, P.E.; Juaristi, E. Synthesis of a New N-Diaminophosphoryl-N’-[(2S)-2-pyrrolidinylmethyl]thiourea as a Chiral Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones—Application in Cascade Processes for the Synthesis of Polycyclic Systems. Eur. J. Org. Chem. 2018, 2018, 6890–6900. [Google Scholar] [CrossRef]
- Wageling, N.B.; Decato, D.A.; Berryman, O.B. Steric effects of pH switchable, substituted (2-pyridinium)urea organocatalysts: A solution and solid phase study. Supramol. Chem. 2018, 30, 1004–1010. [Google Scholar] [CrossRef]
- Limnios, D.; Kokotos, C.G. Ureas and Thioureas as Asymmetric Organocatalysts. In Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 2; Royal Society of Chemistry: London, UK, 2015; Chapter 19; pp. 196–255. [Google Scholar]
- Vakulya, B.; Varga, S.; Soós, T. Epi-cinchona based thiourea organocatalyst family as an efficient asymmetric Michael addition promoter: Enantioselective conjugate addition of nitroalkanes to chalcones and α,β-unsaturated N-acylpyrroles. J. Org. Chem. 2008, 73, 3475–3480. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Highly enantioselective thiourea-catalyzed nitro-Mannich reactions. Angew. Chem. Int. Ed. 2005, 44, 466–468. [Google Scholar] [CrossRef]
- Gao, P.; Wang, C.; Wu, Y.; Zhou, Z.; Tang, C. Sugar-derived bifunctional thiourea organocatalyzed asymmetric Michael addition of acetylacetone to nitroolefins. Eur. J. Org. Chem. 2008, 2008, 4563–4566. [Google Scholar] [CrossRef]
- Lee, H.J.; Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Maruoka, K. Design of New Amino Tf-Amide Organocatalysts: Environmentally Benign Approach to Asymmetric Aldol Synthesis. Synlett 2019, 30, 401–404. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Amide-based bifunctional organocatalysts in asymmetric reactions. Chem. Commun. 2009, 41, 6145–6158. [Google Scholar] [CrossRef]
- Cruz-Hernández, C.; Hernández-González, P.E.; Juaristi, E. Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatalysts for the Enantiodivergent Aldol Reaction of Aryl Aldehydes and Isatins with Cyclohexanone in the Presence of Water. Synthesis 2018, 50, 3445–3459. [Google Scholar] [CrossRef]
- Vega-Peñaloza, A.; Sánchez-Antonio, O.; Escudero-Casao, M.; Tasnádi, G.; Fülöp, F.; Juaristi, E. Stereoselective synthesis of chiral pyrrolidine derivatives of (+)-α-pinene containing a β-amino acid moiety. Synthesis 2013, 45, 2458–2468. [Google Scholar] [CrossRef]
- Gavendova, M.; Lennon, C.M.; Coffey, L.; Manesiotis, P.; Kinsella, M. Novel β-amino Amide Organocatalysts for the Synthesis of Pharmaceutically Relevant Oxindoles. ChemistrySelect 2019, 4, 8246–8254. [Google Scholar] [CrossRef]
- Groselj, U. Camphor-Derivatives in Asymmetric Organocatalysis—Synthesis and Application. Curr. Org. Chem. 2015, 19, 2048–2074. [Google Scholar] [CrossRef]
- Pan, L.; Ding, X.; Ding, J.; Li, D.; Chen, J.; Zuo, X.; An, R. Design and Synthesis of L-Proline Derivatives as Enantioselective Organocatalysts for Synthesis of the (S)-Wieland-Miescher Ketone. ChemistrySelect 2017, 2, 11999–12005. [Google Scholar] [CrossRef]
- Haldar, S.; Saha, S.; Mandal, S.; Jana, C.K. C-H functionalization enabled stereoselective Ugi-azide reaction to α-tetrazolyl alicyclic amines. Green Chem. 2018, 20, 3463–3467. [Google Scholar] [CrossRef]
- Rani, R.; Peddinti, R.K. Camphor-10-sulfonamide-based prolinamide organocatalyst for the direct intermolecular aldol reaction between ketones and aromatic aldehydes. Tetrahedron Asymmetry 2010, 21, 775–779. [Google Scholar] [CrossRef]
- Lin, J.-H.; Xiao, J.-C. Recent advances in asymmetric fluorination and fluoroalkylation reactions via organocatalysis. Tetrahedron Lett. 2014, 55, 6147–6155. [Google Scholar] [CrossRef]
- Obregón-Zúñiga, A.; Milán, M.; Juaristi, E. Improving the Catalytic Performance of (S)-Proline as Organocatalyst in Asymmetric Aldol Reactions in the Presence of Solvate Ionic Liquids: Involvement of a Supramolecular Aggregate. Org. Lett. 2017, 19, 1108–1111. [Google Scholar] [CrossRef]
- Reyes-Rangel, G.; Vargas-Caporali, J.; Juaristi, E. In search of diamine analogs of the α,α-diphenyl prolinol privileged chiral organocatalyst. Synthesis of diamine derivatives of α,α-diphenyl-(S)-prolinol and their application as organocatalysts in the asymmetric Michael and Mannich reactions. Tetrahedron 2016, 72, 379–391. [Google Scholar] [CrossRef]
- Reyes-Rangel, G.; Jiménez-González, E.; Olivares-Romero, J.L.; Juaristi, E. Enantioselective synthesis of β-amino acids using hexahydrobenzoxazolidinones as chiral auxiliaries. Tetrahedron Asymmetry 2008, 19, 2839–2849. [Google Scholar] [CrossRef]
- Luan, Y.; Zheng, N.; Qi, Y.; Tang, J.; Wang, G. Merging metal-organic framework catalysis with organocatalysis: A thiourea functionalized heterogeneous catalyst at the nanoscale. Catal. Sci. Technol. 2014, 4, 925–929. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, T.Y.; Telfer, S.G. Modulating the performance of an asymmetric organocatalyst by tuning its spatial environment in a metal-organic framework. J. Am. Chem. Soc. 2017, 139, 13936–13943. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, K.; Takigawa, S.; Mano, E.; Kudo, K. Sequential oxidation/asymmetric aldol reaction of primary alcohols by resin-supported catalysts. Tetrahedron Lett. 2011, 52, 770–773. [Google Scholar] [CrossRef]
- Bartók, M. Advances in immobilized organocatalysts for the heterogeneous asymmetric direct aldol reactions. Catal. Rev. Sci. Eng. 2015, 57, 192–255. [Google Scholar] [CrossRef]
- Xie, Y.; Sharma, K.K.; Anan, A.; Wang, G.; Biradar, A.V.; Asefa, T. Efficient solid-base catalysts for aldol reaction by optimizing the density and type of organoamine groups on nanoporous silica. J. Catal. 2009, 265, 131–140. [Google Scholar] [CrossRef]
- Elmekawy, A.A.; Sweeney, J.B.; Brown, D.R. Efficient synthesis of supported proline catalysts for asymmetric aldol reactions. Catal. Sci. Technol. 2015, 5, 690–696. [Google Scholar] [CrossRef]
- Andreae, M.R.M.; Davis, A.P. Heterogeneous catalysis of the asymmetric aldol reaction by solid-supported proline-terminated peptides. Tetrahedron Asymmetry 2005, 16, 2487–2492. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Nanoporous silica-supported organocatalyst: A heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 2014, 4, 28238–28248. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Marculescu, A.M.; Noto, R. Novel prolinamide-supported polystyrene as highly stereoselective and recyclable organocatalyst for the aldol reaction. Adv. Synth. Catal. 2008, 350, 1397–1405. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M.; Marculescu, A.M.; Noto, R. Polystyrene-supported proline and prolinamide. Versatile heterogeneous organocatalysts both for asymmetric aldol reaction in water and α-selenenylation of aldehydes. Tetrahedron Lett. 2007, 48, 255–259. [Google Scholar] [CrossRef]
- Price, G.A.; Hassan, A.; Chandrasoma, N.; Bogdan, A.R.; Djuric, S.W.; Organ, M.G. Pd-PEPPSI-IPent-SiO2: A Supported Catalyst for Challenging Negishi Coupling Reactions in Flow. Angew. Chem. Int. Ed. 2017, 56, 13347–13350. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.E.; Vestli, K.; Fredriksen, K.A.; Hansen, F.K.; Hansen, T. Synthesis of acrylic polymer beads for solid-supported proline-derived organocatalysts. Org. Lett. 2009, 11, 2968–2971. [Google Scholar] [CrossRef]
- Sasidharan, M.; Kiyozumi, Y.; Mal, N.K.; Mizukami, F. Synthesis, characterization, and application of mesoporous silica functionalized with alkyl-hydroperoxides. Adv. Funct. Mater. 2006, 16, 1853–1858. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Marculescu, A.M.; Salvo, A.M.P.; Noto, R. Stereoselective aldol reaction catalyzed by a highly recyclable polystyrene supported substituted prolinamide catalyst. ARKIVOC 2009, 8, 5–15. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Shahidzadeh, E.S.; Sarikhani, S.; Panahi, F. A new silica-supported organocatalyst based on L-proline: An efficient heterogeneous catalyst for one-pot synthesis of spiroindolones in water. J. Mol. Catal. A Chem. 2013, 379, 1–8. [Google Scholar] [CrossRef]
- Ferré, M.; Cattoën, X.; Wong Chi Man, M.; Pleixats, R. Recyclable Silica-Supported Proline Sulphonamide Organocatalysts for Asymmetric Direct Aldol Reaction. ChemistrySelect 2016, 1, 6741–6748. [Google Scholar] [CrossRef]
- Rajput, A.P.; Nagarale, D.V. Modern Synthetic Tool L-Proline as an Organocatalyst. J. Chem. Pharm. Res. 2016, 8, 557–575. [Google Scholar]
- Corma, A.; Garcia, H. Silica-bound homogenous catalysts as recoverable and reusable catalysts in organic synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Rodríguez-Escrich, C.; Pericàs, M.A. Organocatalysis on Tap: Enantioselective Continuous Flow Processes Mediated by Solid-Supported Chiral Organocatalysts. Eur. J. Org. Chem. 2015, 2015, 1173–1188. [Google Scholar] [CrossRef]
- Sánchez-Antonio, O.; Juaristi, E. Synthesis of a new chiral organocatalyst derived from (S)-proline containing a 1,2,4-triazolyl moiety and its application in the asymmetric aldol reaction. Importance of one molecule of water generated in situ. Tetrahedron Lett. 2019, 60, 151128. [Google Scholar] [CrossRef]
- Han, Y.R.; Park, J.W.; Kim, H.; Ji, H.; Lim, S.H.; Jun, C.H. A one-step co-condensation method for the synthesis of well-defined functionalized mesoporous SBA-15 using trimethallylsilanes as organosilane sources. Chem. Commun. 2015, 51, 17084–17087. [Google Scholar] [CrossRef]
- Ferré, M.; Pleixats, R.; Wong Chi Man, M.; Cattoën, X. Recyclable organocatalysts based on hybrid silicas. In Proceedings of the Green Chemistry; Royal Society of Chemistry: London, UK, 2016; Volume 18, pp. 881–922. [Google Scholar] [CrossRef]
- Putz, A.M.; Almásy, L.; Len, A.; Ianăşi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Scatena, G.S.; de la Torre, A.F.; Cass, Q.B.; Rivera, D.G.; Paixão, M.W. Multicomponent Approach to Silica-Grafted Peptide Catalysts: A 3 D Continuous-Flow Organocatalytic System with On-line Monitoring of Conversion and Stereoselectivity. ChemCatChem 2014, 6, 3208–3214. [Google Scholar] [CrossRef]
- Puglisi, A.; Benaglia, M.; Annunziata, R.; Chiroli, V.; Porta, R.; Gervasini, A. Chiral hybrid inorganic-organic materials: Synthesis, characterization, and application in stereoselective organocatalytic cycloadditions. J. Org. Chem. 2013, 78, 11326–11334. [Google Scholar] [CrossRef]
- Brühwiler, D. Postsynthetic functionalization of mesoporous silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef]
- Jiang, D.M.; Gao, J.S.; Yang, Q.H.; Li, C. Large-pore mesoporous ethane-silicas as efficient heterogeneous asymmetric catalysts. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2007; Volume 170, pp. 1252–1259. [Google Scholar] [CrossRef]
- Sunada, K.; Takenaka, K.; Shiomi, T. Synthesis of polychloroprene-silica composites by sol-gel method in the presence of modified polychloroprene containing triethoxysilyl group. J. Appl. Polym. Sci. 2005, 97, 1545–1552. [Google Scholar] [CrossRef]
- Fischer, T.; Duong, Q.N.; García Mancheño, O. Triazole-Based Anion-Binding Catalysis for the Enantioselective Dearomatization of N-Heteroarenes with Phosphorus Nucleophiles. Chem. Eur. J. 2017, 23, 5983–5987. [Google Scholar] [CrossRef]
- Riente, P.; Yadav, J.; Pericàs, M.A. A click strategy for the immobilization of Macmillan organocatalysts onto polymers and magnetic nanoparticles. Org. Lett. 2012, 14, 3668–3671. [Google Scholar] [CrossRef]
- García Mancheño, O.; Asmus, S.; Zurro, M.; Fischer, T. Highly Enantioselective Nucleophilic Dearomatization of Pyridines by Anion-Binding Catalysis. Angew. Chem. Int. Ed. 2015, 54, 8823–8827. [Google Scholar] [CrossRef] [PubMed]
- Zurro, M.; Asmus, S.; Bamberger, J.; Beckendorf, S.; García Mancheño, O. Chiral Triazoles in Anion-Binding Catalysis: New Entry to Enantioselective Reissert-Type Reactions. Chem. Eur. J. 2016, 22, 3785–3793. [Google Scholar] [CrossRef] [PubMed]
- Goren, K.; Kehat, T.; Portnoy, M. Elucidation of Architectural Requirements from a Spacer in Supported Proline-Based Catalysts of Enantioselective Aldol Reaction. Adv. Synth. Catal. 2009, 351, 59–65. [Google Scholar] [CrossRef]
- Vega-Peñaloza, A.; Sánchez-Antonio, O.; Ávila-Ortiz, C.G.; Escudero-Casao, M.; Juaristi, E. An alternative synthesis of chiral (S)-proline derivatives that contain a thiohydantoin moiety and their application as organocatalysts in the asymmetric Michael addition reaction under solvent-free conditions. Asian J. Org. Chem. 2014, 3, 487–496. [Google Scholar] [CrossRef]
- Sousa, C.A.D.; Sampaio-Dias, I.E.; Rizzo-Aguiar, F.; Garcia-Mera, X.; Rodríguez-Borges, J.E. Enantiopure synthesis of 7-(1-pyrindanyl)propargyl ethers as rasagiline analogues via chemical or enzymatic resolution of 1-pyrindan-7-ol. RSC Adv. 2015, 5, 104509–104515. [Google Scholar] [CrossRef]
- Bew, S.P.; Hiatt-Gipson, G.D. Synthesis of C-propargylic esters of N-protected amino acids and peptides. J. Org. Chem. 2010, 75, 3897–3899. [Google Scholar] [CrossRef]
- Escudero-Casao, M.; Vega-Penaloza, A.; Juaristi, E. Enantiopure 1,2,3-Triazolyl-β-amino Acids via Click Cycloaddition Reaction on Racemic Alkynyl Precursors Followed by Separation of Stereoisomers. Curr. Top. Med. Chem. 2014, 14, 1257–1270. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Organocatalytic Michael Reactions in Aqueous Media. Curr. Org. Chem. 2018, 21, 323–344. [Google Scholar] [CrossRef]
- Chen, M.; Sun, J. How Understanding the Role of an Additive Can Lead to an Improved Synthetic Protocol without an Additive: Organocatalytic Synthesis of Chiral Diarylmethyl Alkynes. Angew. Chem. Int. Ed. 2017, 56, 11966–11970. [Google Scholar] [CrossRef]
- Hong, L.; Sun, W.; Yang, D.; Li, G.; Wang, R. Additive Effects on Asymmetric Catalysis. Chem. Rev. 2016, 116, 4006–4123. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; López-Ortiz, M.; Vega-Peñaloza, A.; Regla, I.; Juaristi, E. Use of (R)-Mandelic Acid as Chiral Co-Catalyst in the Michael Addition Reaction Organocatalyzed by (1S,4S)-2-Tosyl-2,5-diazabicyclo[2.2.1]heptane under Solvent-Free Conditions. Asymmetric Catal. 2015, 2, 37–44. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Shaw, D.M.; Ley, S.V. 5-Pyrrolidin-2-yltetrazole: A New, Catalytic, More Soluble Alternative to Proline in an Organocatalytic Asymmetric Mannich-type Reaction. Synlett 2004, 15, 558–560. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Shaw, D.M.; Longbottom, D.A.; Gold, J.B.; Ley, S.V. Organocatalysis with proline derivatives: Improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions. Org. Biomol. Chem. 2005, 3, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Asymmetric direct aldol reaction assisted by water and a proline-derived tetrazole catalyst. Angew. Chem. Int. Ed. 2004, 43, 1983–1986. [Google Scholar] [CrossRef]
- Hartikka, A.; Arvidsson, P.I. Rational design of asymmetric organocatalysts—Increased reactivity and solvent scope with a tetrazolic acid. Tetrahedron Asymmetry 2004, 15, 1831–1834. [Google Scholar] [CrossRef]
- Nakashima, E.; Yamamoto, H. Process Catalyst Mass Efficiency by Using Proline Tetrazole Column-Flow System. Chem. Eur. J. 2018, 24, 1076–1079. [Google Scholar] [CrossRef]
- Nakashima, E.; Yamamoto, H. Asymmetric Aldol Synthesis: Choice of Organocatalyst and Conditions. Chem. Asian J. 2017, 12, 41–44. [Google Scholar] [CrossRef]
- Krištofíková, D.; Modrocká, V.; Mečiarová, M.; Šebesta, R. Green Asymmetric Organocatalysis. ChemSusChem 2020, 13, 2828–2858. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, R.; Pavlidis, I.V.; Chen, B.; Tan, T. Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: Inspiration from bio- and organo-catalysis. Green Chem. 2016, 18, 1240–1248. [Google Scholar] [CrossRef]
- Chauhan, P.; Singh Chimni, S. Mechanochemistry assisted asymmetric organo-catalysis: A sustainable approach. Beilstein J. Org. Chem. 2012, 8, 2132–2141. [Google Scholar] [CrossRef]
- Chowdari, N.S.; Ramachary, D.B.; Barbas, C.F., III. Organocatalysis in Ionic Liquids: Highly Efficient L-Proline-Catalyzed Direct Asymmetric Mannich Reactions Involving Ketone and Aldehyde Nucleophiles. Synlett 2003, 2003, 1906–1909. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Deon, M.; Benvenutti, E.V.; Barros, V.A.; de Melo, D.C.; Franceschi, E.; Egues, S.M.; De Conto, J.F. Effect of microwave irradiation on the structural, chemical, and hydrophilicity characteristics of ordered mesoporous silica SBA-15. J. Sol Gel Sci. Technol. 2020, 94, 708–718. [Google Scholar] [CrossRef]
- Corresponding Specification Data about the Commercial Silica. Available online: https://www.mn-net.com/LCadsorbents/Irregularsilica/StandardsilicaforLC/tabid/6009/language/en-US/Default.aspx (accessed on 12 September 2020).
Sample Availability: Samples described in the manuscript are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).