UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles
Abstract
:1. Introduction
2. Results
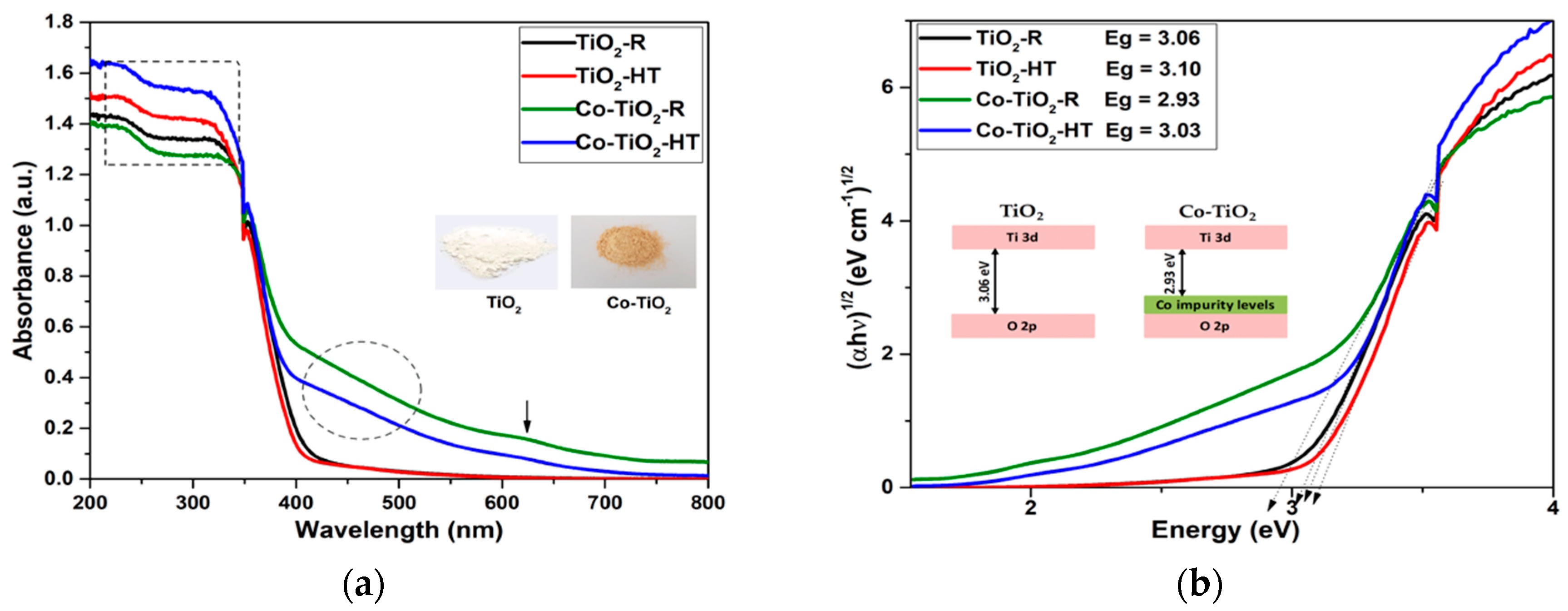
2.1. Photocatalysts Characterizations
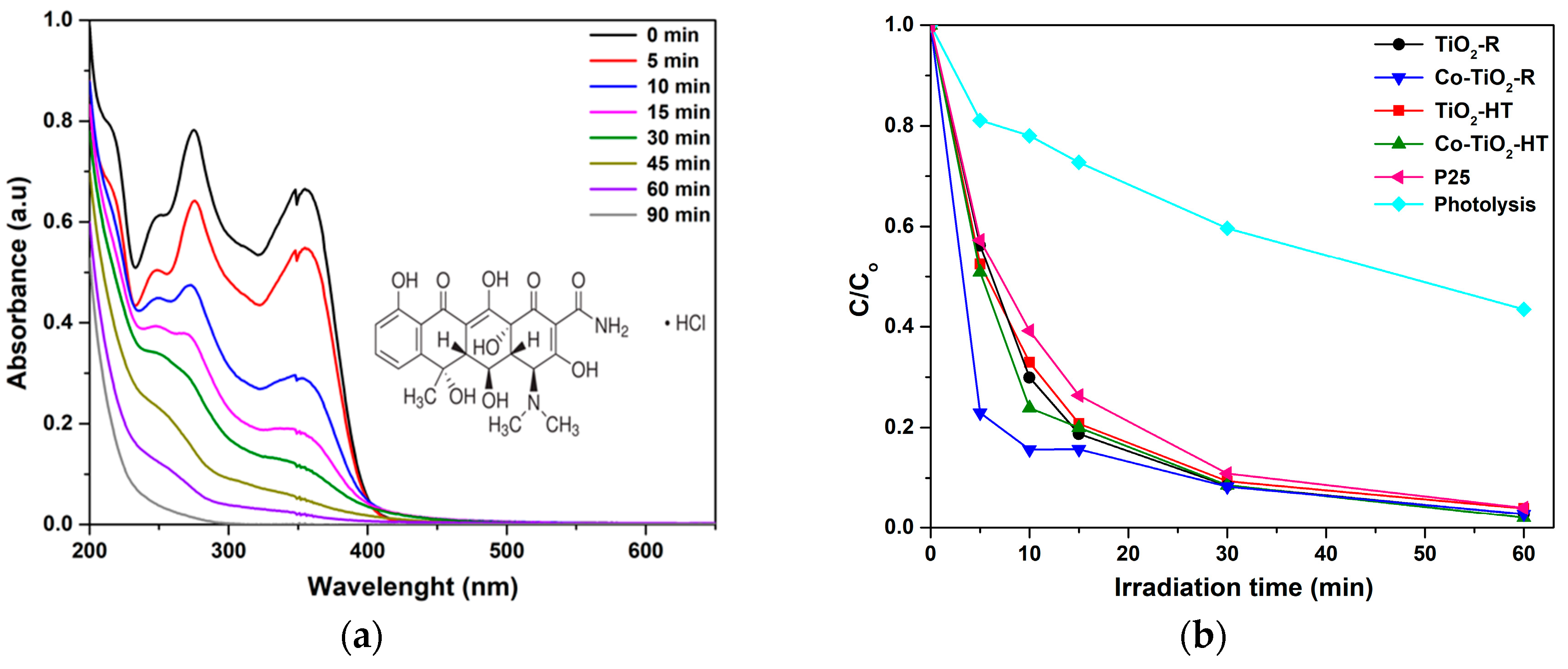
2.2. Photocatalytic Activities of Co-TiO2-R and Co-TiO2-HT on UV/Vis Light-Induced OTC HCl Degradation
3. Discussion
3.1. Characterization of Co-TiO2-R and Co-TiO2-HT Composites
3.2. UV/Vis Light-Induced Oxytetracycline Hydrochloride Degradation over Co-TiO2 Composites
3.3. Proposed Mechanisms of UV/Vis Light-Induced Oxytetracycline Hydrochloride Degradation Using Co-TiO2 Catalysts
4. Materials and Methods
4.1. Materials’ Composites
4.2. Photocatalysts Synthesis
4.2.1. High Temperature Synthesis of Cobalt-Doped TiO2
4.2.2. Reflux Synthesis of Cobalt-doped TiO2
4.3. Photocatalysts Characterization
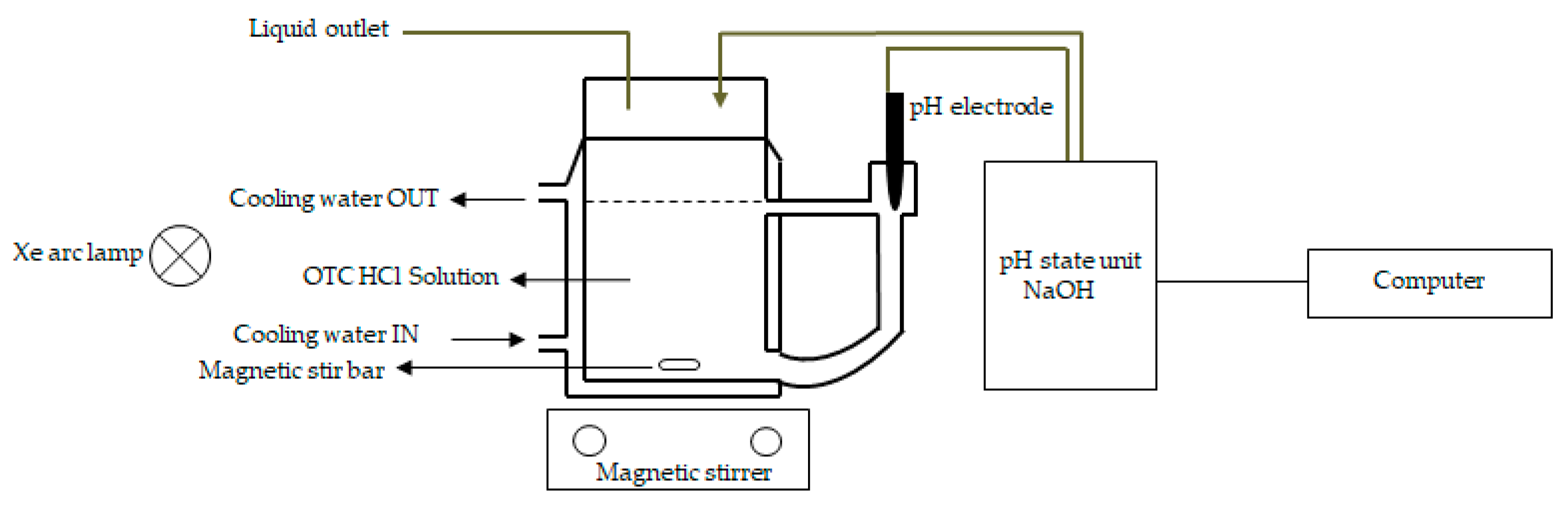
4.4. UV/Vis Light-Induced Oxytetracycline Hydrochloride (OTC HCl) Degradation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in The Aquatic Environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.; Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and Removal Methods of Antibiotics from Aqueous Matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Pupo Nogueira, R.F. Degradation of Tetracycline by Photo-Fenton Process—Solar Irradiation and Matrix Effects. J. Photochem. Photobiol. A Chem. 2007, 187, 33–39. [Google Scholar] [CrossRef]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and Inactivation of Tetracycline by TiO2 Photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and Mechanism Investigation on The Destruction of Oxytetracycline by UV-254 nm Activation of Persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, H.; Li, Y.; Li, Z. Photodegradation of Oxytetracycline in Aqueous by 5A and 13X Loaded with TiO2 Under UV Irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical Degradation of Oxytetracycline: Influence of pH and Role of Carbonate Radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Pereira, J.H.; Queirós, D.B.; Reis, A.C.; Nunes, O.C.; Borges, M.T.; Boaventura, R.A.; Vilar, V.J. Process Enhancement at Near Neutral pH of a Homogeneous Photo-Fenton Reaction Using Ferricarboxylate Complexes: Application to Oxytetracycline Degradation. Chem. Eng. J. 2014, 253, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.H.; Bae, H.; Jung, J. Tetracycline Degradation by Ozonation in The Aqueous Phase: Proposed Degradation Intermediates and Pathway. J. Hazard. Mater 2010, 181, 659–665. [Google Scholar] [CrossRef]
- Dodd, M.C.; Kohler, H.E.; Gunten, U. Oxidation of Antibacterial Compounds by Ozone and Hydroxyl Radical: Elimination of Biological Activity during Aqueous Ozonation Processes. Environ. Sci. Technol. 2009, 43, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pacheco, C.V.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Lopez-Peñalver, J.J. Tetracycline Degradation in Aqueous Phase by Ultraviolet Radiation. Chem. Eng. J. 2012, 187, 89–95. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Fatta-Kassinos, D.; Dionysiou, D.D. Significant Role of UV and Carbonate Radical on the Degradation of Oxytetracycline in UV-AOPs: Kinetics and Mechanism. Water Res. 2016, 95, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Penũela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic Oxidation of The Antibiotic Tetracycline on TiO2 and ZnO Suspensions. Catal. Today. 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Reis, A.C.; Queirós, D.; Nunes, O.C.; Borges, M.T.; Vilar, V.J.P.; Boaventura, R.A.R. Insights into Solar TiO2-Assisted Photocatalytic Oxidation of Two Antibiotics Employed in Aquatic Animal Production, Oxolinic Acid and Oxytetracycline. Sci. Total Environ. 2013, 463–464, 274–283. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and Low Cost Tetracycline Degradation Processes by Nanometric and Immobilized TiO2 Systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Yahiat, S.; Fourcade, F.; Brosillon, S.; Amrane, A. Removal of Antibiotics by An Integrated Process Coupling Photocatalysis and Biological Treatment-Case of Tetracycline and Tylosin. Int. Biodeterior. Biodegrad. 2011, 65, 997–1003. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Wang, Y.-J.; Sun, R.-J.; Zhou, D.-M. Photocatalytic Degradation of Tetracycline in Aqueous Solution by Nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Serpone, N.; Lawless, D.; Khairutdinov, R.; Pelizzetti, E. Subnanosecond Relaxation Dynamics in TiO2 Colloidal Sols (Particle Sizes Rp = 1.0-13.4 Nm). Relevance to Heterogeneous Photocatalysis. J. Phys. Chem. 1995, 99, 16655–16661. [Google Scholar] [CrossRef]
- Choi, W.Y.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Naik, B.; Parida, K.M.; Gopinath, C.S. Facile Synthesis of N- and S-Incorporated Nanocrystalline TiO2 and Direct Solar-Light-Driven Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 19473–19482. [Google Scholar] [CrossRef]
- Wang, P.; Yap, P.-S.; Lim, T.-T. C–N–S Tridoped TiO2 for Photocatalytic Degradation of Tetracycline under Visible-Light Irradiation. Appl. Catal. A 2011, 399, 252–261. [Google Scholar] [CrossRef]
- Jo, W.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt Promoted TiO2/GO for The Photocatalytic Degradation of Oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Litter, M.I. Heterogeneous Photocatalysis Transition Metal Ions in Photocatalytic Systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Dvoranova, D.; Brezova, V.; Mazur, M.; Malati, M. Investigations of Metal-Doped Titanium Dioxide Photocatalysts. Appl. Catal. B Environ. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A High Performance Cobalt-Doped ZnO Visible Light Photocatalyst and Its Photogenerated Charge Transfer Properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A Study on The Mechanism for The Interaction of Light With Noble Metal-Metal Oxide Semiconductor Nanostructures for Various Photophysical Applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Noble Metal Modified Reduced Graphene Oxide/TiO2 Ternary Nanostructures for Efficient Visible Light-Driven Photoreduction of Carbon Dioxide into Methane. Appl. Catal. B Environ. 2014, 166–167, 251–259. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, S.; Xin, Y. Synthesis of Au–CuS–TiO2 Nanobelts Photocatalyst for Efficient Photocatalytic Degradation of Antibiotic Oxytetracycline. Chem. Eng. J. 2016, 302, 377–387. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-Modified Semiconductor TiO2 Nanotube Arrays for Photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced Photoactivity with Nanocluster-Grafted Titanium Dioxide Photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Y.; Wu, J.; Zhen, Q. Photocatalytic Degradation and Pathway of Oxytetracycline in Aqueous Solution by Fe2O3–TiO2 Nanopowder. RSC Adv. 2015, 5, 40764–40771. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-Abundant Cocatalysts for Semiconductor Based Photocatalytic Water Splitting. Chem. Soc. Rev. 2014, 43, 778. [Google Scholar] [CrossRef]
- Alamgir, W.; Khan, S.; Ahmad, M.M.; Hassan, A.H. Naqvi. Structural Phase Analysis, Band Gap Tuning and Fluorescence Properties of Co Doped TiO2 Nanoparticles. Opt. Mater. 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Zhang, F.; Yamakata, A.; Maeda, K.; Moriya, Y.; Takata, T.; Kubota, J.; Teshima, K.; Oishi, S.; Domen, K. Cobalt-Modified Porous Single-Crystalline LaTiO2N for Highly Efficient Water Oxidation under Visible Light. J. Am. Chem. Soc. 2012, 134, 8348–8351. [Google Scholar] [CrossRef]
- Lee, J.; Jackson, D.H.K.; Li, T.; Winans, R.E.; Dumesic, J.A.; Kuech, T.F.; Huber, G.W. Enhanced Stability of Cobalt Catalysts by Atomic Layer Deposition for Aqueous-Phase Reactions. Energy Environ. Sci. 2014, 7, 1657–1660. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Gallagher, J.R.; Enache, D.I.; Smith, P.; Boldrin, P.; Davies, T.E.; Bartley, J.K.; Combes, G.B.; Williams, P.B.; et al. Preparation of Fischer–Tropsch Supported Cobalt Catalysts Using a New Gas Anti-Solvent Process. ACS Catal. 2013, 3, 764–772. [Google Scholar] [CrossRef]
- Siddiqa, A.; Masih, D.; Anjum, D.; Siddiq, M. Cobalt and Sulfur Co-Doped Nano-Size TiO2 for Photodegradation of Various Dyes and Phenol. J. Environ. Sci. 2015, 37, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, M.A.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic Degradation of 2-Chlorophenol by Co-doped TiO2 Nanoparticles. Appl. Catal. B Environ. 2005, 57, 23–30. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of Single Metal-Ion Doping on The Visible-Light Photoreactivity of TiO2. J. Phys. Chem. C. 2010, 114, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Bouras, P.; Stathatos, E.; Lianos, P. Pure Versus Metal-Ion-Doped Nanocrystalline Titania for Photocatalysis. Appl. Catal. B. Environ. 2007, 73, 51–59. [Google Scholar] [CrossRef]
- Karthik, K.; Kesava, P.S.; Suresh Kumar, K.; Victor Jaya, N. Influence of Dopant Level on Structural, Optical and Magnetic Properties of Co-Doped Anatase TiO2 Nanoparticles. Appl. Surf. Sci. 2010, 256, 4757. [Google Scholar] [CrossRef]
- Samet, L.; Nasseur, J.B.; Chtourou, R.; March, K.; Stephan, O. Heat Treatment Effect on The Physical Properties of Cobalt Doped TiO2 Sol-gel Materials. Mater. Charact. 2013, 85, 1–12. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.; Liu, Y.; Wang, Y. Room Temperature Ferromagnetism of Co-Doped TiO2 Nanotube Arrays Prepared by Sol-Gel Template Synthesis. Chem. Phys. Lett. 2006, 432, 468–472. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Luminescence Characteristics of Cobalt Doped TiO2 Nanoparticles. J. Lumin. 2012, 132, 178–184. [Google Scholar] [CrossRef]
- Khurana, C.; Pandey, O.P.; Chudasama, B. Synthesis of Visible Light-Responsive Cobalt-Doped TiO2 Nanoparticles with Tunable Optical Band Gap. J. Sol.-Gel Sci. Technol. 2015, 75, 424–435. [Google Scholar] [CrossRef]
- Hamadanian, A.; Reisi-Vanani, A.; Majedi, A. Sol-Gel Preparation and Characterization of Co/TiO2 Nanoparticles: Application to The Degradation of Methyl Orange. J. Iran. Chem. Soc. 2010, 7, S52–S58. [Google Scholar] [CrossRef]
- Castro, A.L.; Nunes, M.R.; Carvalho, M.D.; Ferreira, L.P.; Jumes, J.-C.; Costa, F.M.; Florencio, M.H. Doped Titanium Dioxide Nanocrystalline Powders with High Photocatalytic Activity. J. Solid State Chem. 2009, 182, 1838–1845. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, T.; Liu, Y.; Cai, J. Preparation and Characterization of Room Temperature Ferromagnetic Co-Doped Anatase TiO2 Nanobelts. J. Phys. Chem. C 2008, 112, 8604–8608. [Google Scholar] [CrossRef]
- Yermakov, A.Y.; Zakharova, G.S.; Uimin, M.A.; Kuznetsov, M.V.; Molochnikov, L.S.; Konev, S.F.; Konev, A.S.; Minin, A.S.; Mesilov, V.V.; Galakhov, V.R.; et al. Surface Magnetism of Cobalt-Doped Anatase TiO2 Nanopowders. J. Phys. Chem. C 2016, 120, 28857–28866. [Google Scholar] [CrossRef]
- Mesilov, V.V.; Galakhov, V.R.; Gubkin, A.F.; Sherstobitova, E.A.; Zakharova, G.S.; Uimin, M.A.; Yermakov, A.Y.e.; Kvashnina, K.O.; Smirnov, D.A. X-ray Diffraction and X-ray Spectroscopy Studies of Cobalt-Doped Anatase TiO2: Co Nanopowders. J. Phys. Chem. C 2017, 121, 24235–24244. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Transition metal Ion Impregnated Mesoporous TiO2 for Photocatalytic Degradation of Organic Contaminants in Water. Ind. Eng. Chem. Res. 2006, 45, 5231–5238. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Ikeda, S.; Marcì, G.; Ohtani, B.; Palmisano, L. Photocatalytic Degradation of Organic Compounds in Aqueous Systems by Transition Metal Doped Polycrystalline TiO2. Catal. Today 2002, 75, 87–93. [Google Scholar] [CrossRef]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayıoglu, K.; Gedik, F.; Akın, A.N.; Özkara-Aydınoglu, S. A Comparative Study for Removal of Different Dyes Over M/TiO2 (M = Cu, Ni, Co, Fe, Mn, and Cr) Photocatalysts Under Visible Light Irradiation. J. Photochem. Photobiol. A 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Miao, Y.; Zhai, Z.; Jiang, L.; Shi, Y.; Yan, Z.; Duana, D.; Zhen, K.; Wang, J. Facile and New Synthesis of Cobalt Doped Mesoporous TiO2 With High Visible-Light Performance. Powder Technol. 2014, 266, 365–371. [Google Scholar] [CrossRef]
- Jiang, P.; Xiang, W.; Kuang, J.; Liu, W.; Cao, W. Effect of Cobalt Doping on the Electronic, Optical and Photocatalytic Properties of TiO2. Solid State Sci. 2015, 46, 27–32. [Google Scholar] [CrossRef]
- Rashad, M.M.; Elsayed, E.M.; Al-Kotb, M.S.; Shalan, A.E. The Structural, Optical, Magnetic and Photocatalytic Properties of Transition Metal Ions Doped TiO2 Nanoparticles. J. Alloys Compd. 2013, 581, 71–78. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Taday, H.; Ito, S. Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light. J. Colloid Interface Sci. 2000, 224, 202–204. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumra, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.; Koinuma, H. Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science 2001, 291, 854. [Google Scholar] [CrossRef]
- Toyosaki, H.; Fukumura, T.; Yamada, Y.; Nakajima, K.; Chikyow, T.; Hasegawa, T.; Koinuma, H.; Kawasaki, M. Anomalous Hall Effect Governed by Electron Doping in a Room-Temperature Transparent Ferromagnetic Semiconductor. Nature 2004, 3, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.A.; Pakhomov, A.B.; Wang, C.M.; Heald, S.M.; Krishnan, K.M. Intrinsic Ferromagnetism in Insulating Cobalt Doped Anatase TiO2. Phys. Rev. Lett. 2005, 94, 157204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Talavera, R.; Vargas, S.; Arroyo-Murillo, R.; Montiel- Campos, R.; Haro-Poniatowski, E. Modification of the Phase Transition Temperatures in Titania Doped with Various Cations. J. Mater. Res. 1997, 12, 439–443. [Google Scholar] [CrossRef]
- Zhao, C.; Shu, X.; Zhu, D.; Wei, S.; Wang, Y.; Tu, M.; Gao, W. High Visible Light Photocatalytic Property of Co2þ-Doped TiO2 Nanoparticles with Mixed Phases. Superlattice Microst 2015, 88, 32–42. [Google Scholar] [CrossRef]
- Martinelli, A.; Alberti, S.; Caratto, V.; Lova, P.; Locardi, F.; Pampararo, G.; Villa, S.; Ferretti, M. Structural studies on copper and nitrogen-doped nanosized anatase. Z. Kristallogr. 2018, 233, 867–876. [Google Scholar] [CrossRef]
- Le, T.T.; Akhtar, M.S.; Park, D.M.; Lee, J.C.; Yang, O.B. Water Splitting on Rhodamine-B Dye-Sensitized Co-doped TiO2 Catalyst Under Visible Light. Appl. Catal. B Environ. 2012, 111–112, 397–401. [Google Scholar] [CrossRef]
- Qiu, M.; Tian, Y.; Chen, Z.; Yang, Z.; Li, W.; Wang, K.; Wang, L.; Wang, K.; Zhang, W. Synthesis of Ti3+ Self-Doped TiO2 Nanocrystals Based on Le Chatelier’s Principle and Their Application in solar light photocatalysis. RSC Adv. 2016, 6, 74376–74383. [Google Scholar] [CrossRef]
- Ohno, Y.; Tomita, K.; Komatsubara, Y.; Taniguchi, T.; Katsumata, K.; Matsushita, N.; Kogure, T.; Okada, K. Pseudo-Cube Shaped Brookite (TiO2) Nanocrystals Synthesized by an Oleate-Modified Hydrothermal Growth Method. Cryst. Growth Des. 2011, 11, 4831–4836. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Fan, W.-S.; Chen, W.-Y.; Lin, J.-Y. Adsorption and Visible-Light-Derived Photocatalytic Kinetics of Organic Dye on Co-Doped Titania Nanotubes Prepared by Hydrothermal Synthesis. Sep. Purif. Technol. 2009, 67, 312–318. [Google Scholar] [CrossRef]
- Lee, J.D. Concise Inorganic Chemistry, 5th ed.; Blackwell Science: Hoboken, NJ, USA, 1996. [Google Scholar]
- Jing, L.; Xin, B.; Yuan, F.; Xue, L.; Wang, B.; Fu, H. Effects of Surface Oxygen Vacancies on Photophysical and Photochemical Processes of Zn-Doped TiO2 Nanoparticles and Their Relationships. J. Phys. Chem. B 2006, 110, 17860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on Photolytic and Photocatalytic Degradation of Antibiotic Oxytetracycline in Aqueous Solution Under Visible/Solar Light: Kinetics and Mechanism Studies. Appl. Catal. B Environ. 2013, 134–135, 83–92. [Google Scholar] [CrossRef]
- Sassman, S.A.; Lee, L.S. Sorption of Three Tetracyclines by Several Soils: Assessing the Role of pH and Cation Exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Chu, W. The Hydrogen Peroxide-Assisted Photocatalytic Degradation of Alachlor in TiO2 Suspensions. Environ. Sci. Technol. 2003, 37, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Sato, T.; Katoh, R.; Furube, A.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Molecular Design of Coumarin Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. B 2003, 107, 597–606. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Co-TiO2-R, Co-TiO2-HT, TiO2-R, and TiO2-HT are available from the authors. |



| Catalysts | XRD Size (nm) | TEM Size (nm) | SSA (m2·g−1) | Band Gap (eV) | r0 [OTC HCl] UV/vis (mg·L−1· min−1) |
|---|---|---|---|---|---|
| TiO2-R | 9.5 | 9.8 ± 0.2 | 160 ± 5 | 3.06 | 3.45 |
| Co-TiO2-R | 9.2 | 9.5 ± 0.2 | 153 ± 5 | 2.93 | 8.83 |
| TiO2-HT | 9.9 | 10.5 ± 0.2 | 109 ± 5 | 3.10 | 3.87 |
| Co-TiO2-HT | 8.4 | 9.7 ± 0.2 | 126 ± 5 | 3.03 | 4.05 |
| P25 | 21 | 20.0 ± 0.2 | 50 ± 5 | 3.06 | 3.34 |
| Photolysis | - | - | - | - | 1.26 |
| Catalysts | Eg Anatase | A1g Brookite | B1g Brookite | B2g Brookite | B1g Anatase | A1g/B1g Anatase | Eg Anatase |
|---|---|---|---|---|---|---|---|
| TiO2-R | 32.8 | 41 | 75.5 | 26 | 93.5 | 96.5 | 129.2 |
| Co-TiO2-R | 34.8 | 46.5 | 71 | 23.5 | 96 | 94.5 | 172 |
| TiO2-HT | 19.5 | 33.5 | 83 | 27 | 97 | 97 | 84.8 |
| Co-TiO2-HT | 20.5 | 31.5 | 82 | 28 | 96.5 | 92.5 | 91.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akel, S.; Boughaled, R.; Dillert, R.; El Azzouzi, M.; Bahnemann, D.W. UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles. Molecules 2020, 25, 249. https://doi.org/10.3390/molecules25020249
Akel S, Boughaled R, Dillert R, El Azzouzi M, Bahnemann DW. UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles. Molecules. 2020; 25(2):249. https://doi.org/10.3390/molecules25020249
Chicago/Turabian StyleAkel, Soukaina, Redouan Boughaled, Ralf Dillert, Mohamed El Azzouzi, and Detlef W. Bahnemann. 2020. "UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles" Molecules 25, no. 2: 249. https://doi.org/10.3390/molecules25020249
APA StyleAkel, S., Boughaled, R., Dillert, R., El Azzouzi, M., & Bahnemann, D. W. (2020). UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles. Molecules, 25(2), 249. https://doi.org/10.3390/molecules25020249






