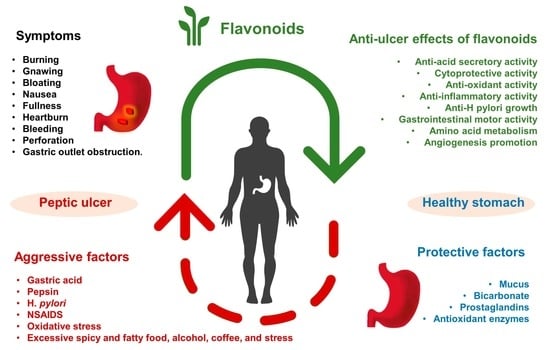
Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers
Abstract
:1. Introduction
2. Anti-Ulcer Mechanisms of Flavonoids
2.1. Flavonoids Exert Anti-Ulcer Effects by Regulating Gastric Secretion Pathways
2.2. Flavonoids Show Gastric Cytoprotective Activity by Regulating Prostaglandins Levels
2.3. Antioxidant Properties of Flavonoids in Peptic Ulcer
2.4. Flavonoids Ameliorate Peptic Ulcer by Regulating Inflammatory Pathways
2.5. Flavonoids Possess Anti-H Pylori Activities for Peptic Ulcer Healing
2.6. Other Mechanisms
3. Alternative Strategies for the Treatment of Peptic Ulcer with Flavonoids
3.1. Combination Therapy of Flavonoids and Approved Drugs
3.2. Bioavailability Improvement of Flavonoids on Peptic Ulcer
4. Safety Assessment of Flavonoids on Peptic Ulcer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lanas, A.; Chan, F.K.L. Peptic Ulcer Disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Najm, W.I. Peptic Ulcer Disease. Prim. Care Clin. Off. Pract. 2011, 38, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sridhar, S.; Hunt, R.H. Role of Helicobacter Pylori Infection and Non-Steroidal Anti-Inflammatory Drugs in Peptic-Ulcer Disease: A Meta-Analysis. Lancet 2002, 359, 14–22. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Kasim, K.F.; Ma’Radzi, A.H.; Gopinath, S.C.B. Peptic Ulcer: Current Prospects of Diagnostic and Nanobiotechnological Trends on Pathogenicity. Process Biochem. 2019, 85, 51–59. [Google Scholar] [CrossRef]
- Stewart, D.J.; Ackroyd, R. Peptic Ulcers and Their Complications. Surgery 2011, 29, 568–574. [Google Scholar]
- Milosavljevic, T.; Kostić-Milosavljević, M.; Jovanović, I.; Krstić, M. Complications of Peptic Ulcer Disease. Dig. Dis. 2011, 29, 491–493. [Google Scholar] [CrossRef]
- Imhof, M.; Epstein, S.; Ohmann, C.; Röher, H.D. Duration of Survival after Peptic Ulcer Perforation. World J. Surg. 2008, 32, 408–412. [Google Scholar] [CrossRef]
- Yuan, Y.; Padol, I.T.; Hunt, R.H. Peptic Ulcer Disease Today. Nat. Clin. Pract. Gastroenterol. Hepatology 2006, 3, 80–89. [Google Scholar]
- Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548724/ (accessed on 26 March 2018).
- Black, M. Possible Ranitidine Hepatotoxicity. Ann. Intern. Med. 1984, 101, 208. [Google Scholar] [CrossRef]
- Donovan, J.W. Hepatotoxic and Hepatoprotective Potential of Histamine (H2)-Receptor Antagonists. Am. J. Med. 1988, 85, 893. [Google Scholar] [CrossRef]
- Garcia Rodríguez, L.A.; Wallander, M.A.; Ch Stricker, B.H. The Risk of Acute Liver Injury Associated with Cimetidine and Other Acid-Suppressing Anti-Ulcer Drugs. Br. J. Clin. Pharmacol. 1997, 43, 183–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter Pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter Pylori Infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, A.; Terzo, S.; Mulè, F. Natural Compounds as Beneficial Antioxidant Agents in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, R.; Mosca, L.; Sánchez-Lamar, A.; Tempera, I.; Hausmann, R. Natural Bioactive Compounds Acting against Oxidative Stress in Chronic, Degenerative, and Infectious Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1–2. [Google Scholar] [CrossRef]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal Plants and Bioactive Natural Compounds in the Treatment of Non-Alcoholic Fatty Liver Disease: A Clinical Review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef]
- Serrano, A.; Ros, G.; Nieto, G. Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects. Medicines 2018, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Fokou, P.; Sharopov, F.; Martorell, M.; Ademiluyi, A.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer Agents: From Plant Extracts to Phytochemicals in Healing Promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [Green Version]
- De Sousa Falcão, H.; Leite, J.; Barbosa-Filho, J.; de Athayde-Filho, P.; de Oliveira Chaves, M.; Moura, M.; Ferreira, A.; De Almeida, A.; Souza-Brito, A.; De Fátima Formiga Melo Diniz, M.; et al. Gastric and Duodenal Antiulcer Activity of Alkaloids: A Review. Molecules 2008, 13, 3198–3223. [Google Scholar] [CrossRef] [Green Version]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer Properties of Fruits and Vegetables: A Mechanism Based Perspective. Food Chem. Toxicol. 2017, 108, 104–119. [Google Scholar] [CrossRef]
- Mohd, A.; Ahmad, M.A.; Sumbul, S.; Mohd, A. Role of Phenolic Compounds in Peptic Ulcer: An Overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-Derived Polysaccharides and Terpenoids: Recent Update on the Gastroprotective Effects and Mechanisms. Front. Pharmacol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-S. Recent Advances in Natural Antifungal Flavonoids and Their Derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Saleem Kalhoro, M.; Hussain Kalhoro, D.; Adebowale, T.O.; Usman Mazhar, M.; ur Rehman, Z.; et al. Flavonoids and Type 2 Diabetes: Evidence of Efficacy in Clinical and Animal Studies and Delivery Strategies to Enhance Their Therapeutic Efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research Trends in Flavonoids and Health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Gutiérrez-Venegas, G. Flavonoids Exert Multiple Periodontic Benefits Including Anti-Inflammatory, Periodontal Ligament-Supporting, and Alveolar Bone-Preserving Effects. Life Sci. 2018, 209, 435–454. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the Gastrointestinal Tract: Local and Systemic Effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Llesuy, S.F. Antioxidant Properties of Natural Compounds Used in Popular Medicine for Gastric Ulcers. Braz. J. Med. Biol. Res. 2002, 35, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols Protect the Epithelial Barrier Function of Caco-2 Cells Exposed to Indomethacin through the Modulation of Occludin and Zonula Occludens-1 Expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-Ols and Procyanidins Protect Liposomes against Lipid Oxidation and Disruption of the Bilayer Structure. Free Radic. Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- de Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [Green Version]
- Engel, E.; Guth, P.H.; Nishizaki, Y.; Kaunitz, J.D. Barrier Function of the Gastric Mucus Gel. Am. J. Physiol. Liver Physiol. 1995, 269, G994–G999. [Google Scholar] [CrossRef]
- Shamburek, R.D.; Schubert, M.L. Pharmacology of Gastric Acid Inhibition. Baillieres. Clin. Gastroenterol. 1993, 7, 23–54. [Google Scholar] [CrossRef]
- Schubert, M.L. Regulation of Gastric Acid Secretion. Curr. Opin. Gastroenterol. 1999, 15, 457. [Google Scholar] [CrossRef]
- Sato, H.; Matsui, T.; Arakawa, Y. The Protective Effect of Catechin on Gastric Mucosal Lesions in Rats, and Its Hormonal Mechanisms. J. Gastroenterol. 2002, 37, 106–111. [Google Scholar] [CrossRef]
- Rao, C.V.; Vijayakumar, M. Protective Effect of (+)-Catechin against Gastric Mucosal Injury Induced by Ischaemia-Reperfusion in Rats. J. Pharm. Pharmacol. 2007, 59, 1103–1107. [Google Scholar] [CrossRef]
- Martin, M.J.; Motilva, V.; ÓN de la Lastra, C.A. Quercetin and Naringenin; Effects on Ulcer Formation and Gastric Secretion in Rats. Phyther. Res. 1993, 7, 150–153. [Google Scholar] [CrossRef]
- Kahraman, A.; Erkasap, N.; Köken, T.; Serteser, M.; Aktepe, F.; Erkasap, S. The Antioxidative and Antihistaminic Properties of Quercetin in Ethanol-Induced Gastric Lesions. Toxicology 2003, 183, 133–142. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Ichimura, A.; Sato, S.; Fujii, T.; Oishi, S.; Sakai, H.; Takeshima, H. The Natural Flavonoid Myricetin Inhibits Gastric H+, K+-ATPase. Eur. J. Pharmacol. 2018, 820, 217–221. [Google Scholar] [CrossRef] [PubMed]
- de Barros, M.; Mota da Silva, L.; Boeing, T.; Somensi, L.B.; Cury, B.J.; de Moura Burci, L.; Santin, J.R.; de Andrade, S.F.; Monache, F.D.; Cechinel-Filho, V. Pharmacological Reports about Gastroprotective Effects of Methanolic Extract from Leaves of Solidago Chilensis (Brazilian Arnica) and Its Components Quercitrin and Afzelin in Rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 403–417. [Google Scholar] [CrossRef]
- Shigeru, M.; Makoto, M.; Hironaka, A.; Susumu, O. Inhibition of Gastric H+,K+-ATPase by the Anti-Ulcer Agent, Sofalcone. Biochem. Pharmacol. 1991, 42, 1447–1451. [Google Scholar] [CrossRef]
- Bigoniya, P.; Singh, K. Ulcer Protective Potential of Standardized Hesperidin, a Citrus Flavonoid Isolated from Citrus Sinensis. Rev. Bras. Farmacogn. 2014, 24, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Elshazly, S.M.; Abd El Motteleb, D.M.; Ibrahim, I.A.A.-H. Hesperidin Protects against Stress Induced Gastric Ulcer through Regulation of Peroxisome Proliferator Activator Receptor Gamma in Diabetic Rats. Chem. Biol. Interact. 2018, 291, 153–161. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Tordera, M. Studies on the Gastric Anti-Ulcer Activity of Hypolaetin-8-Glucoside. Phyther. Res. 1988, 2, 85–88. [Google Scholar] [CrossRef]
- Jayaraj, A.P.; Lewin, M.R.; Tovey, F.I.; Kitler, M.E.; Clark, C.G. The Protective Effect of Meciadanol (O-Methyl-3(+)-Catechin) on Experimental Ulceration. Eur. J. Pharmacol. 1988, 147, 265–271. [Google Scholar] [CrossRef]
- Allen, A.; Flemström, G. Gastroduodenal Mucus Bicarbonate Barrier: Protection against Acid and Pepsin. Am. J. Physiol. Physiol. 2005, 288, C1–C19. [Google Scholar] [CrossRef] [Green Version]
- Benvenutti, R.C.; Dalla Vecchia, C.A.; Locateli, G.; Serpa, P.Z.; Lutinski, J.A.; Rodrigues Junior, S.A.; Corralo, V.; Gutiérrez, M.V.; Vilegas, W.; Somensi, L.B.; et al. Gastroprotective Activity of Hydroalcoholic Extract of the Leaves of Urera Baccifera in Rodents. J. Ethnopharmacol. 2020, 250, 112473. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, J.; Mochizuki, M.; Chisaka, T.; Fujimura, H.; Tamai, Y. The Antiulcer Action of Sophora and the Active Constituent in Sophora. II. The Antiulcer Action of Vexibinol. Chem. Pharm. Bull. 1990, 38, 1039–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, L.M.; Pezzini, B.C.; Somensi, L.B.; Bolda Mariano, L.N.; Mariott, M.; Boeing, T.; dos Santos, A.C.; Longo, B.; Cechinel-Filho, V.; de Souza, P.; et al. Hesperidin, a Citrus Flavanone Glycoside, Accelerates the Gastric Healing Process of Acetic Acid-Induced Ulcer in Rats. Chem. Biol. Interact. 2019, 308, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hamaishi, K.; Kojima, R.; Ito, M. Anti-Ulcer Effect of Tea Catechin in Rats. Biol. Pharm. Bull. 2006, 29, 2206–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Kim, Y.-J.; Chae, H.-S.; Chin, Y.-W. In Vivo Gastroprotective Effect along with Pharmacokinetics, Tissue Distribution and Metabolism of Isoliquiritigenin in Mice. Planta Med. 2015, 81, 586–593. [Google Scholar] [CrossRef] [PubMed]
- George, M.Y.; Esmat, A.; Tadros, M.G.; El-Demerdash, E. In Vivo Cellular and Molecular Gastroprotective Mechanisms of Chrysin; Emphasis on Oxidative Stress, Inflammation and Angiogenesis. Eur. J. Pharmacol. 2018, 818, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kakegawa, H.; Ueda, H.; Mutsumoto, H.; Sudo, T.; Miki, T.; Satoh, T. Gastric Cytoprotective Anti-Ulcerogenic Actions of Hydroxychalcones in Rats. Planta Med. 1992, 58, 389–393. [Google Scholar] [CrossRef]
- Redfern, J.S.; Feldman, M. Role of Endogenous Prostaglandins in Preventing Gastrointestinal Ulceration: Induction of Ulcers by Antibodies to Prostaglandins. Gastroenterology 1989, 96, 596–605. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Brzozowska, I.; Pawlik, T. Role of Prostaglandins in Gastroprotection and Gastric Adaptation. J. Physiol. Pharmacol. 2005, 56, 33–55. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Hegab, I.I.; Abd-Ellatif, R.N.; Sadek, M.T. The Gastroprotective Effect of N -Acetylcysteine and Genistein in Indomethacin-Induced Gastric Injury in Rats. Can. J. Physiol. Pharmacol. 2018, 96, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A. Diosmin Protects against Ethanol-Induced Gastric Injury in Rats: Novel Anti-Ulcer Actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, X.; Zhi, W.; Zhang, H.; He, Z.; Wang, Y.; Liu, F.; Niu, X.; Zhang, X. The Gastroprotective Effect of Nobiletin against Ethanol-Induced Acute Gastric Lesions in Mice: Impact on Oxidative Stress and Inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 354–363. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Bao, Y.; Li, T.; Yang, G.; Chang, X.; Meng, X. Anti-Ulcer Effect and Potential Mechanism of Licoflavone by Regulating Inflammation Mediators and Amino Acid Metabolism. J. Ethnopharmacol. 2017, 199, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Salim, A. Scavenging Free Radicals to Prevent Stress-Induced Gastric Mucosal Injury. Lancet 1989, 334, 1390. [Google Scholar] [CrossRef]
- Pihan, G.; Regillo, C.; Szabo, S. Free Radicals and Lipid Peroxidation in Ethanol- or Aspirin-Induced Gastric Mucosal Injury. Dig. Dis. Sci. 1987, 32, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.A.; Crapo, J.D. Biology of Disease: Free Radicals and Tissue Injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar] [PubMed]
- Schraufstätter, I.; Hyslop, P.A.; Jackson, J.H.; Cochrane, C.G. Oxidant-Induced DNA Damage of Target Cells. J. Clin. Investig. 1988, 82, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, V.; Santos, F.; Sobreira, T.; Souza, M.; Melo, C.; Silveira, E. Investigations on the Gastroprotective and Antidiarrhoeal Properties of Ternatin, a Tetramethoxyflavone from Egletes Viscosa. Planta Med. 1997, 63, 146–149. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free Radical Scavenging Activity and Antiulcer Activity of Garcinol from Garcinia Indica Fruit Rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar] [CrossRef]
- Hu, X.T.; Ding, C.; Zhou, N.; Xu, C. Quercetin Protects Gastric Epithelial Cell from Oxidative Damage in Vitro and in Vivo. Eur. J. Pharmacol. 2015, 754, 115–124. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- MatÉs, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.; Abourehab, M.; Khaled, K.; Sarhan, H. Evaluation of Combined Famotidine with Quercetin for the Treatment of Peptic Ulcer: In Vivo Animal Study. Drug Des. Devel. Ther. 2015, 9, 2159. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.E.I.; Hashim, N.M.; Yousif, M.; Ibrahim, A.A.A.; Ismail Adam, H.A. Gastroprotective Effects of (+)-Catechin Hydrate on Ethanol-Induced Gastric Ulcer in Rats. Cienc Tec Vitivinic. 2016, 31. [Google Scholar]
- Mohod, S.M.; Kandhare, A.D.; Bodhankar, S.L. Gastroprotective Potential of Pentahydroxy Flavone Isolated from Madhuca Indica J. F. Gmel. Leaves against Acetic Acid-Induced Ulcer in Rats: The Role of Oxido-Inflammatory and Prostaglandins Markers. J. Ethnopharmacol. 2016, 182, 150–159. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Akinmoladun, A.C. Comparative Gastroprotective Effect of Post-Treatment with Low Doses of Rutin and Cimetidine in Rats. Fundam. Clin. Pharmacol. 2013, 27, 138–145. [Google Scholar]
- Kim, S.-J.; Lee, H.J.; Kim, B.-S.; Lee, D.; Lee, S.-J.; Yoo, S.-H.; Chang, H.I. Antiulcer Activity of Anthocyanins from Rubus Coreanus via Association with Regulation of the Activity of Matrix Metalloproteinase-2. J. Agric. Food Chem. 2011, 59, 11786–11793. [Google Scholar] [CrossRef]
- Karaoğlan, E.S.; Albayrak, A.; Kutlu, Z.; Bayır, Y. Gastroprotective and Antioxidant Effects of Eremurus Spectabilis Bieb. Methanol Extract and Its Isolated Component Isoorientin on Indomethacin Induced Gastric Ulcers in Rats. Acta Cir. Bras. 2018, 33, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Protective Effects of Orange (Citrus Sinensis L.) Peel Aqueous Extract and Hesperidin on Oxidative Stress and Peptic Ulcer Induced by Alcohol in Rat. Lipids Health Dis. 2017, 16, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Almeida, M.O.; Lemos, M.; Arruda, C.; Casoti, R.; Somensi, L.B.; Boeing, T.; Mariott, M.; de Cássia Melo Vilhena de Andrade Fonseca da Silva, R.; de Paoli Stein, B. Artepillin C, Drupanin, Aromadendrin-4′-O-Methyl-Ether and Kaempferide from Brazilian Green Propolis Promote Gastroprotective Action by Diversified Mode of Action. J. Ethnopharmacol. 2018, 226, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hajrezaie, M.; Salehen, N.; Karimian, H.; Zahedifard, M.; Shams, K.; Batran, R.A.; Majid, N.A.; Khalifa, S.A.M.; Ali, H.M.; El-Seedi, H.; et al. Biochanin A Gastroprotective Effects in Ethanol-Induced Gastric Mucosal Ulceration in Rats. PLoS ONE 2015, 10, e0121529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 Defines a Physiologically Important Stress Response Mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.-N. Molecular Mechanisms of Nrf2-Mediated Antioxidant Response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-T.; Wu, C.-H.; Ho, C.-Y.; Yen, G.-C. Catechin Protects against Ketoprofen-Induced Oxidative Damage of the Gastric Mucosa by up-Regulating Nrf2 in Vitro and in Vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar] [CrossRef]
- Arafa Keshk, W.; Zahran, S.M.; Katary, M.A.; Abd-Elaziz Ali, D. Modulatory Effect of Silymarin on Nuclear Factor-Erythroid-2-Related Factor 2 Regulated Redox Status, Nuclear Factor-ΚB Mediated Inflammation and Apoptosis in Experimental Gastric Ulcer. Chem. Biol. Interact. 2017, 273, 266–272. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, J.S.; Wu, W.M.; Liao, T.N.; Chu, P.; Lin, S.H.; Chuang, C.H.; Lin, Y.F. Myeloperoxidase Serves as a Marker of Oxidative Stress during Single Haemodialysis Session Using Two Different Biocompatible Dialysis Membranes. Nephrol. Dial. Transplant. 2005, 20, 1134–1139. [Google Scholar] [CrossRef] [Green Version]
- Asimakopoulos, G.; Taylor, K.M. Effects of Cardiopulmonary Bypass on Leukocyte and Endothelial Adhesion Molecules. Ann. Thorac. Surg. 1998, 66, 2135–2144. [Google Scholar] [CrossRef]
- Arnhold, J. Properties, Functions, and Secretion of Human Myeloperoxidase. Biochemistry 2004, 69, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, X.; Xuan, Y.; Ying, J.; Fei, Y.; Rong, J.; Zhang, Y.; Zhang, J.; Liu, C.; Liu, Z. Kaempferol Protects Ethanol-Induced Gastric Ulcers in Mice via pro-Inflammatory Cytokines and NO. Acta Biochim. Biophys. Sin. 2018, 50, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gou, L.; Fu, X.; Li, S.; Lan, N.; Yin, X. Protective Effect of Rutin against Acute Gastric Mucosal Lesions Induced by Ischemia-Reperfusion. Pharm. Biol. 2013, 51, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Raheem, I.T. Gastroprotective Effect of Rutin against Indomethacin-Induced Ulcers in Rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 742–750. [Google Scholar] [CrossRef]
- Lanas, A. Role of Nitric Oxide in the Gastrointestinal Tract. Arthritis Res. Ther. 2008, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Cho, C. Current Roles of Nitric Oxide in Gastrointestinal Disorders. J. Physiol. 2001, 95, 253–256. [Google Scholar] [CrossRef]
- Wallace, J.L.; Miller, M.J.S. Nitric Oxide in Mucosal Defense: A Little Goes a Long Way. Gastroenterology 2000, 119, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, B.; Yadav, S.K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Role of the COX-Independent Pathways in the Ulcer-Healing Action of Epigallocatechin Gallate. Food Funct. 2011, 2, 338. [Google Scholar] [CrossRef]
- Ryan, S.; McNicholas, W.T.; Taylor, C.T. A Critical Role for P38 Map Kinase in NF-ΚB Signaling during Intermittent Hypoxia/Reoxygenation. Biochem. Biophys. Res. Commun. 2007, 355, 728–733. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-κB Regulation and Beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Peskar, B.M.; Maricic, N.; Gretzer, B.; Schuligoi, R.; Schmassmann, A. Role of Cyclooxygenase-2 in Gastric Mucosal Defense. Life Sci. 2001, 69, 2993–3003. [Google Scholar] [CrossRef]
- Crofford, L.J.; Tan, B.; McCarthy, C.J.; Hla, T. Involvement of Nuclear FactorkB in the Regulation of Cyclooxygenase-2 Expression by Interleukin-1 in Rheumatoid Synoviocytes. Arthritis Rheum. 1997, 40, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Park, J.G.; Sung, G.H.; Yang, S.; Yang, W.S.; Kim, E.; Kim, J.H.; Ha, V.T.; Kim, H.G.; Yi, Y.S.; et al. Kaempferol, a Dietary Flavonoid, Ameliorates Acute Inflammatory and Nociceptive Symptoms in Gastritis, Pancreatitis, and Abdominal Pain. Mol. Nutr. Food Res. 2015, 59, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Hazell, S.; Lee, A. Campylobacter Pyloridis, Urease, Hydrogen Ion back Diffusion, and Gastric Ulcers. Lancet 1986, 328, 15–17. [Google Scholar] [CrossRef]
- Datta De, D. To Be or Not to Be: The Host Genetic Factor and beyond in Helicobacter Pylori Mediated Gastro-Duodenal Diseases. World J. Gastroenterol. 2015, 21, 2883. [Google Scholar] [CrossRef] [PubMed]
- Aspholm-Hurtig, M. Functional Adaptation of BabA, the H. Pylori ABO Blood Group Antigen Binding Adhesin. Science 2004, 305, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ibraghimov, A.; Pappo, J. The Immune Response against Helicobacter Pylori- a Direct Linkage to the Development of Gastroduodenal Disease. Microbes Infect. 2000, 2, 1073–1077. [Google Scholar] [CrossRef]
- Louw, J.A.; Falck, V.; van Rensburg, C.; Zak, J.; Adams, G.; Marks, I.N. Distribution of Helicobacter Pylori Colonisation and Associated Gastric Inflammatory Changes: Difference between Patients with Duodenal and Gastric Ulcers. J. Clin. Pathol. 1993, 46, 754–756. [Google Scholar] [CrossRef]
- Treiber, G.; Lambert, J.R. The Impact of Helicobacter Pylori Eradication on Peptic Ulcer Healing. Am. J. Gastroenterol. 1998, 93, 1080–1084. [Google Scholar] [CrossRef]
- Mabe, K.; Yamada, M.; Oguni, I.; Takahashi, T. In Vitro and in Vivo Activities of Tea Catechins against Helicobacter Pylori. Antimicrob. Agents Chemother. 1999, 43, 1788–1791. [Google Scholar] [CrossRef] [Green Version]
- González-Segovia, R.; Quintanar, J.L.; Salinas, E.; Ceballos-Salazar, R.; Aviles-Jiménez, F.; Torres-López, J. Effect of the Flavonoid Quercetin on Inflammation and Lipid Peroxidation Induced by Helicobacter Pylori in Gastric Mucosa of Guinea Pig. J. Gastroenterol. 2008, 43, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, J.; Kim, K.-T.; Park, Y.-S.; Nah, S.-Y.; Ahn, D.; Paik, H.-D. Antimicrobial Effect of 7-O-Butylnaringenin, a Novel Flavonoid, and Various Natural Flavonoids against Helicobacter Pylori Strains. Int. J. Environ. Res. Public Health 2013, 10, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti-Helicobacter Pylori Flavonoids from Licorice Extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef]
- Ustün, O.; Ozçelik, B.; Akyön, Y.; Abbasoglu, U.; Yesilada, E. Flavonoids with Anti-Helicobacter Pylori Activity from Cistus Laurifolius Leaves. J. Ethnopharmacol. 2006, 108, 457–461. [Google Scholar] [CrossRef]
- Bae, E.-A.; Han, M.J.; Kim, D.-H. In Vitro Anti-Helicobacter Pylori Activity of Irisolidone Isolated from the Flowers and Rhizomes of Pueraria Thunbergiana. Planta Med. 2001, 67, 161–163. [Google Scholar] [CrossRef]
- Takase, H.; Yamamoto, K.; Hirano, H.; Saito, Y.; Yamashita, A. Pharmacological Profile of Gastric Mucosal Protection by Marmin and Nobiletin from a Traditional Herbal Medicine, Aurantii Fructus Immaturus. Jpn. J. Pharmacol. 1994, 66, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Isomoto, H. Sofalcone, a Mucoprotective Agent, Increases the Cure Rate of Helicobacter Pylori Infection When Combined with Rabeprazole, Amoxicillin and Clarithromycin. World J. Gastroenterol. 2005, 11, 1629. [Google Scholar] [CrossRef] [Green Version]
- Echizen, H.; Ishizaki, T. Clinical Pharmacokinetics of Famotidine. Clin. Pharmacokinet. 1991, 21, 178–194. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Kamil, A.; Chen, C.-Y.O.; Blumberg, J.B. The Application of Nanoencapsulation to Enhance the Bioavailability and Distribution of Polyphenols. In Nanotechnology and Functional Foods; Cristina, M.S., Hongda, C., Rickey, Y.Y., Eds.; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Chakraborty, S.; Stalin, S.; Das, N.; Thakur Choudhury, S.; Ghosh, S.; Swarnakar, S. The Use of Nano-Quercetin to Arrest Mitochondrial Damage and MMP-9 Upregulation during Prevention of Gastric Inflammation Induced by Ethanol in Rat. Biomaterials 2012, 33, 2991–3001. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.-A.; EL Sabbagh, H.M. In Vitro–in Vivo Evaluation of Chitosan-PLGA Nanoparticles for Potentiated Gastric Retention and Anti-Ulcer Activity of Diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Choi, J.K.; Jung, C.H.; Koh, H.J.; Heo, P.; Shin, J.Y.; Kim, S.; Park, W.S.; Shin, H.J.; Kweon, D.H. Snare-wedging Polyphenols as Small Molecular Botox. Planta Med. 2012, 78, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J. Critical Review of the Toxicology of Coumarin with Special Reference to Interspecies Differences in Metabolism and Hepatotoxic Response and Their Significance to Man. Food Cosmet. Toxicol. 1979, 17, 277–289. [Google Scholar] [CrossRef]
- Nagao, M.; Morita, N.; Yahagi, T.; Shimizu, M.; Kuroyanagi, M.; Fukuoka, M.; Yoshihira, K.; Natori, S.; Fujino, T.; Sugimura, T. Mutagenicities of 61 Flavonoids and 11 Related Compounds. Environ. Mutagen. 1981, 3, 401–419. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernández, M.; Estela, J.M.; Asensi, M.Á.; Mañes, J.; Picó, Y. Short-Term Oral Toxicity of Quercetin and Pterostibene in Swiss Mice. Toxicol. Lett. 2006, 164, S275–S276. [Google Scholar] [CrossRef]
- Michael McClain, R.; Wolz, E.; Davidovich, A.; Pfannkuch, F.; Edwards, J.A.; Bausch, J. Acute, Subchronic and Chronic Safety Studies with Genistein in Rats. Food Chem. Toxicol. 2006, 44, 56–80. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety Evaluation of Proanthocyanidin-Rich Extract from Grape Seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Féres, C.A.O.; Madalosso, R.C.; Rocha, O.A.; Leite, J.P.V.; Guimarães, T.M.D.P.; Toledo, V.P.P.; Tagliati, C.A. Acute and Chronic Toxicological Studies of Dimorphandra Mollis in Experimental Animals. J. Ethnopharmacol. 2006, 108, 450–456. [Google Scholar] [CrossRef]






| Substance | Structure | Sources | Experimental Assay | Dose | Activity | Ref. |
|---|---|---|---|---|---|---|
| Catechins |  | Tea | Water immersion restraint (WIR) stress-induced gastric mucosal lesion model and isolated rat stomach infusion model in Wistar rats | 0.1% crude catechin-containing water (p.o.) | Active | [40] |
| Absolute ethanol-induced gastric ulcer in Sprague-Dawley strain SPF rats | 50 mg/kg (p.o.) | Inactive | [55] | |||
| 100 mg/kg (p.o.) | Active | |||||
| 200 mg/kg (p.o.) | ||||||
| Restraint plus water immersion stress in Sprague-Dawley strain SPF rats | 100 mg/kg (p.o.) | Active | ||||
| Ethanol-induced gastric ulcer in Sprague-Dawley rats | 25 mg/kg (p.o.) | Active | [78] | |||
| 50 mg/kg (p.o.) | ||||||
| Ketoprofen-induced oxidative damage in the gastrointestinal mucosa in Sprague-Dawley rats | 14 mg/kg (p.o.) | Active | [88] | |||
| 35 mg/kg (p.o.) | ||||||
| Ketoprofen-induced damage in humanInt-407cell line | 100 μM (in vitro) | Active | ||||
| Quercetin |  | Quercus iberica, Dysosma veitchii | Cold restraint-induced gastric ulcer and pylorus-ligate induced gastric ulcer in Wistar rats | 100 mg/kg (i.g.) | Active | [42] |
| Ethanol-induced gastric ulcer in Sprague-Dawley rats | 200 mg/kg (i.g.) | Active | [43] | |||
| Ethanol-induced gastric ulcer in Balb/c mice; | 25 mg/kg (p.o.) | Active | [72] | |||
| H2O2-induced damage in GES-1 cells | 6.25 μM (in vitro) | Inactive | ||||
| 12.5 μM (in vitro) | ||||||
| 25 μM (in vitro) | Active | |||||
| 50 μM (in vitro) | ||||||
| 100 μM (in vitro) | Inactive | |||||
| Ethanol-induced gastric ulcer in Sprague-Dawley rats | Not mentioned | Active | [122] | |||
| Naringenin |  | Grapefruits (Citrus paradise) | Cold-restraint induced gastric ulcer and pylorus-ligate induced gastric ulcer in Wistar rats | 100 mg/kg (i.g.) | Active | [42] |
| Myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone) |  | Berries and red wine | Enzyme assay using freeze-dried tubulovesicles prepared from hog stomach; histamine-induced gastric acid secretion in ICR mice | 50 mg/kg (i.g.) | Active | [44] |
| Quercitrin |  | Solidago chilensis (Brazilian arnica) | Ethanol/HCl-induced gastric ulcer in Swiss mice | 0.46 mg/kg (p.o.) | Inactive | [45] |
| 1.38 mg/kg (p.o.) | Active | |||||
| Afzelin (kaempferol 3-O-glucorhamnoside) |  | Solidago chilensis (Brazilian arnica) | Ethanol/HCl-induced gastric ulcer in Swiss mice | 0.026 mg/kg (p.o.) | Active | [45] |
| 0.078 mg/kg (p.o.) | ||||||
| Hesperidin |  | Citrus sinensis peel, Citrus fruits | Indomethacin-induced gastric ulcer in Wistar rats | 150 mg/kg (i.g.) | Inactive | [47] |
| 300 mg/kg (i.g.) | ||||||
| 450 mg/kg (i.g.) | Active | |||||
| Hypothermic restraint stress-induced ulcer in Wistar rats | 150 mg/kg (i.g.) | Inactive | ||||
| 300 mg/kg (i.g.) | Active | |||||
| 450 mg/kg (i.g.) | ||||||
| Stress-induced gastric ulcer in diabetic rats | 100 mg/kg (i.g.) | Active | [48] | |||
| Ethanol-induced gastric ulcer in Wistar rats | 50 mg/kg (p.o.) | Active | [83] | |||
| Hypolaetin-8-glucoside |  | Sideritis leucantha | Ethanol-induced gastric ulcer in Wistar rats | 60 mg/kg (s.c.) | Active | [49] |
| 80 mg/kg (s.c.) | ||||||
| 100 mg/kg (s.c.) | ||||||
| 100 mg/kg (p.o.) | Inactive | |||||
| 200 mg/kg (p.o.) | Active | |||||
| 300 mg/kg (p.o.) | ||||||
| Meciadanol (O-methyl-3(+)-catechin) |  | Disconfirmation | Ethanol- induced gastric ulcer in rats; South Indian ulcerogenic diet- gastric ulcer in rats; rice bran oil-induced gastric ulcer in pylorus-ligated rats | 150 mg/kg (p.o.) | Active | [50] |
| Diosmetin |  | Urera baccifera | Ethanol-induced gastric ulcer in Wistar rats | 3 mg/kg extract (p.o.) | Inactive | [52] |
| 30 mg/kg extract (p.o.) | Active | |||||
| 300 mg/kg extract (p.o). | ||||||
| Apigenin glucuronide |  | Urera baccifera | Ethanol-induced gastric ulcer in Wistar rats | 3 mg/kg extract (p.o.) | Inactive | [52] |
| 30 mg/kg extract (p.o.) | Active | |||||
| 300 mg/kg extract (p.o.) | ||||||
| Vexibinol |  | Sophara | HCl-ethanol, 0.6 N HCl 0.2 N NaOH, absolute ethanol and 1% NH3-induced gastric ulcers in Wistar rats | 100 mg/kg (p.o.) | Active | [53] |
| 300 mg/kg (p.o.) | ||||||
| Isoliquiritigenin (4,2’.4’-trihydroxychalcone) |  | Glycyrrhiza glabra | Indomethacin-induced gastric ulcer in ICR mice | 100 mg/kg (p.o.) | Active | [56] |
| HCI/ethanol-, NaOH-induced gastric ulcer in Sprague-Dawley rats | 10 mg/kg (p.o.) | Active | [58] | |||
| Chrysin |  | Honey, propolis, and various plants | Indomethacin-induced gastric ulcer in Sprague-Dawley rats | 50 mg/kg (p.o.) | Active | [57] |
| 100 mg/kg (p.o.) | Active | |||||
| 2’,4’-dihydroxychalcone |  | Disconfirmation | HCI/ethanol-, NaOH-, water-immersion stress-induced gastric ulcer in Sprague-Dawley rats | 10 mg/kg (p.o.) | Active | [58] |
| Genistein |  | Soy | Indomethacin-induced gastric ulcer in albino rats | 10 mg/kg (p.o.) | Active | [62] |
| Diosmin (diosmetin 7-O-rutinoside) |  | Citrus fruits | Ethanol-induced gastric ulcer in Wistar rats | 100 mg/kg (p.o.) | Active | [63] |
| 70% ethanol-induced gastric ulcer in Sprague-Dawley rats | Chitosan-coated PLGA nanoparticles dispersion at a dose equivalent to 100 mg/kg of diosmin (p.o.) | Active | [123] | |||
| Nobiletin (5,6,7,8,3;4”-hexamethoxy flavone) |  | Aurantii fructus immaturus citrus fruits | Ethanol-induced gastric ulcer in Kunming mice | 5 mg/kg (p.o.) | Active | [64] |
| 10 mg/kg (p.o.) | ||||||
| 20 mg/kg (p.o.) | ||||||
| Ethanol-induced gastric ulcer in Wistar rats | 10 mg/kg (p.o.) | Active | [117] | |||
| 25 mg/kg (p.o.) | ||||||
| 50 mg/kg (p.o.) | ||||||
| Aspirin-induced gastric ulcer in Wistar rats | 50 mg/kg (p.o.) | |||||
| Ternatin (4’-dihydroxy-3,7,8,3’-Tetramethoxyflavone) |  | Egletes viscosa Less | Ethanol-induced gastric ulcer in Swiss mice | 25 mg/kg (p.o.) | Active | [70] |
| 50 mg/kg (p.o.) | ||||||
| Indomethacin-induced gastric ulcer in Swiss mice | 25 mg/kg (p.o.) | Inactive | ||||
| 50 mg/kg (p.o.) | ||||||
| Stress-induced gastric ulcer in Swiss mice | 25 mg/kg (p.o.) | Inactive | ||||
| 50 mg/kg (p.o.) | ||||||
| Garcinol |  | Garcinia indica | Indomethacin-induced gastric ulcer in Wistar/Crj rats | 200 mg/kg (p.o.) | Active | [71] |
| Anthocyanins (cyanidin-3-glucoside and cyanidin-3-rutinoside: 1:1.5 (w/w)) |   | Rubus coreanus | Naproxen-induced gastric ulcer in Sprague-Dawley rats | 20 mg/kg (p.o.) | Active | [81] |
| 50 mg/kg (p.o.) | ||||||
| 80 mg/kg (p.o.) | ||||||
| Isoorientin |  | Eremurus spectabilis Bieb. | Indomethacin-induced gastric ulcer in Wistar rats | 50 mg/kg (p.o.) | Active | [82] |
| 100 mg/kg (p.o.) | ||||||
| 250 mg/kg (p.o.) | ||||||
| 500 mg/kg (p.o.) | ||||||
| Aromadendrin-4′-O-methyl-ether |  | Brazilian green propolis | Ethanol/HCl-induced ulcer in Swiss mice | 0.3 mg/kg (p.o.) | Inactive | [84] |
| 3 mg/kg (p.o.) | Active | |||||
| 10 mg/kg (p.o.) | ||||||
| 30 mg/kg (p.o.) | ||||||
| Indomethacin-induced ulcer in Swiss mice | 30 mg/kg (p.o.) | Active | ||||
| Kaempferide |  | Brazilian green propolis | Ethanol/HCl-induced ulcer in Swiss mice | 0.3 mg/kg (p.o.) | Inactive | [84] |
| 3 mg/kg (p.o.) | Active | |||||
| 10 mg/kg (p.o.) | ||||||
| 30 mg/kg (p.o.) | ||||||
| Indomethacin-induced ulcer in Swiss mice | 30 mg/kg (p.o.) | Active | ||||
| Biochanin A (5,7-Dihydrox -4’-methoxyisoflavone) |  | Soy and red clover | Ethanol-induced gastric ulcer in Sprague-Dawley rats | 25 mg/kg (p.o.) | Active | [85] |
| 50 mg/kg (p.o.) | ||||||
| Silymarin |  | Silybum marianum (milk thistle) plant | Indomethacin-induced gastric ulcer in albino rats | 50 mg/kg (p.o.) | Active | [89] |
| Kaempferol (3,5,7,4′-tetrahydroxy flavone) |  | Edible plants (e.g., tea, broccoli) and botanical products | Ethanol-induced gastric ulcer in ICR mice | 40 mg/kg (p.o.) | Active | [93] |
| 80 mg/kg (p.o.) | ||||||
| 160 mg/kg (p.o.) | ||||||
| Ethanol/HCl-induced gastric ulcer in mice | 3 mg/kg (p.o.) | Active | [104] | |||
| 30 mg/kg (p.o.) | ||||||
| Rutin (quercetin-3-O-rutinoside) |  | Ruta graveolens | Ischemia reperfusion-induced gastric ulcers in Sprague-Dawley rats | 50 mg/kg (p.o.) | Active | [94] |
| 100 mg/kg (p.o.) | ||||||
| 200 mg/kg (p.o.) | ||||||
| Indomethacin-induced gastric ulcer in Wistar albino rats | 200 mg/kg (p.o.) | Active | [95] | |||
| Marmin (7-(6;7”-dihydroxygeranyloxy) coumarin) |  | Aurantii fructus immaturus | Ethanol-induced gastric ulcer in Wistar rats | 10 mg/kg (p.o.) | Active | [117] |
| 25 mg/kg (p.o.) | ||||||
| 50 mg/kg (p.o.) | ||||||
| Aspirin-induced gastric ulcer in Wistar rats | 50 mg/kg (p.o.) | Active |
| Substance | Structure | Sources | Experimental assay | Dose | Activity | Ref. |
|---|---|---|---|---|---|---|
| Catechins |  | Tea | Ischemia reperfusion-induced gastric ulcers in Sprague-Dawley rats | 50 mg/kg (p.o.) | Active | [41] |
| Acetic acid-induced gastric ulcer in Sprague-Dawley strain SPF rats | 1 mL/100 g (p.o.) | Active | [55] | |||
| H. pylori-infected Mongolian gerbils | 0.5% Catechin diet (p.o.) | Active | [111] | |||
| 1.0% Catechin diet (p.o.) | ||||||
| 2.0% Catechin diet (p.o.) | ||||||
| Chalcone |  | Various plants | H+K+-ATPase activity | IC50 = 4.8 × 10–5M (in vitro) | Active | [46] |
| Sofalcone |  | A synthetic derivative of sophoradine | H+K+-ATPase activity | IC50 = 1.5 × 10–5M (in vitro) | Active | [46] |
| Consecutive outpatients with peptic ulcer and H. pylori infection | Sofalcone (100 mg), rabeprazole (10 mg), clarithromycin (200 mg), and amoxicillin (750 mg) (twice daily for 7 days) (p.o.) | Active | [118] | |||
| Sophoradine |  | Sophora subprostrata root | H+K+-ATPase activity | IC50= 7.4 × 10–7M (in vitro) | Active | [46] |
| Hypolaetin-8-glucoside |  | Sideritis leucantha | Acetylsalicylic acid (ASA)-induced gastric ulcers in Wistar rats | 100 mg/kg (s.c.) | Active | [49] |
| Hesperidin |  | Citrus fruits | Acetic acid-induced chronic gastric ulcer in Wistar rats | 1 mg/kg (p.o.) | Inactive | [54] |
| 3 mg/kg (p.o.) | Active | |||||
| 10 mg/kg (p.o.) | ||||||
| 2’,4’-dihydroxychalcone |  | Disconfirmation | Acetic acid-induced gastric ulcer in Sprague-Dawley rats | 10 mg/kg (p.o.) | Active | [58] |
| Garcinol |  | Garcinia indica | Stress-induced gastric ulcer in Wistar/Crj rats | 200 mg/kg (p.o.) | Active | [71] |
| Quercetin (combined with famotidine) |  | Madhuca indica J. F. Gmel. (Sapotaceae), fruits and vegetables | Indomethacin-induced gastric ulcer in albino rats | 12 mg/kg famotidine beads and 50 mg/kg quercetin (p.o.) | Active | [77] |
| Quercetin (3,5,7,3′,4′- Pentahydroxy flavone) |  | Madhuca indica J. F. Gmel. (Sapotaceae), fruits and vegetables | Acetic acid-induced gastric ulcer in Wistar rats | 2.5 mg/kg (p.o.) | Inactive | [79] |
| 5 mg/kg (p.o.) | Active | |||||
| 10 mg/kg (p.o.) | ||||||
| H. pylori-induced gastric ulcer in guinea pigs | 200 mg/kg (p.o.) | Active | [112] | |||
| Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM | Inactive | [113] | |||
| 5 mM | ||||||
| 10 mM | Inactive (active for H. pylori SS1) | |||||
| 20 mM | Active (inactive for H. pylori 51) | |||||
| Rutin (quercetin-3-O-rutinoside) |  | Buckwheat, Ruta graveolens | Ethanol-induced gastric ulcers in Wistar rats | 20 mg/kg (p.o.) | Active | [80] |
| 40 mg/kg (p.o.) | ||||||
| 80 mg/kg (p.o.) | ||||||
| Acetic acid-induced gastric ulcers in Wistar rats | 20 mg/kg (p.o.) | Active | ||||
| 40 mg/kg (p.o.) | ||||||
| 80 mg/kg (p.o.) | ||||||
| Stress-induced gastric ulcers in Wistar rats | 20 mg/kg (p.o.) | Active | ||||
| 40 mg/kg (p.o.) | ||||||
| 80 mg/kg (p.o.) | ||||||
| Epigallocatechin gallate (EGCG) |  | Tea | Indomethacin-induced gastric ulcer in Swiss albino mice | 2 mg/kg (p.o.) | Active | [99] |
| Killing assay for antibacterial activity (H. pylori 110) | Minimum inhibitory concentration (for 50% of isolates): 8 μg/mL (in vitro) | Active | [111] | |||
| Epicatechin gallate |  | Tea | Killing assay for antibacterial activity (H. pylori 55) | Minimum inhibitory concentration (for 50% of isolates): 16 μg/mL (in vitro) | Active | [111] |
| Epigallocatechin |  | Tea | Killing assay for antibacterial activity (H. pylori 55) | Minimum inhibitory concentration (for 50% of isolates): 64 μg/mL (in vitro) | Active | [111] |
| Epicatechin |  | Tea | Killing assay for antibacterial activity (H. pylori 55) | Minimum inhibitory concentration (for 50% of isolates): 256 μg/mL (in vitro) | Active | [111] |
| Theaflavin |  | Tea | Killing assay for antibacterial activity (H. pylori 55) | Minimum inhibitory concentration (for 50% of isolates): 32 μg/mL (in vitro) | Active | [111] |
| 7-O-Butylnaringenin |  | A novel flavonoid modified from naringenin | Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM (in vitro) | Inactive | [113] |
| 5 mM (in vitro) | Active | |||||
| 10 mM (in vitro) | ||||||
| 20 mM (in vitro) | ||||||
| Kaempferol (3,5,7,4′-tetrahydroxy flavone) | 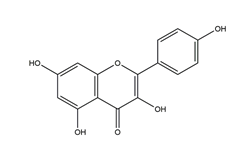 | Kaempferia galanga L | Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM (in vitro) | Inactive | [113] |
| 5 mM (in vitro) | Active (inactive for H. pylori 51) | |||||
| 10 mM (in vitro) | Active | |||||
| 20 mM (in vitro) | ||||||
| Luteolin |  | Resedaceae plants | Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM (in vitro) | Inactive | [113] |
| 5 mM (in vitro) | Active (inactive for H. pylori SS1) | |||||
| 10 mM (in vitro) | Active | |||||
| 20 mM (in vitro) | ||||||
| Naringenin |  | Grapefruits (Citrus paradise) | Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM (in vitro) | Inactive | [113] |
| 5 mM (in vitro) | Active | |||||
| 10 mM (in vitro) | ||||||
| 20 mM (in vitro) | ||||||
| Hesperetin |  | Citrus maxima peel | Antibacterial activity (H. pylori 26695, H. pylori 51, H. pylori SS1) | 2.5 mM (in vitro) | Inactive | [113] |
| 5 mM (in vitro) | Active | |||||
| 10 mM (in vitro) | ||||||
| 20 mM (in vitro) | ||||||
| Vestitol |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 12.5 μg/mL (in vitro) | Active | [114] |
| Licoricone |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 12.5 μg/mL (in vitro) | Active | [114] |
| 1-Methoxyphaseollidin |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 16 μg/mL (in vitro) | Active | [114] |
| Gancaonol C |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 16 μg/mL (in vitro) | Active | [114] |
| Glycyrin |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 50 μg/mL (in vitro) | Active | [114] |
| Formononetin |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: > 100 μg/mL (in vitro) | Active | [114] |
| Isolicoflavonol |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 25 μg/mL (in vitro) | Active | [114] |
| Glyasperin D |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 25 μg/mL (in vitro) | Active | [114] |
| 6,8-Diprenylorobol |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 50 μg/mL (in vitro) | Active | [114] |
| Gancaonin I |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 50 μg/mL (in vitro) | Active | [114] |
| Dihydrolicoisoflavone A |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 25 μg/mL (in vitro) | Active | [114] |
| Gancaonol B |  | Licorice | Anti-H. pylori activity by disk method (H. pylori: ATCC43504, ATCC43526, ZLM1007, GP98) | Minimum inhibitory concentration: 32 μg/mL (in vitro) | Active | [114] |
| Isorhamnetin (quercetin 3-methyl ether) |  | Cistus laurifolius | Anti-H. pylori activity by agar dilution method (H. pylori: NCTC11637) | Minimum inhibitory concentration: 3.9 μg/mL (in vitro) | Active | [115] |
| Quercetin 3,7-dimethyl ether |  | Cistus laurifolius | Anti-H. pylori activity by agar dilution method (H. pylori: NCTC11637) | Minimum inhibitory concentration: 62.5 μg/mL (in vitro) | Active | [115] |
| Kaempferol 3,7-dimethyl ether |  | Cistus laurifolius | Anti-H. pylori activity by agar dilution method (H. pylori: NCTC11637) | Minimum inhibitory concentration: 62.5 μg/mL (in vitro) | Active | [115] |
| Irisolidone |  | Pueraria thunbergiana (Leguminosae) | Growth inhibition assay of H. pylori (H. pylori: ATCC43504, NCTC11637, NCTC11638, 82516, 82548, 4) | Minimum inhibitory concentration: 12.5–25 μg/mL (in vitro) | Active | [116] |
| Tectorigenin |  | Pueraria thunbergiana (Leguminosae) | Growth inhibition assay of H. pylori (H. pylori: ATCC43504, NCTC11637, NCTC11638, 82516, 82548, 4) | Minimum inhibitory concentration: 100 μg/mL (in vitro) | Active | [116] |
| Genistein | 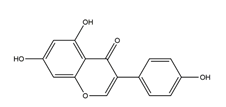 | Pueraria thunbergiana (Leguminosae) | Growth inhibition assay of H. pylori (H. pylori: ATCC43504, NCTC11637, NCTC11638, 82516, 82548, 4) | Minimum inhibitory concentration: > 100 μg/mL (in vitro) | Active | [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lian, Y.; Li, Q.; Sun, L.; Chen, R.; Lai, X.; Lai, Z.; Yuan, E.; Sun, S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules 2020, 25, 4626. https://doi.org/10.3390/molecules25204626
Zhang W, Lian Y, Li Q, Sun L, Chen R, Lai X, Lai Z, Yuan E, Sun S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules. 2020; 25(20):4626. https://doi.org/10.3390/molecules25204626
Chicago/Turabian StyleZhang, Wenji, Yingyi Lian, Qiuhua Li, Lingli Sun, Ruohong Chen, Xingfei Lai, Zhaoxiang Lai, Erdong Yuan, and Shili Sun. 2020. "Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers" Molecules 25, no. 20: 4626. https://doi.org/10.3390/molecules25204626
APA StyleZhang, W., Lian, Y., Li, Q., Sun, L., Chen, R., Lai, X., Lai, Z., Yuan, E., & Sun, S. (2020). Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules, 25(20), 4626. https://doi.org/10.3390/molecules25204626