Transformed Shoots of Dracocephalum forrestii W.W. Smith from Different Bioreactor Systems as a Rich Source of Natural Phenolic Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Culture Growth
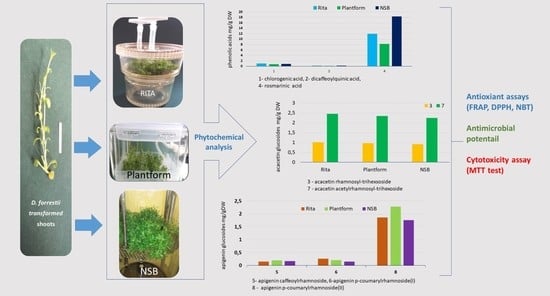
2.2. Phytochemical Analysis
2.3. Antioxidant Potential
2.4. Antimicrobial Potential
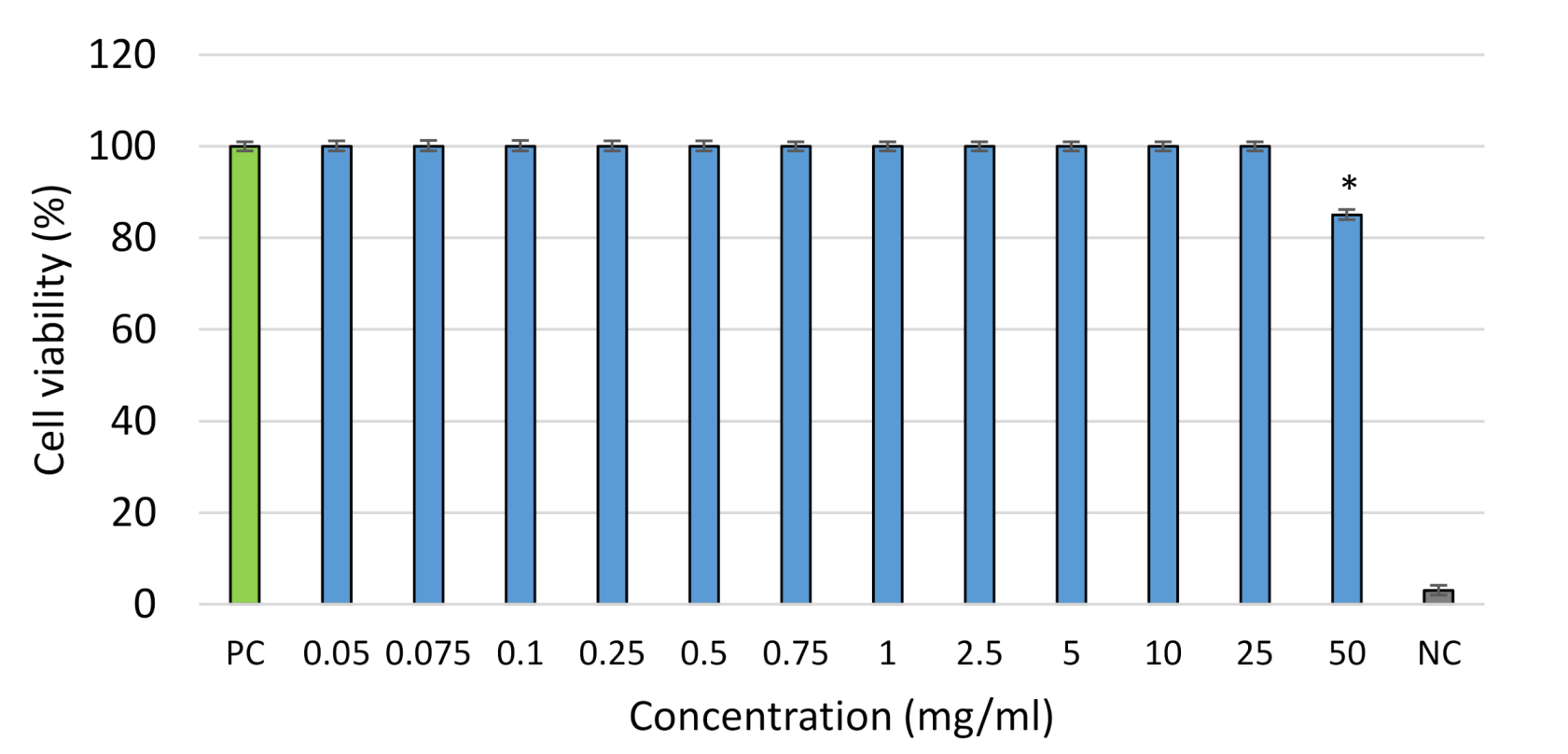
2.5. Cytotoxicity Potential
3. Materials and Methods
3.1. Transformed Shoot Culture
3.2. Transformed Shoot Culture in Bioreactor Systems
3.3. Phytochemical Analysis
3.4. Biological Investigations
3.4.1. Preparation of Samples
3.4.2. Antioxidant Potential
3.4.3. Antimicrobial Potential
3.4.4. Cytotoxicity Properties
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Valdini, A.; Hansen, O.K.; Nielsenn, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Dinanai, E.T.; Abiri, R. Bioreactor-Based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef]
- Preil, W. Application of bioreactors in plant propagation. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Acad. Pub.: Dordrecht, The Netherlands, 1991; Available online: https://link.springer.com/chapter/10.1007/978-94-009-2075-0_25 (accessed on 20 May 2020).
- Welander, M.; Zhu, L.H.; Li, X.Y. Factors influencing conventional and semi-automated micropropagation. Propag. Ornam. Plants. 2007, 7, 103–111. [Google Scholar]
- Businge, E.; Trifonora, A.; Schneider, C.; Rödel, P.; Egersdotter, U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of eucalyptus, birch and fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Máximo, W.P.F.; Santos, P.A.A.; Marins, G.S.; Mendonça, E.G.; Paiva, L.V. In vitro multiplication of Eucalyptus hybrid via temporary immersion bioreactor: Culture media and cytokinin effects. Crop Breed Appl. Biotechnol. 2018, 18, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Perik, R.L. In Vitro Culture of Higher Plants, 3rd ed.; Martinus Nijnhoff off Publishers: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Roels, S.; Noceda, S.; Escalona, M.; Sandoval, J.; Canal, M.J.; Rodriquez, R.; Debergh, P. The effect of headspace renewal in a temporary immersion bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tiss. Organ Cult. 2006, 84, 155–163. [Google Scholar] [CrossRef]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of the new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Scherer, R.F.; Garcia, A.C.; de Freitas Fraga, H.P.; Dal Vesco, L.L.; Steinmacher, D.A.; Guerra, M.P. Nodule cluster cultures and temporary immersion bioreactors as a high performance micropropagation strategy in pineapple (Ananas comosus var. comosus). Sci. Hortic. 2013, 151, 38–45. [Google Scholar] [CrossRef]
- Tapia, M.; Arbizu, C.; Beraún, F.; Lorenzo, J.; Escalona, M. Pre-basic seed potato (Solanum tuberosum L.) production using temporary immersion bioreactors. Peruv. J. Agron. 2018, 2, 9–14. [Google Scholar] [CrossRef]
- Perez Alonso, N.; Capote, A.; Gerth, A.; Jimenez, E. Increased cardenolides by elicitation of Digitalis lanata shoots cultured in temporary immersion system. Plant Cell Tiss. Organ Cult. 2012, 110, 153–162. [Google Scholar] [CrossRef]
- Piątczak, E.; Grzegorczyk-Karolak, I.; Wysokińska, H. Micropropagation of Rehmannia glutinosa Libosch.: Production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol. Plant. 2014, 36, 1693–1702. [Google Scholar]
- López, C.Q.; Corral, P.; Lorran-Lorrette, B.; Martinez-Swatson, K.; Simonsen, H.T. Use of temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot culture of Thapsia garganica. Plant Methods 2018, 14, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Rytczak, P.; Bielecki, S.; Wysokińska, H. The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tiss. Organ Cult. 2017, 128, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Jafernik, K.; Luczkiewicz, M.; Ekiert, H. Bioreactor type affects the accumulation of phenolic acids and flavonoids in microshoot cultures of Schisandra chinensis (Turcz.) Baill. Plant Cell Tiss. Organ Cult. 2019, 139, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.X.; Li, X.W. Flora of China; Since Press: Bejing, China, 1979; Volume 2. [Google Scholar]
- Li, G.P.; Zhao, J.F.; Yang, L.J.; Yang, X.D.; Li, Y.L. New monoterpenoids from Dracocephalum forrestii aerial parts. Indian, J. Chem. 2007, 46B, 1352–1354. [Google Scholar] [CrossRef]
- Li, S.-M.; Yang, X.-W.; Li, Y.-L.; Shen, Y.-H.; Feng, L.; Wang, Y.-H.; Zeng, H.-W.; Liu, X.-H.; Zhang, C.-S.; Long, C.-L.; et al. Chemical constituents of Dracocephalum forrestii. Planta Med. 2009, 75, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selenge, E.; Murata, T.; Tanaka, S.; Sasaki, K.; Bathu, J.; Yoshizaku, F. Flavone tetraglycosides, phenylpropanoids and acacetin glycosides from D. foetidum. Phytochemistry 2014, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Selenge, E.; Murata, T.; Kobayashi, K.; Batkhuu, J.; Yoshizaki, F. Flavone tetraglycosides and benzyl alcohol glycosides from the mongolian medicinal plant Dracocephalum ruyschiana. J. Nat. Prod. 2013, 76, 186–193. [Google Scholar] [CrossRef]
- Faham, N.; Javidnia, K.; Bahmani, M.; Amirghofran, Z. Calycopterin, an immunoinhibitory compound from the extract of Dracocephalum kotschyi. Phytother. Res. 2008, 22, 1154–1158. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Skała, E.; Kuźma, L.; Kiss, A.K.; Grzegorczyk-Karolak, I. The effect of purine-type cytokinin on the proliferation and production of phenolic compounds in transformed shoots of Dracocephalum forrestii. J. Biotechnol. 2019, 306, 125–133. [Google Scholar]
- Wu, W.L.; Li, Y.D.; Cui, Y.K.; Wu, C.; Hong, Y.X.; Li, G.; Wu, Y.; Jie, I.J.; Wang, Y.; Li, G.W. The natural flavone acacetin confers cardiomyocyte/reoxygenation in jury via AMPK – medialesacacetin of Nrf2 signaling pathway. Front. Pharmacol. 2018, 9, 497–501. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-4′- metoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-ƙB/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, W.R. Application of bioreactor design principles to plant micropropagation. Plant Cell Tiss. Organ Cult. 2005, 81, 255–264. [Google Scholar] [CrossRef]
- Preil, W. General introduction: A personal reflection on the use of liquid media for in vitro culture. In Liquid Culture Systems for In Vitro Plant Propagation; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schizandra lignans production regulated by different bioreactor type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kochan, E.; Szymczyk, P.; Lisiecki, P.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W.W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem. Lett. 2019, 30, 254–260. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Thimaraju, R.; Bhagyalakshmi, N.; Narayan, M.S.; Ravishankar, R. Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol. Plant. 2008, 52, 355–360. [Google Scholar] [CrossRef]
- González, R.; Rios, D.; Avillés, F.; Sánchez-Olate, M. In vitro multiplication of Eucalyptus globulus by a temporary immersion system. Bosque 2011, 32, 147–154. [Google Scholar] [CrossRef]
- Ziv, M. Liquid Culture Systems for In Vitro Plant Propagation; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Mehrotra, S.; Goel, K.M.; Kukreja, A.K.; Mishra, B.N. Efficiency of liquid culture systems over conventional micropropagation. A progress towards commercialization. Afr. J. Biotechnol. 2007, 6, 1484–1492. [Google Scholar]
- Zeng, Q.; Qin, J.J.; Jin, H.Z.; Fu, J.J.; Hu, X.J.; Lin, J.H.; Yan, L.; Chen, M.; Zhang, W.D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010, 7, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokińska, H. Antioxidant compounds in Salvia officinalis L. shoot and hairy root cultures in the nutrient sprinkle bioreactor. Acta Soc. Bot. Pol. 2010, 79, 7–10. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. Effect of cytokinins on shoots proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestii W.W. Smith. Acta Physiol. Plant. 2018, 40, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Amoah, S.K.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic acid – pharmaceutical and clinical aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imram, A.; Shahbaz, S.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Alif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Zdařilova, A.; Svobodovà, A.; Šimanek, V.; Ulrichovà, J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide–induced alteration in human gingival fibroblasts. Toxicol. In Vitro. 2009, 23, 386–392. [Google Scholar] [CrossRef]
- Jang, Y.G.; Hwang, K.A.; Choi, K.C. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Gao, R.; Yang, H.; Jing, S.; Liu, B.; Wei, M.; He, P.; Zhang, N. Protective effect of chlorogenic acid on lipopolysaccharide-induced inflammatory response in dairy mammary epithelial cells. Microb. Pathog. 2018, 124, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Padadigorria, C.L.; Bazotte, R.; dos Santos Fereira da Silva, R.S.; de Souza, H.M. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biol. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Fesen, M.R.; Pommier, Y.; Leteurtre, F.; Hiroguchi, S.; Yung, J.; Kohn, K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994, 48, 595–608. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamaski, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar]
- Sanchez-Alonso, I.; Careche, M.; Moreno, P.; Gonzalez, M.J.; Medina, I. Testing caffeic acid as a natural antioxidant infunctional fish-fibre restructured products. Food Sci. Technol. 2011, 44, 1149–1155. [Google Scholar]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Nugroho, A.; Park, J.H.; Choi, J.S.; Park, K.S.; Hong, J.P.; Park, H.J. Structure determination and of a new flavone glycosides with anti-acetylcholinesterase activity from herbs of Elscholtzia ciliate. Former. Nat. Prod. Lett. 2019, 33, 814–821. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, E.J.; Kim, H.J.; Park, J.H.; Choi, S.W. Antioxidative flavonoids from leaves of Carthamus tinctorius. Arch. Pharm. Res. 2002, 25, 313–318. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Yang, X.; He, C.; Wang, W.; Xing, J. Pretreatment with total flavonoid extract from Dracocephalum moldavica L. attenuates ischemia reperfusion-induced apoptosis. Sci. Rep. 2018, 8, 17491–17505. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Ma, Y.; Zhang, W.; Wang, S.; Wang, Y.; Tion, L.; Peng, Z.; Wang, H.; Qingrong, T. Apigenin-7-O-(6′’-p-coumaryl)-glucopyranoside treatment elicits neuroprotective effect against experimental ischemic stroke. Int. J. Biol. Sci. 2016, 12, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, R. Isolation of apigenin-7-O-(6′’-O-E-caffeoyl)-β-d-glucopyranoside from Leucas aspera L. with anti-inflammatory and wound healing activities. J. Pharm. Pharmacogn. Res. 2016, 4, 54–61. [Google Scholar]
- Fattahi, M.; Nazeri, V.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R.M.; Zamani, Z.; Palazon, J. A new biotechnological source of rosmarinic acid and surface flavonoids: Hairy root cultures of Dracocephalum kotschyi Boiss. Ind. Crops Prod. 2013, 50, 256–263. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Rotinberg, P.; Trifan, A.; Luca, S.V.; Petreus, T.; Gille, E.; Miron, A. Antigenotoxic and antioxidant activities of a polyphenolic extract from european Dracocephalum moldavica L. Ind. Crops Prod. 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Khodaei, M.; Amanzadeh, Y.; Faramarzi, M.A.; Hamedani, M.P.; Adhami, H.R. Cholinesterase inhibitory, anti-oxidant and anti-tyrosinase activities of three Iranian species of Dracocephalum. Res. J. Pharmacogn. 2019, 6, 25–31. [Google Scholar]
- Liu, C.-S.; Cheng, Y.; Hu, J.-F.; Zhang, W.; Chen, N.-H.; Zhang, J.-T. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb761). Acta Pharmacol. Sin. 2006, 27, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H. Dietary flavonoid aglycones and their glycosides. Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lou, J.F.; Luo, C.; Zhou, L.G.; Wang, M.G.; Wang, I. Phenolic compounds from Halimodendron halodendron (Pall.) Voss and their antimicrobial and antioxidant activities. Int. J. Mol. Sci. 2012, 13, 11349–11364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compean, K.L.; Yankez, R.A. Antimicrobial activity of plant secondary metabolites: A review. Res. J. Med. Plants 2014, 8, 204–2014. [Google Scholar]
- Farimani, M.M.; Mirzania, F.; Sonboli, A.; Moghaddam, F.M. Chemical composition and antibacterial activity of Dracocephalum kotschyi essential oil obtained by microwave extraction and hydrodistillation. Int. J. Food Prop. 2017, 20, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Cha, K.H.; Kim, S.N.; Altantsetseg, S.; Shatar, S.; Sarangerel, O. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. Microbiol. 2007, 45, 53–57. [Google Scholar]
- Keikhoie, K.R.; Jahantigh, H.R.; Bagheri, R.; Kehkhaie, A.R. The effects of the ethanol extract of Dracocephalum moldavica (Badrashbu) against strains of antibiotic-resistant Escherichia coli and Klebsiella pneumonia. Int. J. Infect. 2018, 5, e65295. [Google Scholar]
- Kamali, M.; Khosroyar, S.; Mohammad, A. Antibacterial activity of various extracts from Dracocephalum kotschyi against food pathogenic microorganisms. Pharm. Tech. 2015, 8, 158–163. [Google Scholar]
- Yu, H.; Liu, M.; Liu, Y.; Qin, L.; Jin, M.; Wang, Z. Antimicrobial activity and mechanism of action of Dracocephalum moldavica extracts against clinical isolates of Staphylococcus aureus. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman, B.N.; Yazdinjed, A.; Salah, S.; Sajedinefad, N. Antifungal activity of essential oils of Prangos frulacea, Ziziphora tenuior, Ferula gummosa and Dracocephalum moldavica against Candida albicans. J. Qazvin Univ. Med. Sci. 2017, 21, 11–40. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sani, T.A.; Mohammadpour, E.; Mohammadi, A.; Memariani, T.; Yazdi, M.V.; Rezaee, R. Cytotoxic and apoptogenic properties of Dracocephalum kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia 2017, 65, 189–199. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia by use of shoot tip culture. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Weremczuk-Jeżyna, I.; Skała, E.; Olszewska, M.A.; Kiss, A.K.; Balcerczak, E.; Wysokińska, H.; Kicel, A. The identification and quantitative determination of rosmarinic acid and salvianolic acid B in hairy root cultures of Dracocephalum forrestii W.W. Smith. Ind. Crops Prod. 2016, 19, 125–131. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Królicka, A.; Wysokińska, H. Hairy roots of Dracocephalum moldavica rosmarinic acid content and antioxidant potential. Acta Physiol. Plant. 2013, 35, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
- Grzegorczyk–Karolak, I.; Kiss, A.K. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Lisiecki, P.; Kiss, A. Accumulation of phenolic compounds in different in vitro cultures of Salvia viridis L. and their antioxidant and antimicrobial. Phytochem. Lett. 2019, 30, 324–332. [Google Scholar] [CrossRef]
- ISO 10993-5. Biological Evaluation of Medical Devices-Part 5: Tests for in vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are not available. |
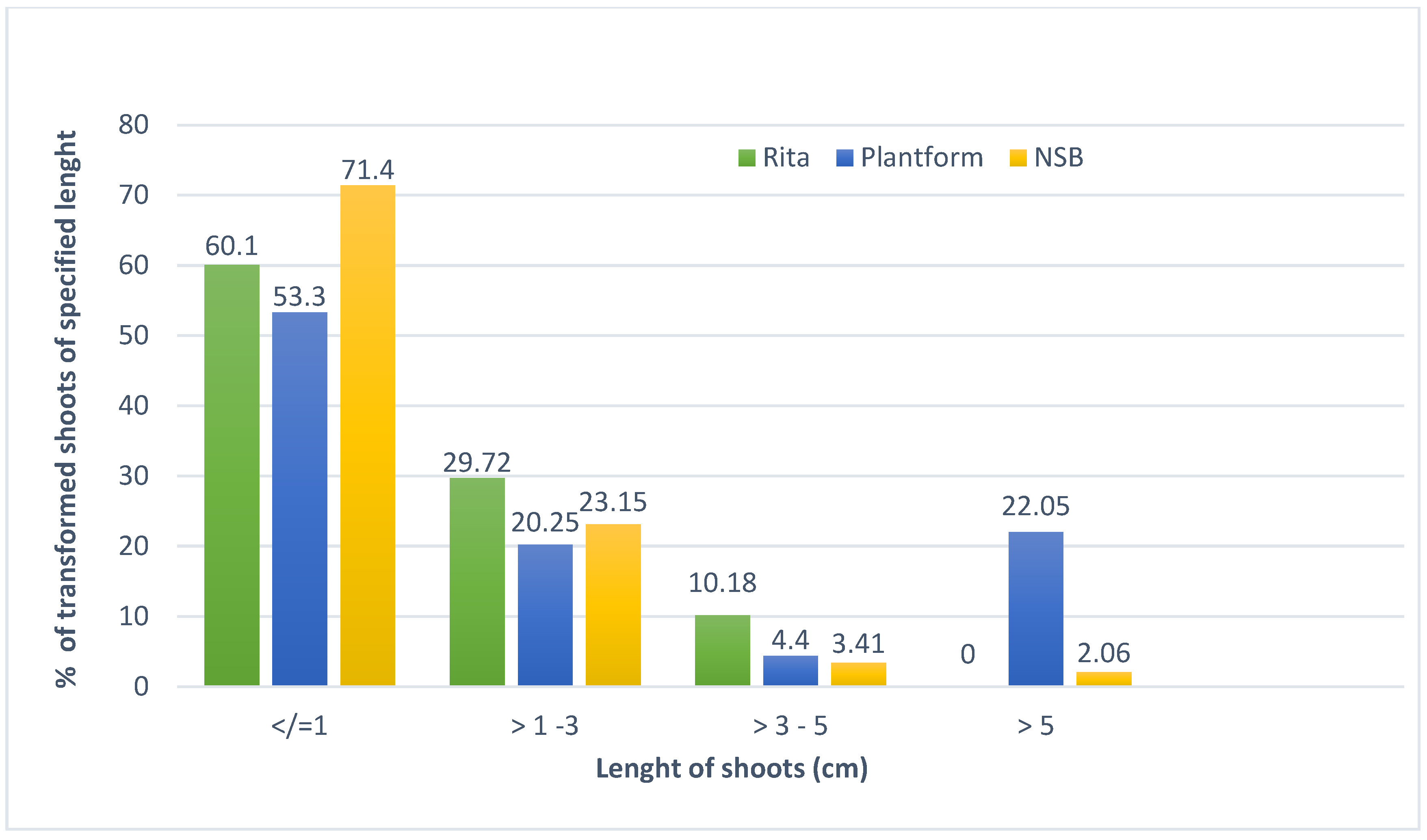

| Type of Bioreactors | Number of Shoots per Explants | Number of Shoots per Bioreactor | Growth Index | Hyperhydricity Shoots (%) | |
|---|---|---|---|---|---|
| FW | DW | ||||
| RITA 1 | 47.6 ± 5.1 | 286 ± 10.2 | 52.06 ± 1.2 | 55.67 ± 4.6 | 16 |
| Plantform 2 | 17.02 ± 3.1 | 204 ± 8.5 | 11.89 ± 0.4 | 8.65 ± 0.3 | 5 |
| NSB2 | 69.7 ± 7.3 | 836 ± 26.9 | 43.48 ± 1.2 | 44.55 ± 0.7 | 24 |
| Bioreactor Type | Content of Compounds | Number of Compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 * | 3 | 4 | 5 * | 6 * | 7 | 8 | ||
| RITA | mg/g DW | 0.99 ± 0.02 a | tr | 1.01 ± 0.03 a | 11.91 ± 0.1 b | tr | tr | 2.45 ± 0.02 a | 1.86 ± 0.01 b |
| mg/L | 13.0 ± 1.3 A | tr | 13.28 ± 1.2 A | 156.6 ± 4.1 A | tr | tr | 32.2 ± 2.6 A | 24.44 ± 2.8 A | |
| Plantform | mg/g DW | 0.78 ± 0.03 c | tr | 0.97 ± 0.05 a,b | 8.23 ± 0.1 c | tr | tr | 2.34 ± 0.04 a | 2.28 ± 0.03 a |
| mg/L | 1.43 ± 0.2 C | tr | 1.74 ± 0.3 C | 29.64 ± 2.8 C | tr | tr | 4.22 ± 0.1 C | 4.11 ± 0.3 C | |
| NSB | mg/g DW | 0.88 ± 0.01 b | tr | 0.91 ± 0.03 b | 18.35 ± 0.2 a | tr | tr | 2.25 ± 0.1 a | 1.76 ± 0.04 b |
| mg/L | 4.09 ± 0.2 B | tr | 8.81 ± 0.1 B | 96.97 ± 2.7 B | tr | tr | 11.89 ± 0.4 B | 9.29 ± 0.5 B | |
| Assay | BHT | Plant Extract |
|---|---|---|
| FRAP (μM Fe(II)/g DW of extract) | 3667.4 ± 52.0 | 724.9 ± 16.8 |
| DPPH (EC50 μg/mL) | 29.42 ± 0.1 | 74.9 ± 0.9 |
| NBT (EC50 μg/mL) | 42.7 ± 0.1 | 116.1 ± 9.0 |
| Microorganism | MIC mg/mL | MBC mg/mL | RA MIC/MBC mg/mL | Amikacine MIC = MBC µg/mL | Fluconazole MIC/MB µg/mL |
|---|---|---|---|---|---|
| Gram-Positive Bacteria | |||||
| Staphylococcus aureus ATCC 6538 | >20 | >20 | 1/>1 | 0.5 | - |
| Staphylococcus epidermidis ATCC 12228 | 10 | 10 | 1/>1 | 0.125 | - |
| Enterococcus faecalis ATTC 29212 | >20 | >20 | 1/>1 | 8 | - |
| Enterococcus faecium PCM 1859 | >20 | >20 | 1/>1 | 2.5 | - |
| Bacillus cereus PCM 1948 | 10 | 10 | 1/1 | 0.03 | - |
| Gram-Negative Bacteria | |||||
| Escherichia coli ATCC 25922 | 10 | 10 | 1/1 | 0.625 | - |
| Pseudomonas aeruginosa ATCC 27853 | >20 | >20 | 1/>1 | 20 | - |
| Proteus vulgaris CCM 1799 | >20 | >20 | 1/1 | 0.625 | - |
| Fungi | |||||
| Candida albicans ATTC 10231 | >20 | >20 | 1/>1 | - | 5/>5 |
| Candida albicans ZMF 1 | >20 | >20 | 1/>1 | 5/>5 | |
| Candida glabrata 2 ZMF | >20 | >20 | 0.5/0.5 | - | 2.5/>5 |
| Aspergillus brasilinsis ATCC 16404 | >20 | >20 | 1/>1 | - | 5/>5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weremczuk-Jeżyna, I.; Lisiecki, P.; Gonciarz, W.; Kuźma, Ł.; Szemraj, M.; Chmiela, M.; Grzegorczyk-Karolak, I. Transformed Shoots of Dracocephalum forrestii W.W. Smith from Different Bioreactor Systems as a Rich Source of Natural Phenolic Compounds. Molecules 2020, 25, 4533. https://doi.org/10.3390/molecules25194533
Weremczuk-Jeżyna I, Lisiecki P, Gonciarz W, Kuźma Ł, Szemraj M, Chmiela M, Grzegorczyk-Karolak I. Transformed Shoots of Dracocephalum forrestii W.W. Smith from Different Bioreactor Systems as a Rich Source of Natural Phenolic Compounds. Molecules. 2020; 25(19):4533. https://doi.org/10.3390/molecules25194533
Chicago/Turabian StyleWeremczuk-Jeżyna, Izabela, Paweł Lisiecki, Weronika Gonciarz, Łukasz Kuźma, Magdalena Szemraj, Magdalena Chmiela, and Izabela Grzegorczyk-Karolak. 2020. "Transformed Shoots of Dracocephalum forrestii W.W. Smith from Different Bioreactor Systems as a Rich Source of Natural Phenolic Compounds" Molecules 25, no. 19: 4533. https://doi.org/10.3390/molecules25194533
APA StyleWeremczuk-Jeżyna, I., Lisiecki, P., Gonciarz, W., Kuźma, Ł., Szemraj, M., Chmiela, M., & Grzegorczyk-Karolak, I. (2020). Transformed Shoots of Dracocephalum forrestii W.W. Smith from Different Bioreactor Systems as a Rich Source of Natural Phenolic Compounds. Molecules, 25(19), 4533. https://doi.org/10.3390/molecules25194533