Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Enological Tannins Used
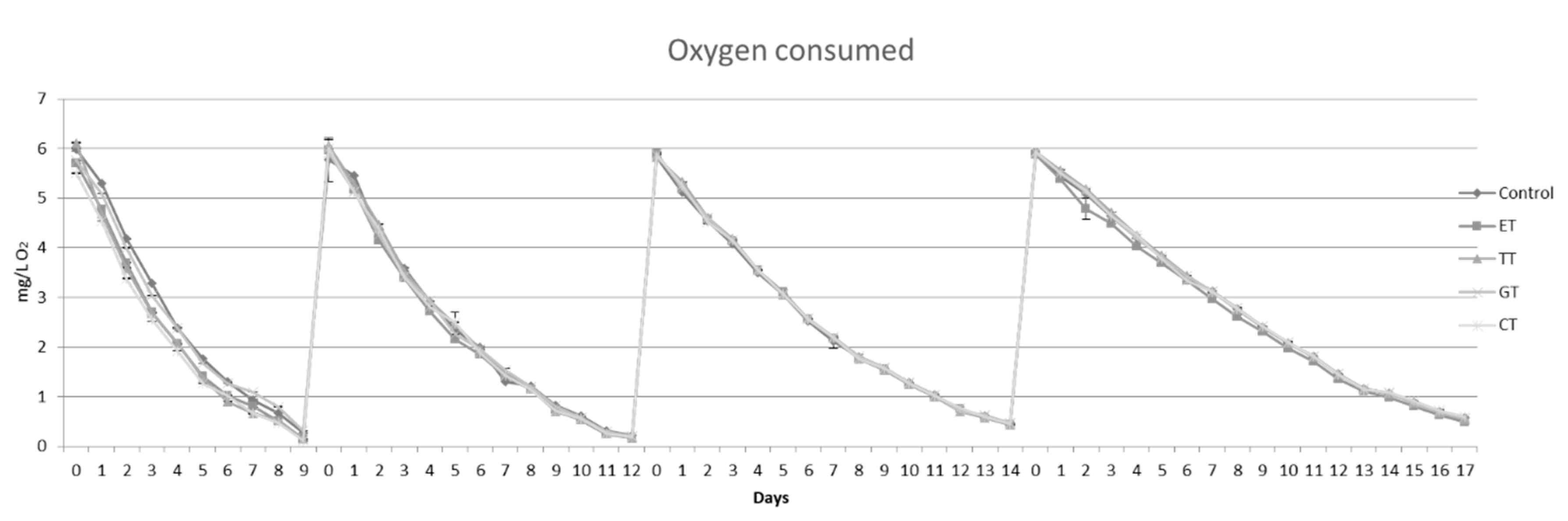
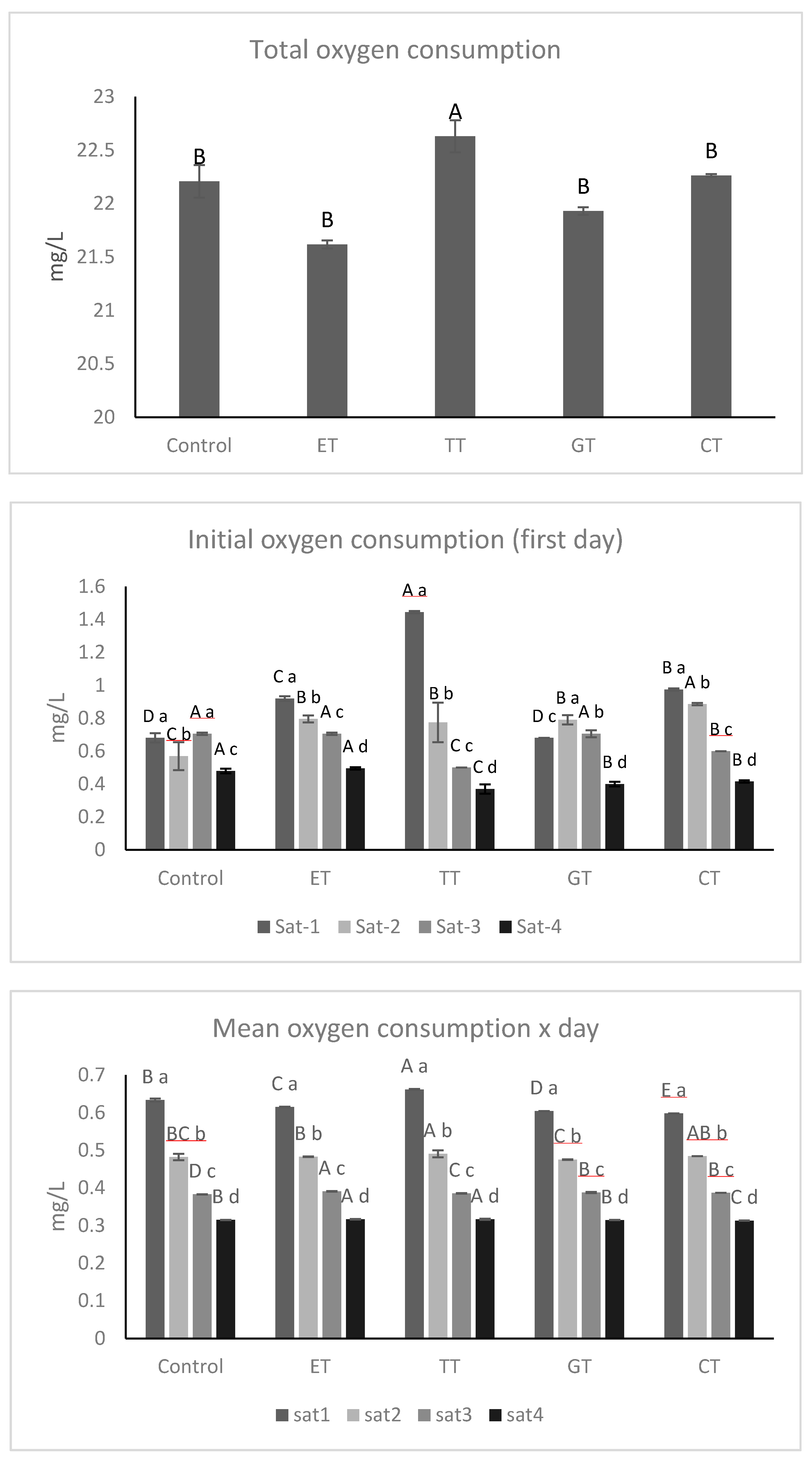
2.2. Oxygen Saturations Kinetics and Sulfur Dioxide Consumption
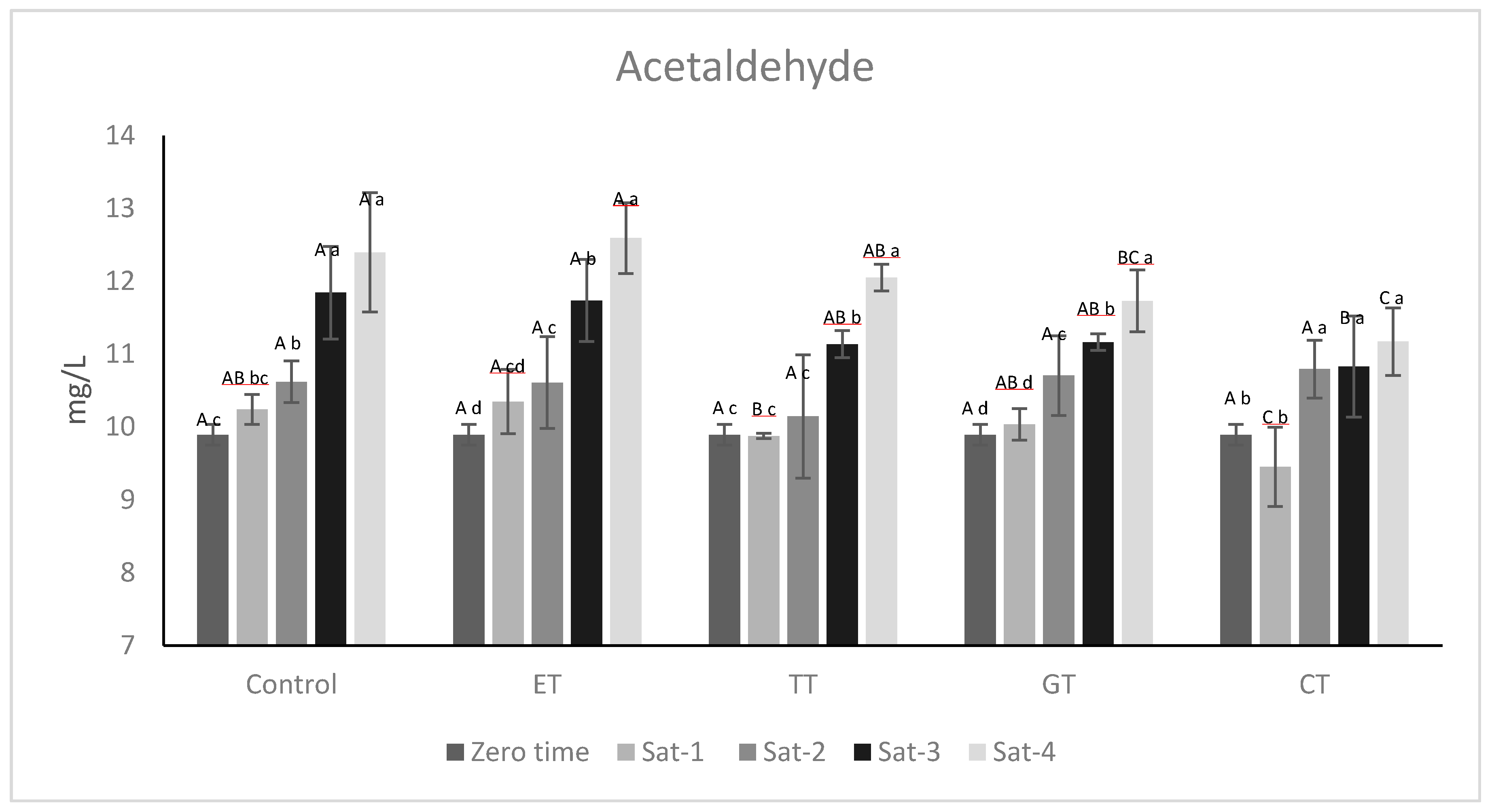
2.3. Effects of Wine Oxygen Saturations on Acetaldehyde
2.4. Effects of Different Enological Tannins on Wine Phenolics and Color Parameters during Oxygen Saturations
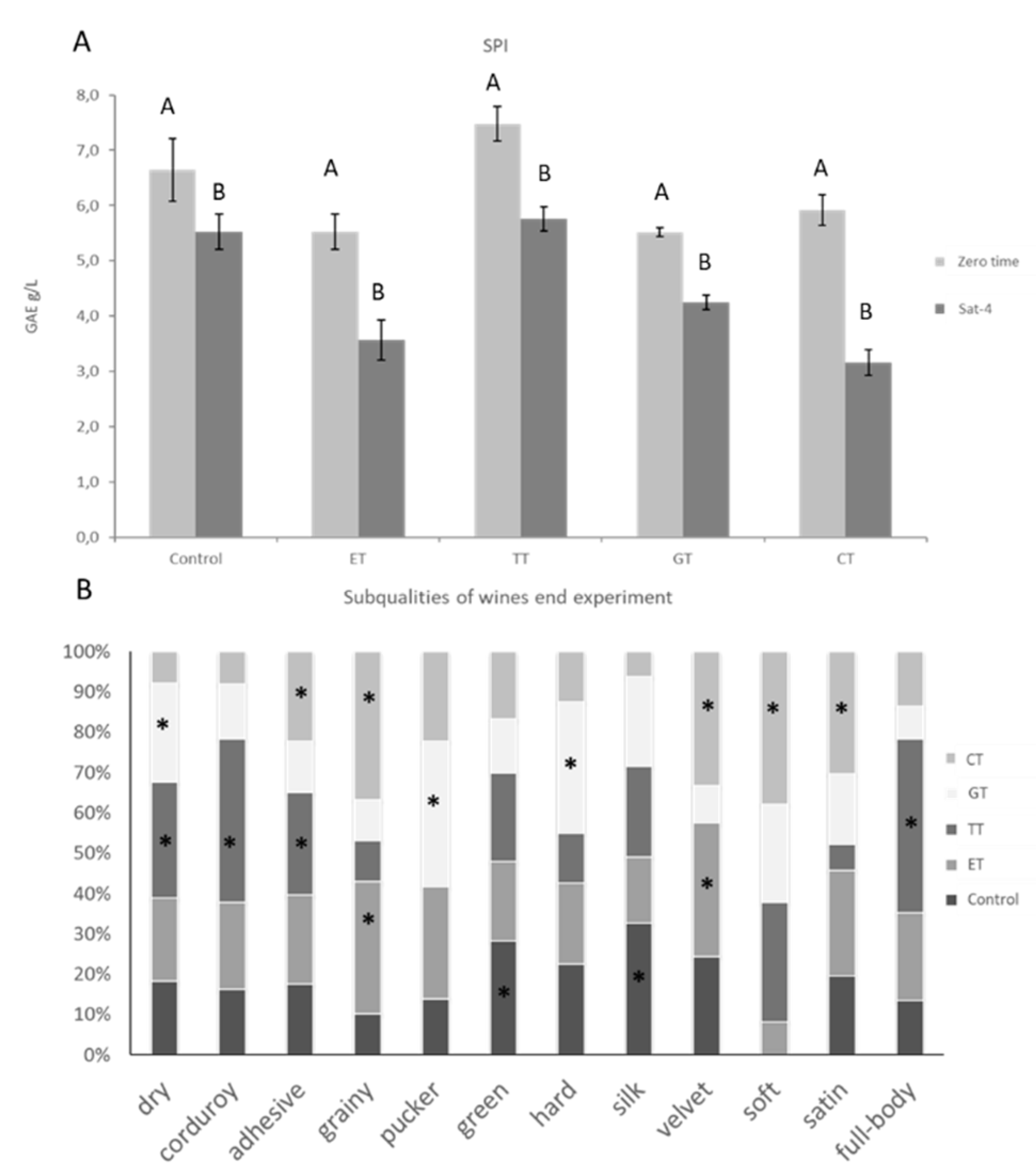
2.5. Effects of Different Enological Tannins on SPI and Astringency Subqualities at the End of the Experiment
3. Materials and Methods
3.1. Experimental Wines
3.2. Aglianico Wine
3.3. Determination of Sulfur Dioxide
3.4. High-Performance Liquid Chromatography Analysis of Acetaldehyde
3.5. High-Performance Liquid Chromatography Analyses of Anthocyanins
3.6. Analysis of the Chromatic Characteristics and Phenolic Compounds of the Wine
3.7. The Saliva Precipitation Index
3.8. Wine Evaluation
3.9. NMR Experiments
3.10. MS Experiments
3.11. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vignault, A.; Pascual, O.; Gombau, J.; Jourdes, M.; Moine, V.; Fermaud, M.; Roudet, J.; Canals, J.M.; Teissedre, P.L.; Zamora, F. New insight about the functionality of oenological tannins; Main results of the working group on oenological tannins. BIO W. Conf. 2019, 12. [Google Scholar] [CrossRef]
- Giacosa, S.; Segade, S.R.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in Wines: Rational Use and Possible Alternative. R. Win. Tech. 2019, 21, 309–321. [Google Scholar]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The effects of enological practices in anthocyanins, phenolic compounds and wine colour and their dependence on grape characteristics. J. Food. Comp. Ana. 2007, 20, 546–552. [Google Scholar] [CrossRef]
- Versari, A.; Du Toit, W.; Parpinello, G.P. Oenological tannins: A review. Aust. J. Grape Win. Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Conde, E.; Cadahia, E.; Valejo, G. Low-molecular-weight phenolic compounds in woods of Spanish, French and American oak. J. Sci. Tech. Ton. 1996, 1, 1–23. [Google Scholar]
- Saucier, C.; Jourdes, M.; Glories, Y.; Quideau, S. Extraction, detection, and quantification of flavano-ellagitannins and ethylvescalagin in a Bordeaux red wine aged in oak barrels. J. Agric. Food Chem. 2006, 19, 7349–7354. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compound. In Handbook of Enology; John Wiley & Sons: Chichester, UK, 2006; Volume 8, pp. 141–203. [Google Scholar]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nut. 2005, 81, 243–255. [Google Scholar] [CrossRef]
- Ugliano, M.; Slaghenaufi, D.; Picariello, L.; Olivieri, G. Oxygen and SO2 consumption of different enological tannins in relationship to their chemical and electrochemical characteristics. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Ramos, I.I.; Reis, S.; Segundo, M.A. Antioxidant profile of commercial oenological tannins determined by multiple chemical assays. Aust. J. Grape Win. Res. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Jeremic, J.; Vongluanngam, I.; Ricci, A.; Parpinello, G.P.; Versari, A. The oxygen consumption kinetics of commercial oenological tannins in model wine solution and Chianti red wine. Molecules 2020, 25, 1215. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Flavan-3-ols and Condensed Tannin. In Understanding Wine Chemistry; John Wiley & Sons: Chichester, UK, 2016; Volume 14, pp. 117–125. [Google Scholar]
- Elias, R.J.; Waterhouse, A.L. Controlling the Fenton reaction in wine. J. Agric. Food. Chem. 2010, 58, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.L.; Waterhouse, A.L. 1H NMR: A novel approach to determining the thermodynamic properties of acetaldehyde condensation reactions with glycerol, (+)-catechin, and glutathione in model wine. J. Agric. Food Chem. 2016, 64, 6869–6878. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Petracca, F.; Rinaldi, A.; Moio, L. Enological tannins affect acetaldehyde evolution, colour stability and tannin reactivity during forced oxidation of red wine. Int. J. Food Sci. Tech. 2018, 53, 228–236. [Google Scholar]
- Wildenradt, H.L.; Singleton, V.L. The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. Am. J. Enol. Vitic. 1974, 25, 119–126. [Google Scholar]
- Picariello, L.; Gambuti, A.; Picariello, B.; Moio, L. Evolution of pigments, tannins and acetaldehyde during forced oxidation of red wine: Effect of tannins addition. LWT 2017, 77, 370–375. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A. Relationship between polyphenolic content, antioxidant properties and oxygen consumption rate of different tannins in a model wine solution. Food Chem. 2020, 313, 126045. [Google Scholar] [CrossRef]
- Rinaldi, A.; Moio, L. Effect of enological tannin addition on astringency subqualities and phenolic content of red wines. J. Sens. Stud. 2018, 33, 12325. [Google Scholar] [CrossRef]
- Marzouk, M.S.; Moharram, F.A.; Gamal-Eldeen, A.; Damlakhy, I.M. Spectroscopic Identifi cation of New Ellagitannins and a Trigalloylglucosylkaempferol from an Extract of Euphorbia cotinifolia L. with Antitumour and Antioxidant Activity. Z. Nat. C 2012, 67, 151–162. [Google Scholar]
- Jin, H.J.; Lee, J.H.; Kim, K.T.; Lee, G.W.; Choi, S.J.; Chang, P.S.; Paik, H.D. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci. Biol. 2015, 24, 1555–1558. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; da Costa, D.L.M.G.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from Caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef]
- Davis, A.L.; Cai, Y.; Davies, A.P.; Lewis, J.R. 1H and 13C NMR assignments of some green tea polyphenols. Magn. Reson. Chem. 1996, 34, 887–890. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Moio, L. Evolution of Sangiovese wines with varied tannin and anthocyanin ratios during oxidative aging. Front. Chem. 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Nikolantonaki, M.; Waterhouse, A.L. A method to quantify quinone reaction rates with wine relevant nucleophiles: A key to the understanding of oxidative loss of varietal thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Vignault, A.; Pascual, O.; Jourdes, M.; Moine, V.; Fermaud, M.; Canals, J.M.; Teissedre, P.L.; Roudet, J.; Zamora, F. Impact of enological tannins on laccase activity. OENO One 2019, 53, 27–38. [Google Scholar] [CrossRef]
- Sheridan, M.K.; Elias, R.J. Exogenous acetaldehyde as a tool for modulating wine color and astringency during fermentation. Food Chem. 2015, 177, 17–22. [Google Scholar] [CrossRef]
- Gambuti, A.; Han, G.; Peterson, A.L.; Waterhouse, A.L. Sulfur dioxide and glutathione alter the outcome of microoxygenation. Am. J. Enol. Vitic. 2015, 66, 411–423. [Google Scholar] [CrossRef][Green Version]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions between anthocyanins, phenolic compounds, and acetaldehyde and their significance in red wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- Cano-López, M.; Pardo-Minguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Effect of microoxygenation on anthocyanin and derived pigment content and chromatic characteristics of red wines. Am. J. Enol. Vitic. 2006, 57, 325–331. [Google Scholar]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Fulcrand, H.; dos Santos, P.J.C.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc. Perkin Trans. 1996, 1, 735–739. [Google Scholar] [CrossRef]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Jöbstl, E.; O’Connell, J.; Fairclough, J.P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- de Freitas, V.; Carvalho, E.; Mateus, N. Study of carbohydrate influence on protein–tannin aggregation by nephelometry. Food Chem. 2003, 81, 503–509. [Google Scholar] [CrossRef]
- Carrascón, V.; Vallverdú-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition. Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef]
- Basile, B.; Caccavello, G.; Giaccone, M.; Forlani, M. Effects of early shading and defoliation on bunch compactness, yield components, and berry composition of Aglianico grapevines under warm climate conditions. Am. J. Enol. Vitic. 2015, 66, 234–243. [Google Scholar] [CrossRef]
- Bonfante, A.; Alfieri, S.M.; Albrizio, R.; Basile, A.; De Mascellis, R.; Gambuti, A.; Giorio, P.; Langella, G.; Manna, P.; Monaco, E.; et al. Evaluation of the effects of future climate change on grape quality through a physically based model application: A case study for the Aglianico grapevine in Campania region, Italy. Agric. Syst. 2017, 152, 100–109. [Google Scholar] [CrossRef]
- Gambuti, A.; Capuano, R.; Lecce, L.; Fragasso, M.G.; Moio, L. Extraction of phenolic compounds from ‘Aglianico’and ‘Uva di Troia’grape skins and seeds in model solutions: Influence of ethanol and maceration time. Vitis 2009, 48, 193–200. [Google Scholar]
- Gambuti, A.; Strollo, D.; Erbaggio, A.; Lecce, L.; Moio, L. Effect of winemaking practices on color indexes and selected bioactive phenolics of Aglianico wine. J. Food Sci. 2007, 72, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Rinaldi, A.; Pessina, R.; Moio, L. Evaluation of aglianico grape skin and seed polyphenol astringency by SDS–PAGE electrophoresis of salivary proteins after the binding reaction. Food Chem. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- Rinaldi, A.; Gambuti, A.; Moio, L. Application of the SPI (Saliva Precipitation Index) to the evaluation of red wine astringency. Food Chem. 2012, 135, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Lisanti, M.T.; Gambuti, A.; Piombino, P.; Moio, L. Relationship between sensory perception and aroma compounds of monovarietal red wines. Int. Workshop Adv. Grapevine Wine Res. 2005, 75, 549–556. [Google Scholar] [CrossRef]
- Han, G.; Wang, H.; Webb, M.R.; Waterhouse, A.L. A rapid, one step preparation for measuring selected free plus SO2-bound wine carbonyls by HPLC-DAD/MS. Talan 2015, 134, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Rinaldi, A.; Iturmendi, N.; Gambuti, A.; Jourdes, M.; Teissedre, P.L.; Moio, L. Chip electrophoresis as a novel approach to measure the polyphenols reactivity toward human saliva. Electrophoresis 2014, 35, 1735–1741. [Google Scholar] [CrossRef]

| ET-Mixture | TT-Mixture | GT-Mixture | CT-Mixture | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| m/z Formula RDB | Compd | m/z Formula RDB | Compd | m/z Formula RDB | Compd | m/z Formula RDB | Compd | m/z Formula RDB | Compd | m/z Formula RDBa | Compd |
| 169.0132 C7H5O5 RDB = 5 | Gallic acid | 289.0697 C15H13O6 RDB=9 | EC monomer | 169.0133 C7H5O5 RDB=5 | Gallic acid (G) | 289.0697 C15H13O6 RDB=9 | (E)C monomer | 865.1932 C45H37O18 RDB=27 | 3 (E)C trimer | 1451.2636 C75H55O31 RDB=48 | 4 (E)C + (E)GC pentamer (3 A-type bonds) |
| 300.9968 C14H5O8 RDB = 12 | Ellagic acid | 305.0645 C15H13O7 RDB=9 | EGC monomer | 191.0549 C7H11O6 RDB=2 | Quinic acid (Q) | 305.0644 C15H13O7 RDB=9 | (E)GC monomer | 877.1555 C45H33O19 RDB=29 | 2 (E)C + (E)GC trimer (2 A-type bonds) | 1453.2787 C75H57O31 RDB=47 | 4 (E)C + (E)GC pentamer (2 A-type bonds) |
| 481.0586 C20H17O14 RDB = 12 | Hexahydroxy diphenoyl-glucose | 441.0794 C22H17O10 RDB=14 | ECG monomer | 343.0646 C14H15O10 RDB=7 | 1 Q + 1 G | 441.0794 C22H17O10 RDB=14 | (E)CG monomer | 879.1717 C45H35O19 RDB=28 | 2 (E)C + (E)GC trimer (1 A-type bond) | 1455.2912 C75H59O31 RDB=46 | 4 (E)C + (E)GC pentamer (1 A-type bond) |
| 631.0528 C27H19O18 RDB = 18 | Castalin | 457.0742 C22H17O11 RDB=14 | EGCG monomer | 495.0742 C21H19O14 RDB=12 | 1 Q + 2 G | 457.0741 C22H17O11 RDB=14 | (E)GCG monomer | 1147.2048 C60H43O24 RDB=39 | 4 (E)C tetramer (3 A-type bonds) | 1723.3264 C90H67O36 RDB=57 | 6 (E)C hexamer (3 A-type bonds) |
| 633.0658 C27H21O18 RDB = 17 | Corilagin | 591.0946 C30H23O13 RDB=19 | EC + EGC dimer (1 A-type bond) | 647.0840 C28H23O18 RDB=17 | 1 Q + 3 G | 575.1150 C30H23O12 RDB=19 | 2 (E)C dimer (1 A-type bond) | 1149.2216 C60H45O24 RDB=38 | 4 (E)C tetramer (2 A-type bonds) | 1725.3431 C90H69O36 RDB=56 | 6 (E)C hexamer (2 A-type bonds) |
| 635.0837 C27H23O18 RDB = 16 | Tri-O-galloyl-glucose | 607.0893 C30H23O14 RDB=19 | 2 EGC dimer (1 A-type bond) | 799.0937 C35H27O22 RDB=22 | 1 Q + 4 G | 577.1306 C30H25O12 RDB=19 | 2 (E)C dimer | 1151.2376 C60H47O24 RDB=37 | 4 (E)C tetramer (1 A-type bond) | 1727.3500 C90H71O36 RDB=55 | 6 (E)C hexamer (1 A-type bond) |
| 783.0625 C34H23O22 RDB = 23 | Pedunculagin | 647.0839 C30H24KO14 RDB=18 | 2 EGC dimer | 951.1036 C42H31O26 RDB=27 | 1 Q + 5 G | 591.0946 C30H23O13 RDB=19 | (E)C + (E)GC dimer (1 A-type bond) | 1153.2532 C60H47O24 RDB=36 | 4 (E)C tetramer | 1729.3663 C90H73O36 RDB=54 | 6 (E)C hexamer |
| 785.0763 C34H25O22 RDB = 22 | Tellimagrandin I | 799.0936 C37H28KO18 RDB=23 | EGC + EGCG dimer | 1103.1134 C49H35O30 RDB=32 | 1 Q + 6 G | 607.0893 C30H23O14 RDB=19 | 2 (E)GC dimer (1 A-type bond) | 1165.2165 C60H45O25 RDB=38 | 3 (E)C + (E)GC tetramer (2 A-type bonds) | 1739.3183 C90H67O37 RDB=57 | 5 (E)C + (E)GC hexamer (3 A-type bonds) |
| 933.0565 C41H25O26 RDB = 29 | Castalagin and/or Vescalagin | 951.1034 C45H36KO21 RDB=27 | 3 EGC trimer | 1255.1230 C56H39O34 RDB=37 | 1 Q + 7 G | 647.0839 C30H24KO14 RDB=18 | 2 (E)GC dimer | 1435.2684 C75H55O30 RDB=48 | 5 (E)C pentamer (3 A-type bonds) | 1741.3300 C90H69O37 RDB=56 | 5 (E)C + (E)GC hexamer (2 A-type bonds) |
| 1065.0978 C46H33O30 RDB = 30 | Grandinin | 1103.1133 C52H40KO25 RDB=32 | 2 EGC + EGCG trimer | 861.1605 C45H33O18 RDB=29 | 3 (E)C trimer (2 A-type bonds) | 1437.2822 C75H57O30 RDB=47 | 5 (E)C pentamer (2 A-type bonds) | 1743.3071 C90H71O37 RDB=55 | 5 (E)C + (E)GC hexamer (1 A-type bond) | ||
| 863.1763 C45H35O18 RDB=28 | 3 (E)C trimer (1 A-type bond) | 1439.2955 C75H59O30 RDB=46 | 5 (E)C pentamer (1 A-type bond) | ||||||||
| Samples | Total Anthocyanins mg/L | Short Polymeric PigmentsAbs | Color IntensityAbs | Tonality | |
|---|---|---|---|---|---|
| Zero Time | Control | 1795.13 ± 17.37 A a | 0.28 ± 0.01 A b | 6.84 ± 0.05 B e | 0.51 ± 0.00 ABC c |
| ET | 1795.13 ± 17.37 A a | 0.28 ± 0.00 A c | 6.82 ± 0.00 BC e | 0.51 ± 0.00 C d | |
| TT | 1795.13 ± 17.37 A a | 0.28 ± 0.00 A d | 6.77 ± 0.01 C e | 0.51 ± 0.00 BC d | |
| GT | 1795.13 ± 17.37 A a | 0.28 ± 0.02 A d | 6.82 ± 0.04 BC e | 0.52 ± 0.01 AB c | |
| CT | 1795.13 ± 17.37 A a | 0.28 ± 0.00 A c | 6.94 ± 0.01 A e | 0.52 ± 0.00 A d | |
| Sat.-1 (9d) | Control | 1715.06 ± 74.88 BC b | 0.26 ± 0.00 B c | 7.32 ± 0.09 A d | 0.51 ± 0.00 B c |
| ET | 1681.19 ± 29.41 C b | 0.27 ± 0.00 A d | 7.39 ± 0.06 A d | 0.52 ± 0.00 A c | |
| TT | 1758.16 ± 33.91 AB a | 0.26 ± 0.00 B e | 7.35 ± 0.11 A d | 0.51 ± 0.00 B d | |
| GT | 1813.51 ± 44.49 A a | 0.27 ± 0.00 A e | 7.35 ± 0.03 A d | 0.51 ± 0.00 B c | |
| CT | 1741.04 ± 35.88 BC a | 0.27 ± 0.00 A d | 7.37 ± 0.03 A d | 0.51 ± 0.00 B e | |
| Sat.-2 (12d) | Control | 1679.00 ± 4.31 A b | 0.28 ± 0.01 C b | 7.79 ± 0.05 B c | 0.51 ± 0.00 B c |
| ET | 1621.58 ± 45.35 A b | 0.28 ± 0.00 C c | 7.92 ± 0.00 A c | 0.52 ± 0.00 A c | |
| TT | 1672.76 ± 23.72 A b | 0.30 ± 0.00 A c | 7.96 ± 0.10 A c | 0.52 ± 0.01 A c | |
| GT | 1681.54 ± 3.96 A b | 0.29 ± 0.00 B c | 8.02 ± 0.07 A c | 0.53 ± 0.00 A c | |
| CT | 1532.24 ± 107.80 B b | 0.29 ± 0.00 B b | 8.00 ± 0.09 A c | 0.52 ± 0.00 A c | |
| Sat.-3 (14d) | Control | 1517.78 ± 55.37 A c | 0.31 ± 0.00 B a | 8.70 ± 0.33 A a | 0.56 ± 0.00 A b |
| ET | 1420.47 ± 64.09 B c | 0.30 ± 0.01 B b | 8.76 ± 0.09 A a | 0.55 ± 0.00 A b | |
| TT | 1428.70 ± 40.49 B c | 0.31 ± 0.00 A b | 8.88 ± 0.04 A a | 0.55 ± 0.00 A b | |
| GT | 1446.34 ± 0.75 B c | 0.30 ± 0.00 C b | 9.00 ± 0.46 A a | 0.54 ± 0.02 A b | |
| CT | 1467.64 ± 21.04 AB b | 0.32 ± 0.00 A a | 8.75 ± 0.06 A a | 0.55 ± 0.00 A b | |
| Sat.-4(17d) | Control | 1168.75 ± 44.13 A d | 0.32 ± 0.01 B a | 8.14 ± 0.21 B b | 0.60 ± 0.01 B a |
| ET | 1121.57 ± 29.36 BC d | 0.33 ± 0.01 A a | 8.58 ± 0.07 A b | 0.60 ± 0.01 AB a | |
| TT | 1133.60 ± 3.46 ABC d | 0.34 ± 0.01 A a | 8.53 ± 0.07 A b | 0.61 ± 0.00 A a | |
| GT | 1099.69 ± 1.86 C d | 0.34 ± 0.01 A a | 8.50 ± 0.01 A b | 0.61 ± 0.00 A a | |
| CT | 1160.10 ± 36.23 AB c | 0.32 ± 0.00 B a | 8.56 ± 0.11 A b | 0.61 ± 0.00 A a |
| Samples | Total Phenols mg/L | Flavans Reactive to Vanillin mg/L | |
|---|---|---|---|
| Zero Time | Control | 2039.05 ± 33.41 A a | 1090.89 ± 47.54 A a |
| ET | 2058.54 ± 88.33 A a | 1101.11 ± 51.92 A a | |
| TT | 2034.87 ± 18.40 A b | 1112.89 ± 59.53 A a | |
| GT | 2020.95 ± 58.11 A b | 1099.53 ± 42.86 A a | |
| CT | 2069.68 ± 43.25 A a | 1094.04 ± 43.49 A a | |
| Sat.-4 | Control | 2033.48 ± 89.21 B b | 1062.61 ± 89.04 AB a |
| ET | 2121.18 ± 53.29 AB a | 1049.26 ± 38.14 BC b | |
| TT | 2151.81 ± 27.42 A a | 1136.46 ± 18.39 A a | |
| GT | 2126.75 ± 39.73 A a | 1024.12 ± 48.34 BC b | |
| CT | 2126.75 ± 67.03 A a | 984.84 ± 18.59 C b |
| Code | Description |
|---|---|
| Experimental wines | |
| Control | Wine control |
| ET | Wine with addition of ellagitannin (30 g hL−1) |
| TT | Wine with addition of tea tannins (30 g hL−1) |
| GT | Wine with addition of gallotannins (30 g hL−1) |
| CT | Wine with addition of condensed (tannin 30 g hL−1) |
| Saturations time | |
| Zero Time | Zero analysis time |
| Sat.-1 | First oxygen saturation |
| Sat.-2 | Second oxygen saturation |
| Sat.-3 | Third oxygen saturation |
| Sat.-4 | Fourth oxygen saturation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picariello, L.; Rinaldi, A.; Forino, M.; Errichiello, F.; Moio, L.; Gambuti, A. Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine. Molecules 2020, 25, 4607. https://doi.org/10.3390/molecules25204607
Picariello L, Rinaldi A, Forino M, Errichiello F, Moio L, Gambuti A. Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine. Molecules. 2020; 25(20):4607. https://doi.org/10.3390/molecules25204607
Chicago/Turabian StylePicariello, Luigi, Alessandra Rinaldi, Martino Forino, Francesco Errichiello, Luigi Moio, and Angelita Gambuti. 2020. "Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine" Molecules 25, no. 20: 4607. https://doi.org/10.3390/molecules25204607
APA StylePicariello, L., Rinaldi, A., Forino, M., Errichiello, F., Moio, L., & Gambuti, A. (2020). Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine. Molecules, 25(20), 4607. https://doi.org/10.3390/molecules25204607







