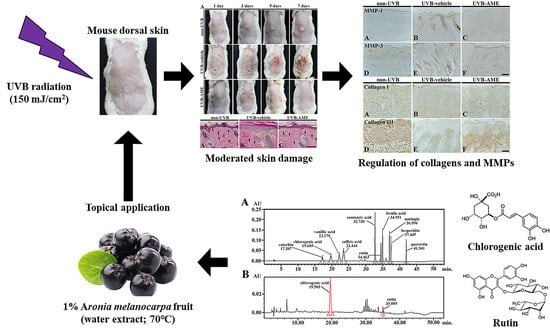
Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice
Abstract
:1. Introduction
2. Results
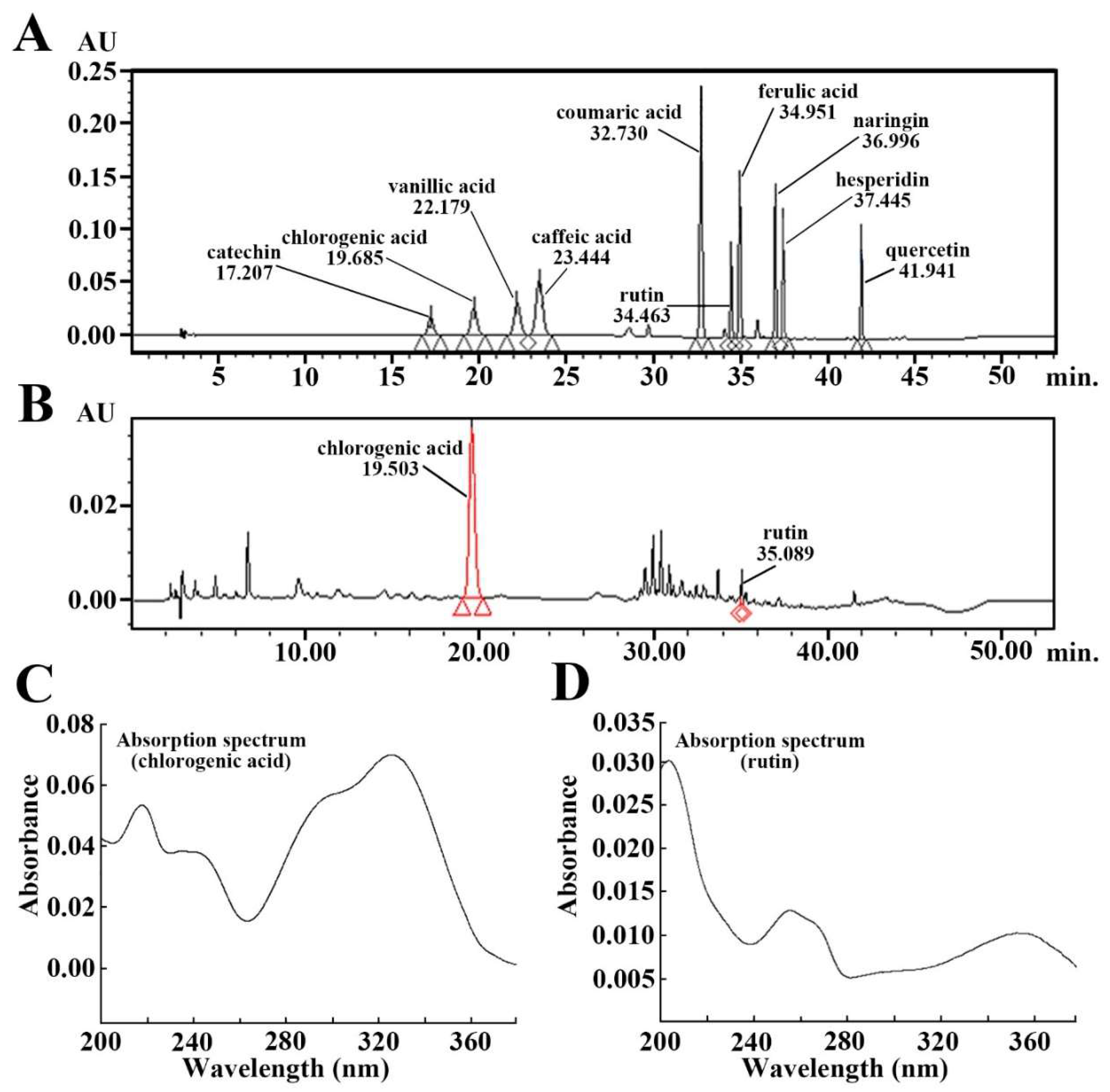
2.1. Ingredients of Aronia Melanocarpa Extract (AME)
2.2. Clinical Skin Severity (CSS) Score
2.3. Epithermal Thickness and Fibroblasts by Hematoxylin and Eosin (H and E) Staining
2.4. Collagen Fibers by Masson’s Trichrome Staining
2.5. Collagen Fibers by Immunohistochemistry
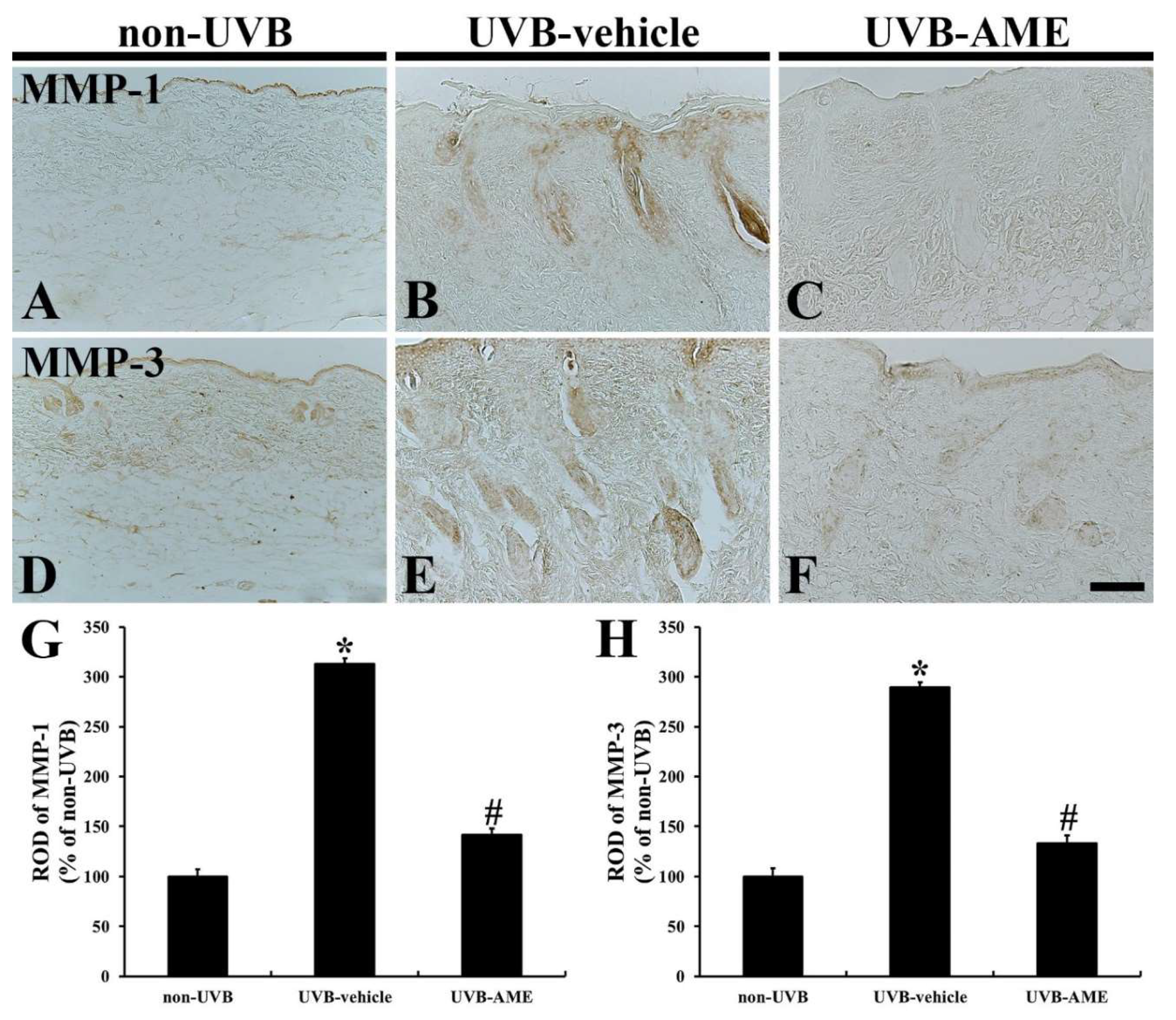
2.6. MMPs by Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Preparation and Treatment of AME
4.3. Qualitative Analysis of AME
4.4. Experimental Groups and AME Treatment
4.5. Observation of CSS
4.6. Tissue Preparation for Histological Analysis
4.7. H and E Staining
4.8. Masson’s Trichrome Staining
4.9. Immunohistochemistry
4.10. Data Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.; Shin, B.-N.; Lee, Y.L.; Park, J.H.; Kim, D.W.; Kim, K.S.; Kim, H.; Song, M.; Kim, J.D.; Won, M.-H.; et al. Oenanthe Javanica Extract Protects Mouse Skin from UVB Radiation via Attenuating Collagen Disruption and Inflammation. Int. J. Mol. Sci. 2019, 20, 1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.-H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxidative Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.-K.; Lee, J.-C.; Yang, G.E.; Her, Y.; et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Larrosa, M.; García-Cantalejo, J.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.-T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-Rich Extracts Inhibit Multiple Biomarkers of Colon Cancer in Rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.V.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2006, 48, 11–17. [Google Scholar]
- Ho, G.T.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating Activity of Aronia melanocarpa Polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef] [Green Version]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V. Antidiabetic Effects of Aronia melanocarpa and Its Other Therapeutic Properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Verhoef, L.; Vennema, H.; Van Pelt, W.; Lees, D.; Boshuizen, H.; Henshilwood, K.; Koopmans, M.; Network, F.-B.V.I.E. Use of Norovirus Genotype Profiles to Differentiate Origins of Foodborne Outbreaks. Emerg. Infect. Dis. 2010, 16, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2006, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [Green Version]
- Urso, V.; Signorini, M.A.; Tonini, M.; Bruschi, P. Wild medicinal and food plants used by communities living in Mopane woodlands of southern Angola: Results of an ethnobotanical field investigation. J. Ethnopharmacol. 2016, 177, 126–139. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Škrovánková, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavová, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Medica 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-mediated Oxidative Stress in Human HaCaT Keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-S.; Kundu, J.K.; Chun, K.-S.; Na, H.-K.; Dong, Z. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Yogianti, F.; Kunisada, M.; Nakano, E.; Ono, R.; Sakumi, K.; Oka, S.; Nakabeppu, Y.; Nishigori, C. Inhibitory Effects of Dietary Spirulina platensis on UVB-Induced Skin Inflammatory Responses and Carcinogenesis. J. Investig. Dermatol. 2014, 134, 2610–2619. [Google Scholar] [CrossRef] [Green Version]
- Paulrayer, A.; Adithan, A.; Lee, J.-H.; Moon, K.H.; Kim, D.G.; Im, S.-Y.; Kang, C.-W.; Kim, N.S.; Kim, J.-H. Aronia melanocarpa (Black Chokeberry) Reduces Ethanol-Induced Gastric Damage via Regulation of HSP-70, NF-κB, and MCP-1 Signaling. Int. J. Mol. Sci. 2017, 18, 1195. [Google Scholar] [CrossRef]
- Piotrowska-Kempisty, H.; Nowicki, M.; Jodynis-Liebert, J.; Kurpik, M.; Ewertowska, M.; Adamska, T.; Oszmiański, J.; Kujawska, M. Assessment of Hepatoprotective Effect of Chokeberry Juice in Rats Treated Chronically with Carbon Tetrachloride. Molecules 2020, 25, 1268. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Wang, Y.; Jiao, X.; Chou, S.; Li, E.; Li, B. Preparative Purification of Polyphenols from Aronia melanocarpa (Chokeberry) with Cellular Antioxidant and Antiproliferative Activity. Molecules 2018, 23, 139. [Google Scholar] [CrossRef] [Green Version]
- Sidor, A.; Gramza-Michałowska, A.; Michałowska, G. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [Green Version]
- Yamate, Y.; Hiramoto, K.; Sato, E.F. The Preventive Effect of Coffee Compounds on Dermatitis and Epidermal Pigmentation after Ultraviolet Irradiation in Mice. Ski. Pharmacol. Physiol. 2017, 30, 24–35. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Bin Kwon, S.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.L.; Park, S.M.; Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.-H.; Lee, C.-H.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits uvb-induced oxidative damage and inflammation through map kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, C.S.; Yang, J.; Shin, C.-Y.; Chung, J.H. Topical or oral treatment of peach flower extract attenuates UV-induced epidermal thickening, matrix metalloproteinase-13 expression and pro-inflammatory cytokine production in hairless mice skin. Nutr. Res. Pr. 2018, 12, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Han, M.; Shin, H.S.; Kim, M.-K.; Shin, C.-Y.; Lee, D.-H.; Chung, J.H. Perilla frutescens leaves extract ameliorates ultraviolet radiation-induced extracellular matrix damage in human dermal fibroblasts and hairless mice skin. J. Ethnopharmacol. 2017, 195, 334–342. [Google Scholar] [CrossRef]
- Choi, S.; Jung, T.; Cho, B.; Choi, S.; Sim, W.; Han, X.; Lee, S.J.; Kim, Y.; Lee, O. Anti-photoaging effect of fermented agricultural by-products on ultraviolet B-irradiated hairless mouse skin. Int. J. Mol. Med. 2019, 44, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Goh, A.R.; Youn, G.S.; Yoo, K.Y.; Won, M.H.; Han, S.-Z.; Lim, S.S.; Lee, K.-W.; Choi, S.Y.; Park, J. Aronia melanocarpa Concentrate Ameliorates Pro-Inflammatory Responses in HaCaT Keratinocytes and 12-O-Tetradecanoylphorbol-13-Acetate-Induced Ear Edema in Mice. J. Med. Food 2016, 19, 654–662. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, J.Y.; Jeong, M.S.; Park, K.Y.; Park, K.H.; Lee, M.W.; Joo, S.S.; Seo, S.J. Effect of topical application of quercetin-3-o-(2’’-gallate)-α-l-rhamnopyranoside on atopic dermatitis in NC/Nga mice. J. Dermatol. Sci. 2015, 77, 166–172. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, J.H.; Ahn, J.H.; Kim, J.D.; Cho, J.H.; Lee, T.-K.; Won, M.-H. Stronger antioxidant enzyme immunoreactivity of Populus tomentiglandulosa extract than ascorbic acid in rat liver and kidney. Iran. J. Basic Med Sci. 2019, 22, 963–967. [Google Scholar]
- Cho, J.H.; Moon, J.B.; Park, C.W.; Ohk, T.G.; Shin, M.C.; Won, M.H. Differential activation of c-Fos in the paraventricular nuclei of the hypothalamus and thalamus of the rat following myocardial infarction. Resuscitation 2016, 106, e90. [Google Scholar] [CrossRef]
- Carpenter, J.; Marion, C. Exotic animal formulary; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- Park, Y.; Tae, H.-J.; Cho, J.H.; Kim, I.-S.; Ohk, T.G.; Park, C.W.; Moon, J.B.; Shin, M.C.; Lee, T.-K.; Lee, J.-C.; et al. The relationship between low survival and acute increase of tumor necrosis factor α expression in the lung in a rat model of asphyxial cardiac arrest. Anat. Cell Biol. 2018, 51, 128–135. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Her, Y.; Lee, T.-K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.-C.; Ahn, J.H.; Park, J.H.; Lee, J.-W.; Hong, J.; et al. Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25, 4577. https://doi.org/10.3390/molecules25194577
Her Y, Lee T-K, Kim JD, Kim B, Sim H, Lee J-C, Ahn JH, Park JH, Lee J-W, Hong J, et al. Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules. 2020; 25(19):4577. https://doi.org/10.3390/molecules25194577
Chicago/Turabian StyleHer, Young, Tae-Kyeong Lee, Jong Dai Kim, Bora Kim, Hyejin Sim, Jae-Chul Lee, Ji Hyeon Ahn, Joon Ha Park, Ji-Won Lee, Junkee Hong, and et al. 2020. "Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice" Molecules 25, no. 19: 4577. https://doi.org/10.3390/molecules25194577