Strong Affinity of Triazolium-Appended Dipyrromethenes (TADs) for BF4−
Abstract
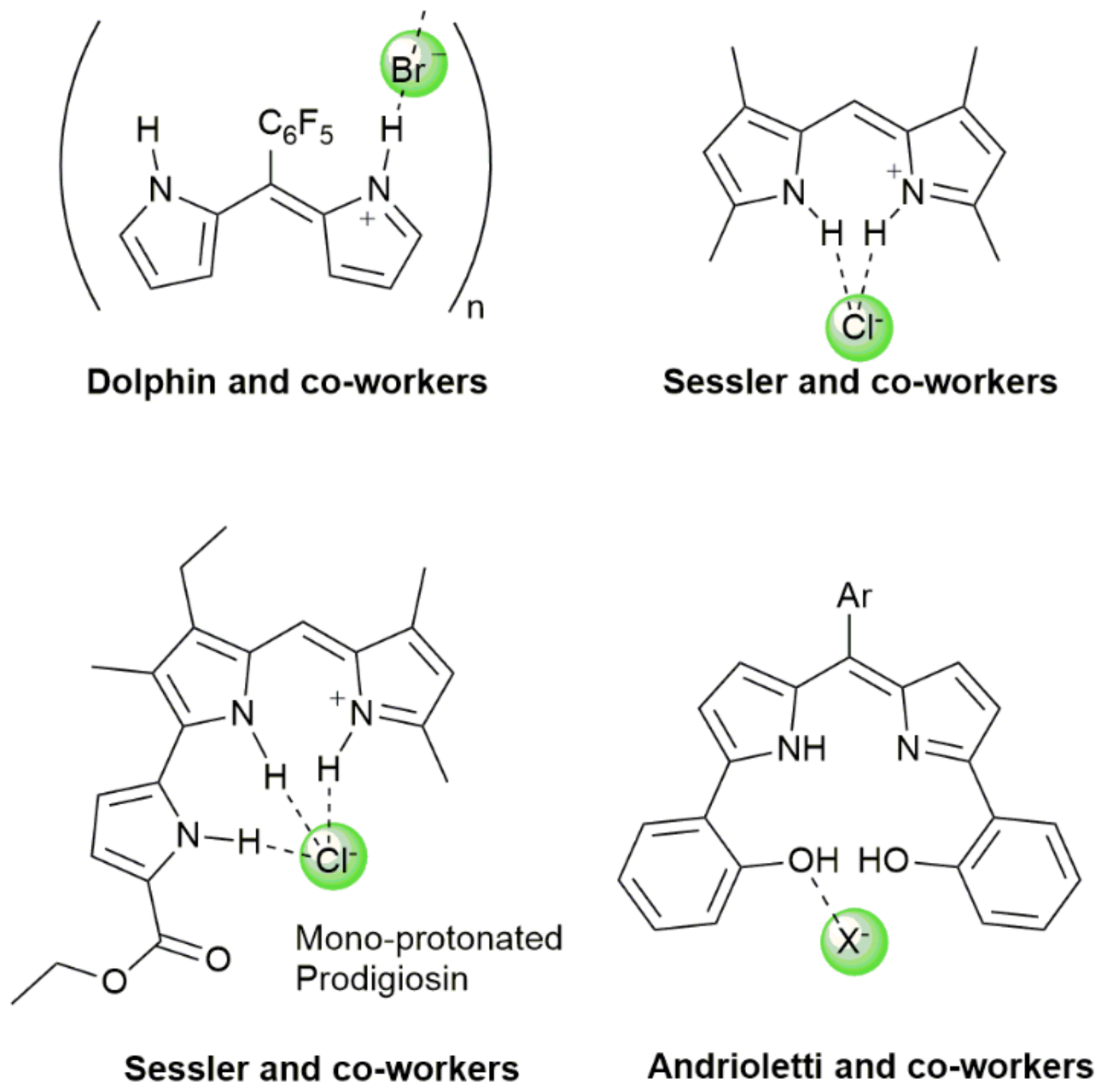
1. Introduction
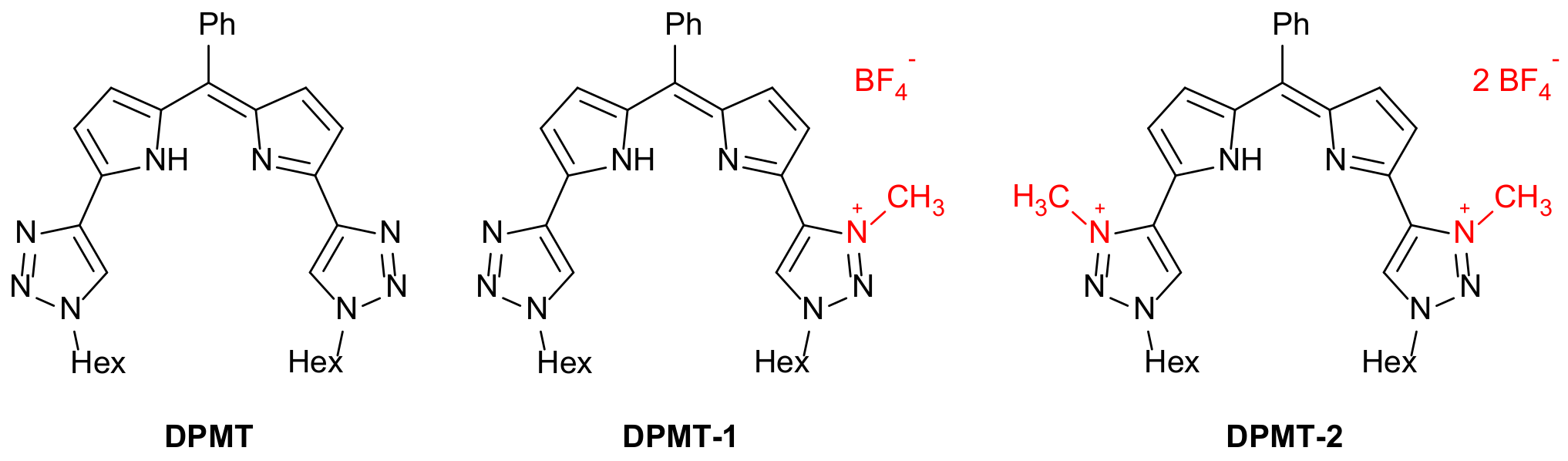
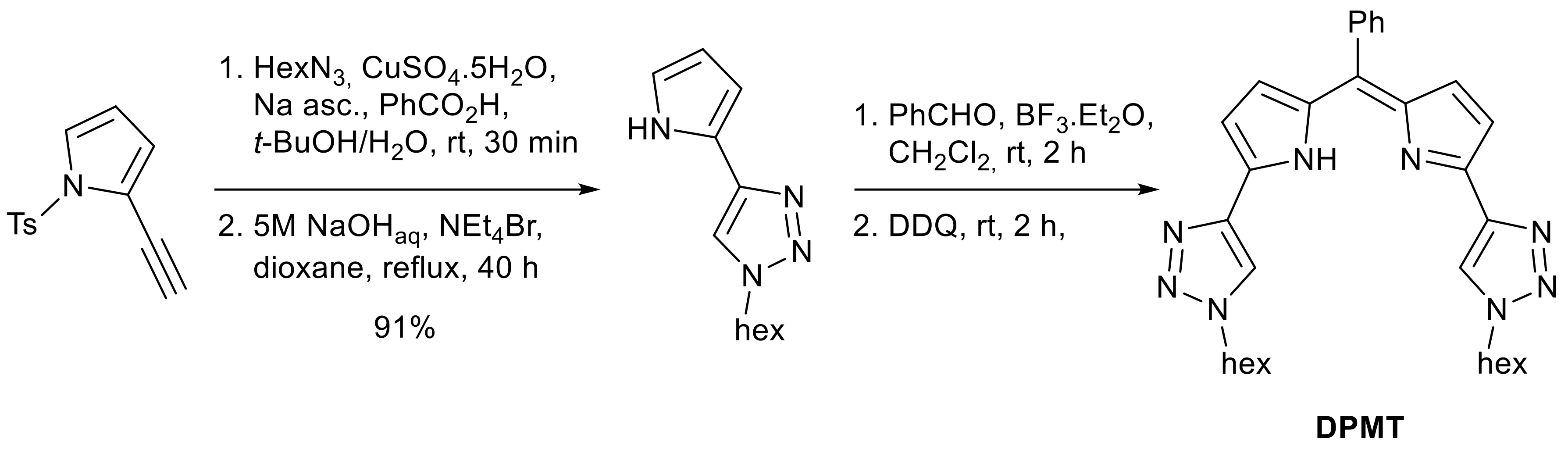
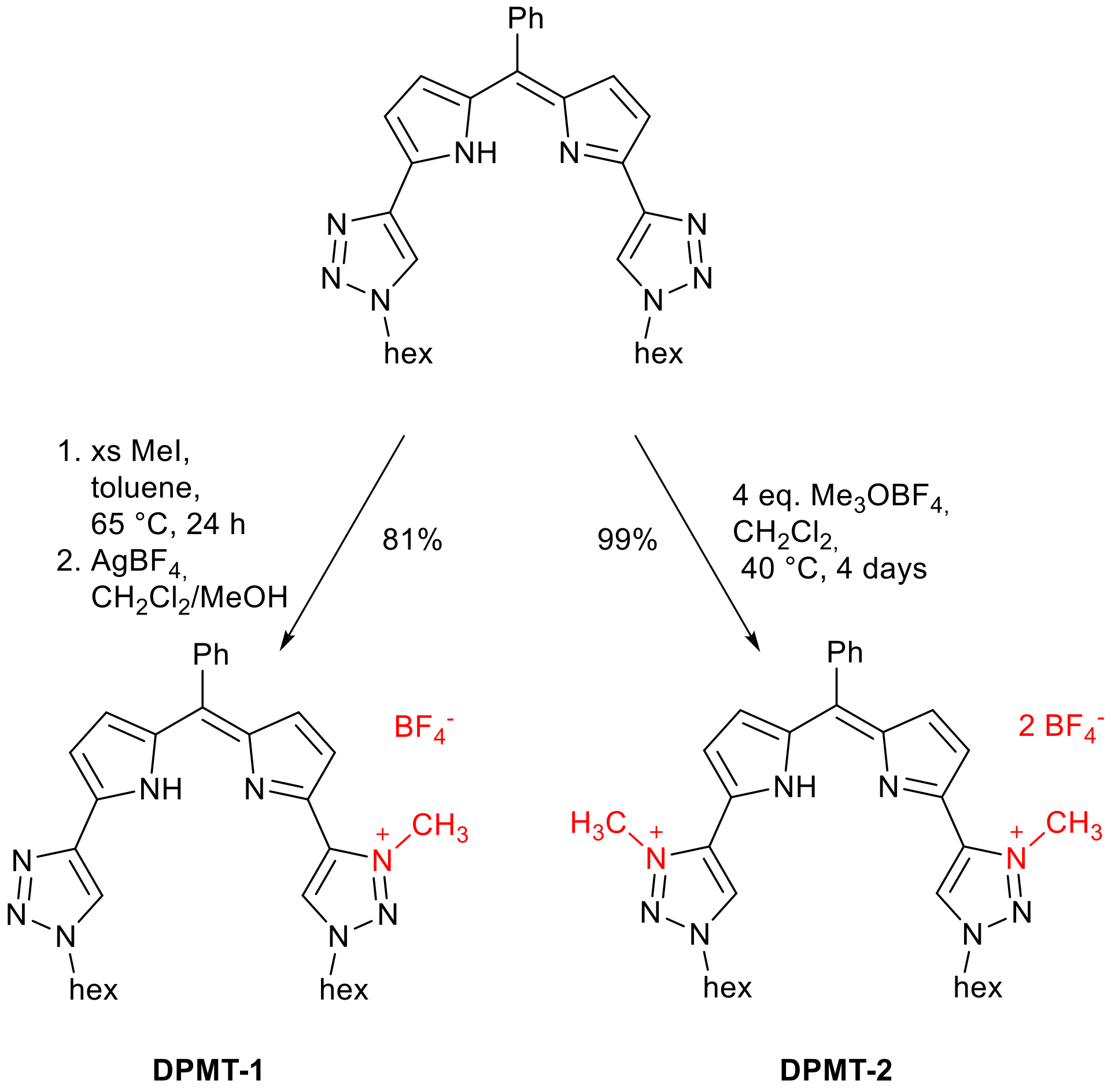
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caballero, A.; Zapata, F.; González, L.; Molina, P.; Alkorta, I.; Elguero, J. Discovery of anion-π interactions in the recognition mechanism of inorganic anions by 1,2,3-triazolium rings. Chem. Commun. 2014, 50, 4680–4682. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; Caballero, A.; Molina, P.; Alkorta, I.; Elguero, J. Open bis(triazolium) structural motfs as a benchmark to study combined hydrogen- and halogen-bonding interactions in oxoanion recognition processes. J. Org. Chem. 2014, 79, 6959–6969. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Farrán, M.A.; Santa María, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Jaime, C.; Elguero, J. Pyrrole-pyrridine and pyrrole-naphthyridine hosts for anion recognition. Molecules 2015, 20, 9862–9878. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Gale, P.A.; Cho, W.-J. Anion Receptor Chemistry; Royal Society of Chemistry Publishing: Cambridge, UK, 2006. [Google Scholar]
- Fyfe, M.C.T.; Glink, P.T.; Menzer, S.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Anion-assisted self assembly. Angew. Chem. Int. Ed. Engl. 1997, 36, 2068–2070. [Google Scholar] [CrossRef]
- Fleming, J.S.; Mann, K.L.V.; Carraz, C.-A.; Psillakis, E.; Jeffery, J.C.; McCleverty, J.A.; Ward, M.D. Anion-templated assembly of a supramolecular cage complex. Angew. Chem. Int. Ed. 1998, 37, 1279–1281. [Google Scholar] [CrossRef]
- Campos-Fernández, C.S.; Clérac, R.; Dunbar, K.R. A one-pot, high-yield synthesis of a paramagnetic nickel square from divergent precursors by anion template assembly. Angew. Chem. Int. Ed. 1998, 38, 3477–3479. [Google Scholar] [CrossRef]
- Nabeshima, T.; Saiki, T.; Iwabuchi, J.; Akine, S. Stepwise and dramatic enhancement of anion recognition with a triple-site receptor based on the calix[4]arene framework using two different cationic effectors. J. Am. Chem. Soc. 2005, 127, 5507–5511. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.; Grantham, W.; Levason, W.; Light, M.E.; Reid, G. Hexafluorosilicate and tetrafluoroborate coordination to lead(II) di- and tri-imine complexes – Unusual fluoroanion coordination modes. Polyhedron 2015, 85, 530–536. [Google Scholar] [CrossRef]
- Maeda, H. Anion Recognition in Supramolecular Chemistry; Gale, P.A., Dehaen, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 115–119. [Google Scholar]
- Shin, J.-Y.; Dolphin, D.; Patrick, B.O. Protonated dipyrromethenes and tetrahalozinc anions as synthons in the solid state. Cryst. Growth Des. 2004, 4, 659–661. [Google Scholar] [CrossRef]
- Sessler, J.L.; Eller, L.R.; Cho, W.-S.; Nicolaou, S.; Aguilar, A.; Tae Lee, J.; Lynch, V.M.; Magda, D.J. Synthesis, anion-binding properties, and In Vitro anticancer activity of prodigiosin analogues. Angew. Chem. Int. Ed. 2005, 44, 5989–5992. [Google Scholar] [CrossRef] [PubMed]
- Copey, L.; Jean-Gérard, L.; Framery, E.; Pilet, G.; Andrioletti, B. Synthesis, solid-state analyses, and anion-binding properties of meso-aryldipyrrin-5,5′-diylbis(phenol) and-bis(aniline) ligands. Eur. J. Org. Chem. 2014, 2014, 4759–4766. [Google Scholar] [CrossRef]
- Guérin, C.; Jean-Gérard, L.; Octobre, G.; Pascal, S.; Maury, O.; Pilet, G.; Ledoux, A.; Andrioletti, B. Bis-triazolyl BODIPYs: a simple dye with strong red-light emission. RSC Adv. 2015, 5, 76342–76345. [Google Scholar] [CrossRef]
- Li, Y.; Flood, A.H. Pure C-H hydrogen bonding to chloride ions: a preorganized and rigid macrocyclic receptor. Angew. Chem. Int. Ed. 2008, 47, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, P.S. Anion recognition by 1,2,3-triazolium receptors: Application of click chemistry in anion recognition. Org. Lett. 2008, 10, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Schubert, U.S. Beyond click chemistry–supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Friebe, C.; Hager, M.D.; Günther, W.; Köhn, U.; Jahn, B.O.; Görls, H.; Schubert, U.S. Anion complexation by triazolium “ligands”: mono- and bis-tridentate complexes of sulfate. Org. Lett. 2010, 12, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
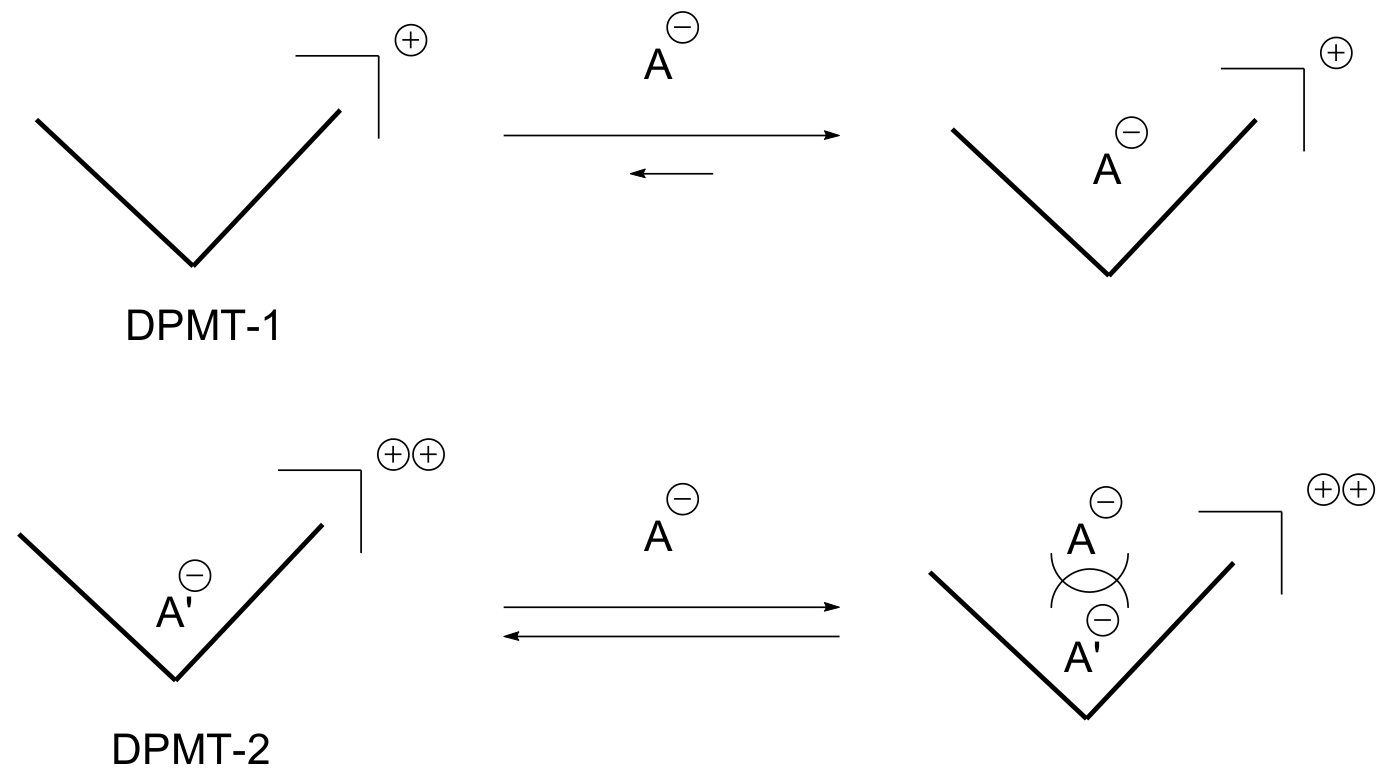
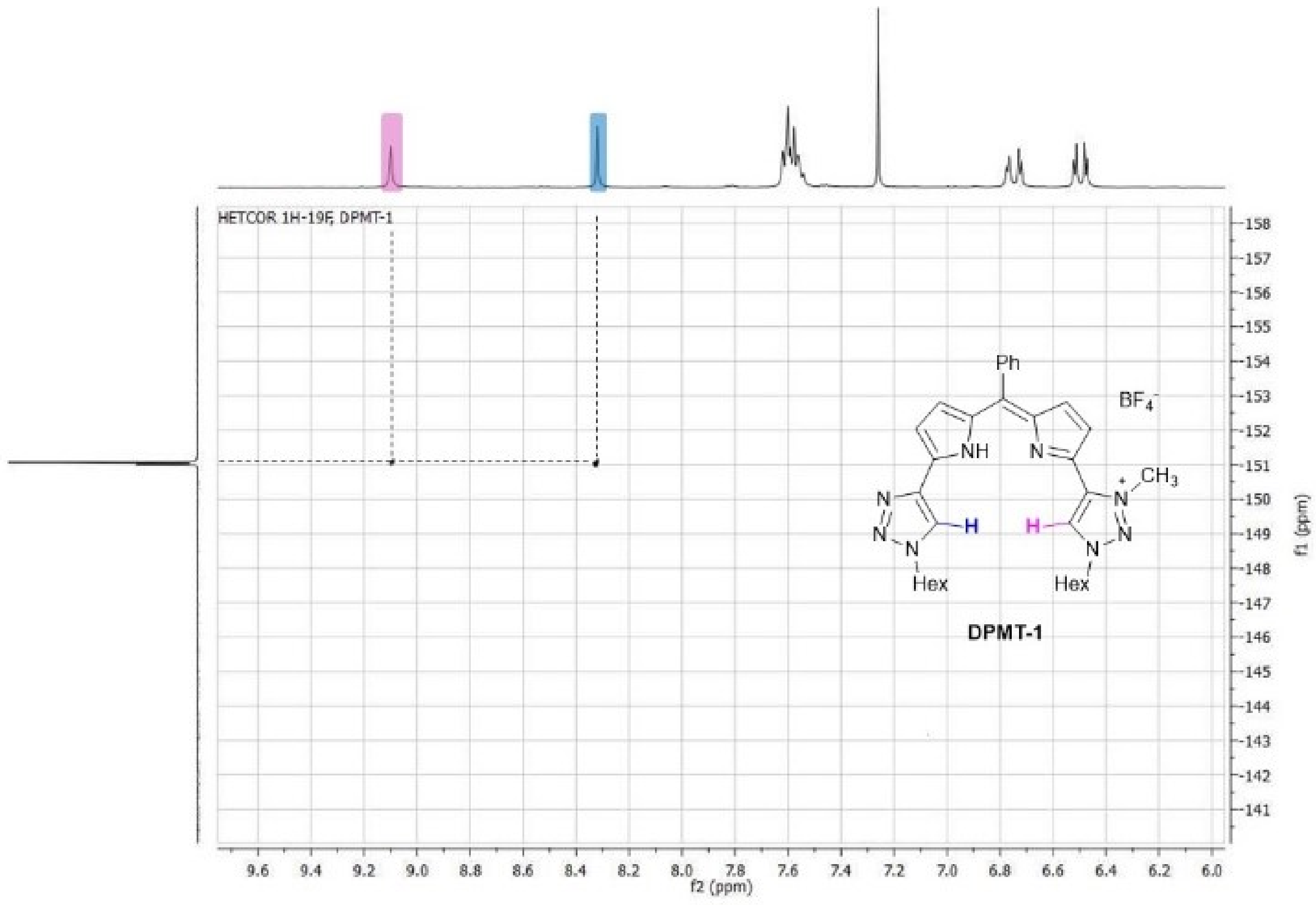
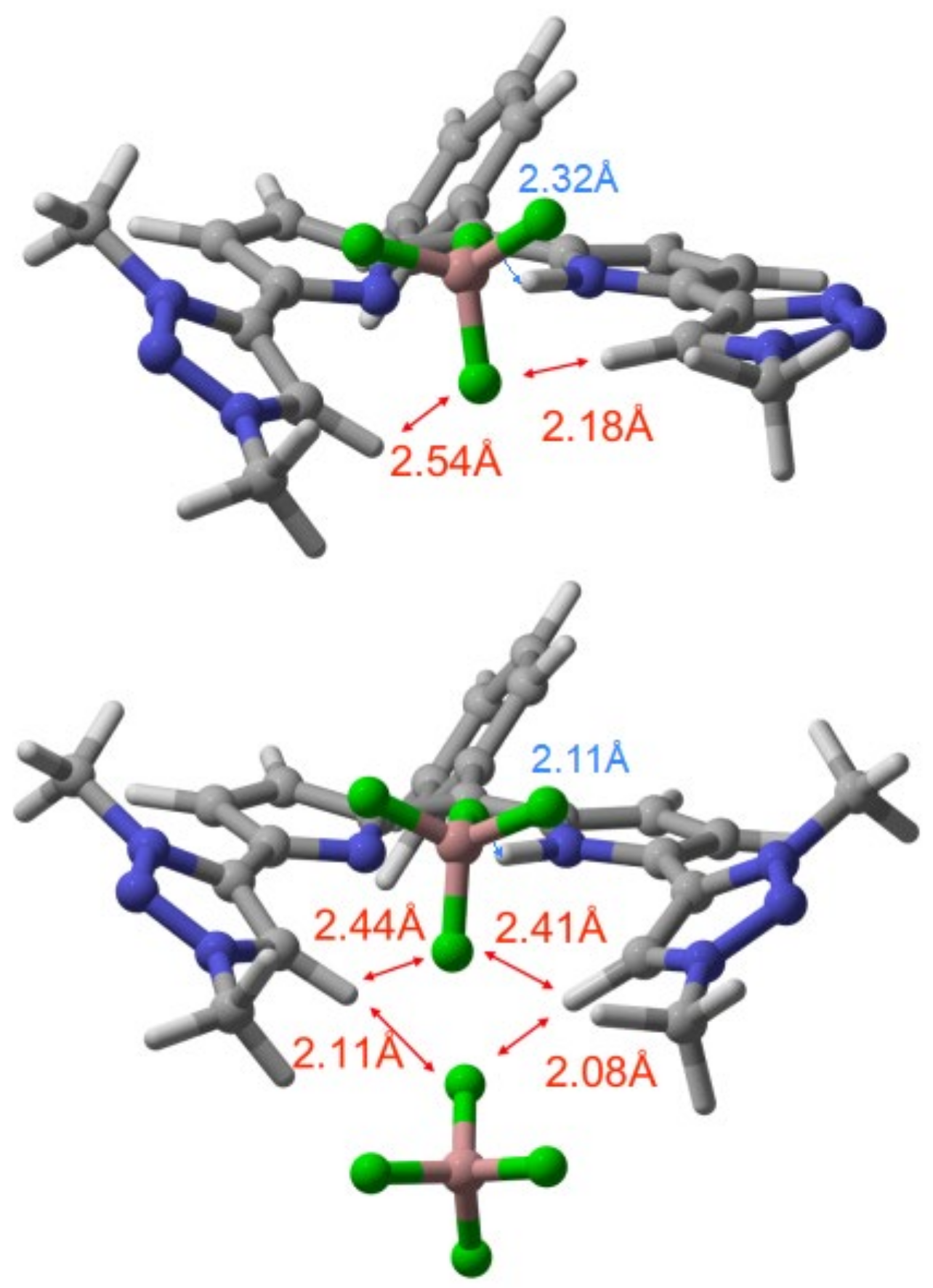
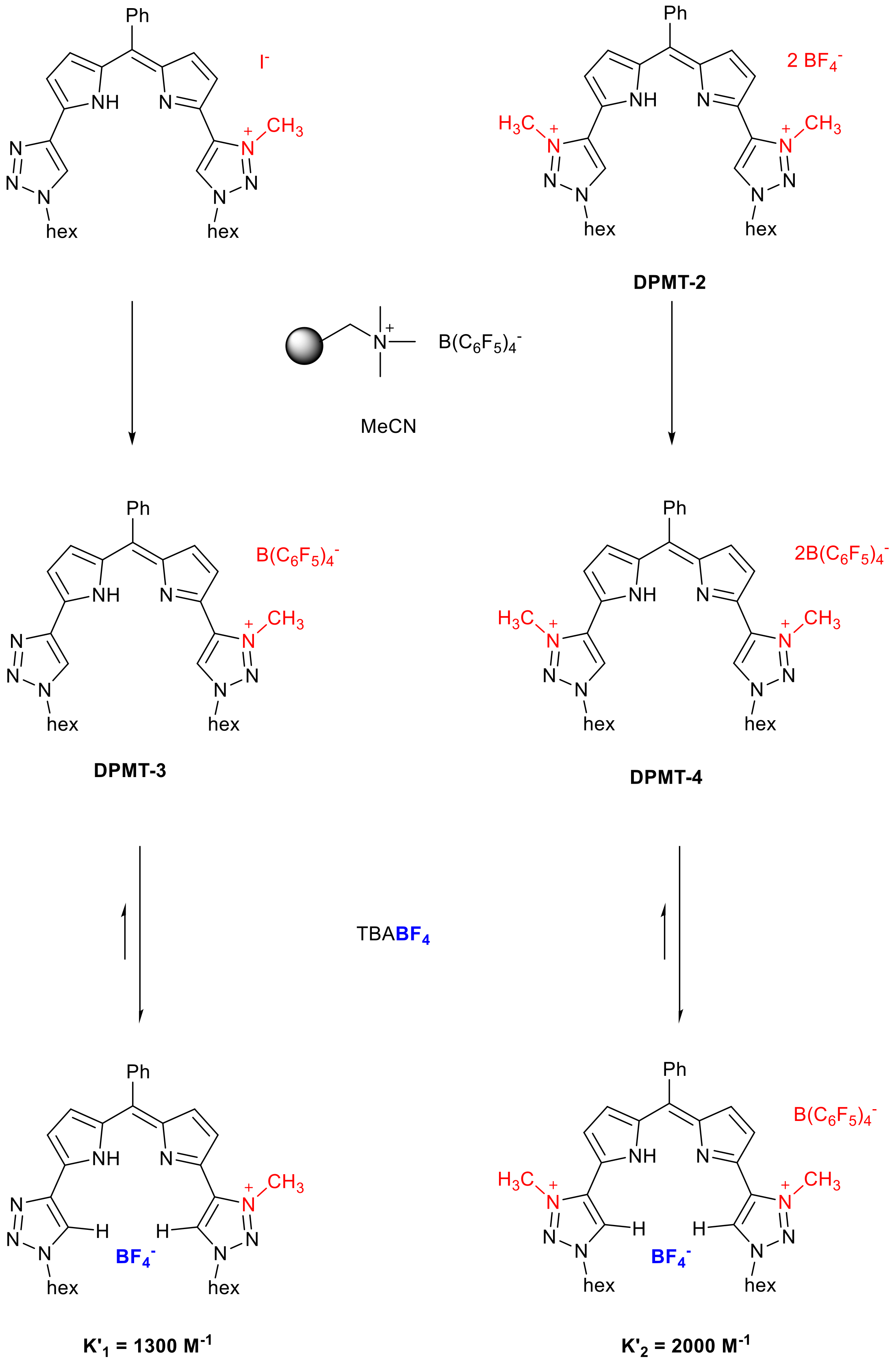
| X− (TBAX) | K1 (DPMT-1) | K2 (DPMT-2) |
|---|---|---|
| F− | Degradation | Degradation |
| Cl− | 630 | 164 |
| Br− | 652 | 129 |
| I− | 460 | 98 |
| NO3− | 444 | 148 |
| HSO4− | Degradation | Degradation |
| H2PO4− | Degradation | Degradation |
| AcO− | Degradation | Degradation |
| X− (TBAX) | K1 (DPMT-1) | K2 (DPMT-2) |
|---|---|---|
| Cl− | 8 × 105 | 3 × 105 |
| Br− | 8.5 × 105 | 2.5 × 105 |
| I− | 6 × 105 | 2 × 105 |
| NO3− | 5 × 105 | 3 × 105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guérin, C.; Zhang, Z.; Jean-Gérard, L.; Steinmann, S.; Michel, C.; Andrioletti, B. Strong Affinity of Triazolium-Appended Dipyrromethenes (TADs) for BF4−. Molecules 2020, 25, 4555. https://doi.org/10.3390/molecules25194555
Guérin C, Zhang Z, Jean-Gérard L, Steinmann S, Michel C, Andrioletti B. Strong Affinity of Triazolium-Appended Dipyrromethenes (TADs) for BF4−. Molecules. 2020; 25(19):4555. https://doi.org/10.3390/molecules25194555
Chicago/Turabian StyleGuérin, Charles, Zhan Zhang, Ludivine Jean-Gérard, Stephan Steinmann, Carine Michel, and Bruno Andrioletti. 2020. "Strong Affinity of Triazolium-Appended Dipyrromethenes (TADs) for BF4−" Molecules 25, no. 19: 4555. https://doi.org/10.3390/molecules25194555
APA StyleGuérin, C., Zhang, Z., Jean-Gérard, L., Steinmann, S., Michel, C., & Andrioletti, B. (2020). Strong Affinity of Triazolium-Appended Dipyrromethenes (TADs) for BF4−. Molecules, 25(19), 4555. https://doi.org/10.3390/molecules25194555








