Hydrogen Bond and Other Lewis Acid–Lewis Base Interactions as Preliminary Stages of Chemical Reactions
Abstract
:1. Introduction
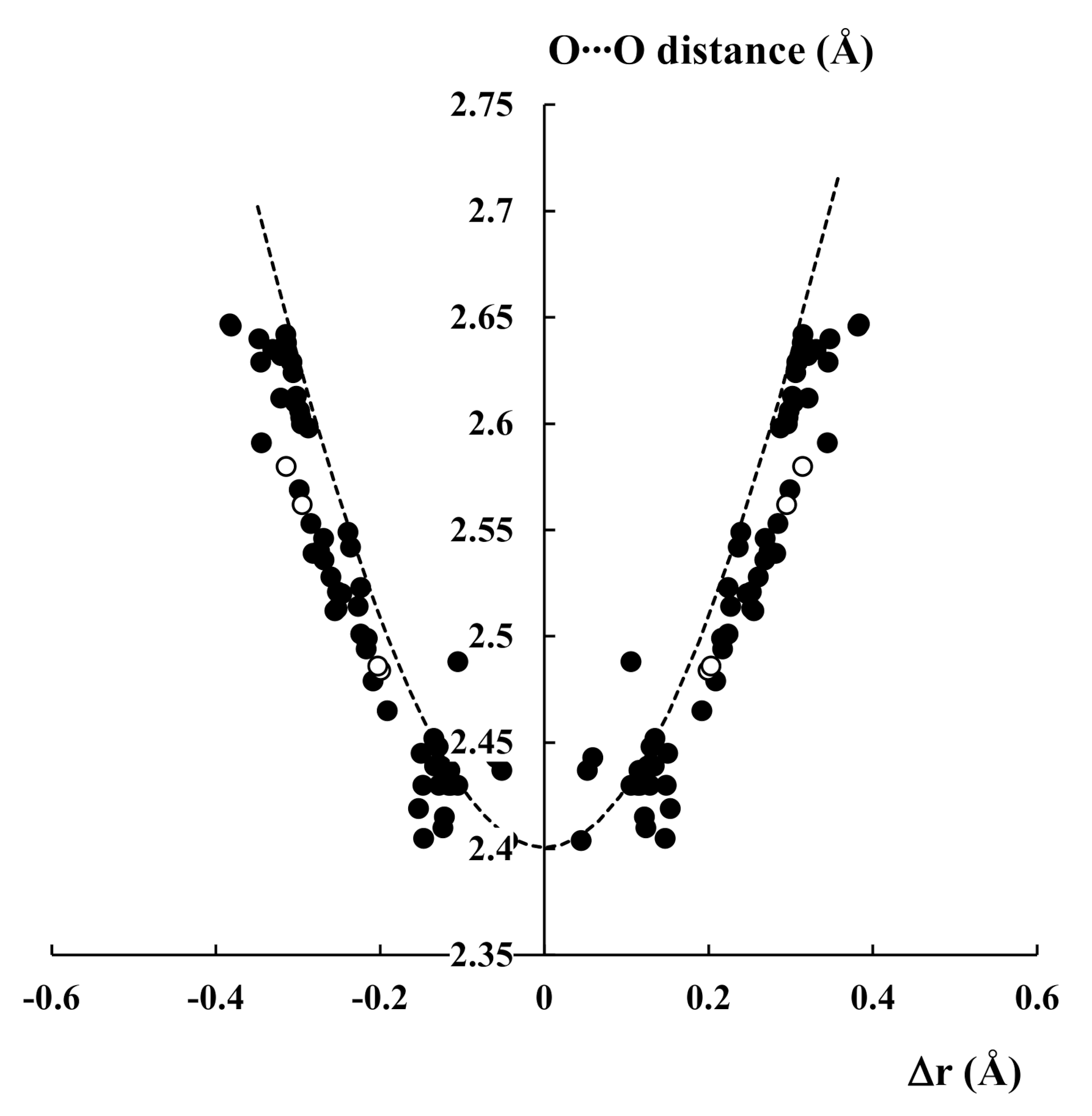
2. The Hydrogen Bond as a Preliminary Stage of the Proton Transfer Process
3. The Case of Halogen Bonds
4. The Dihydrogen Bond as a Stage of the Molecular Hydrogen Uptake
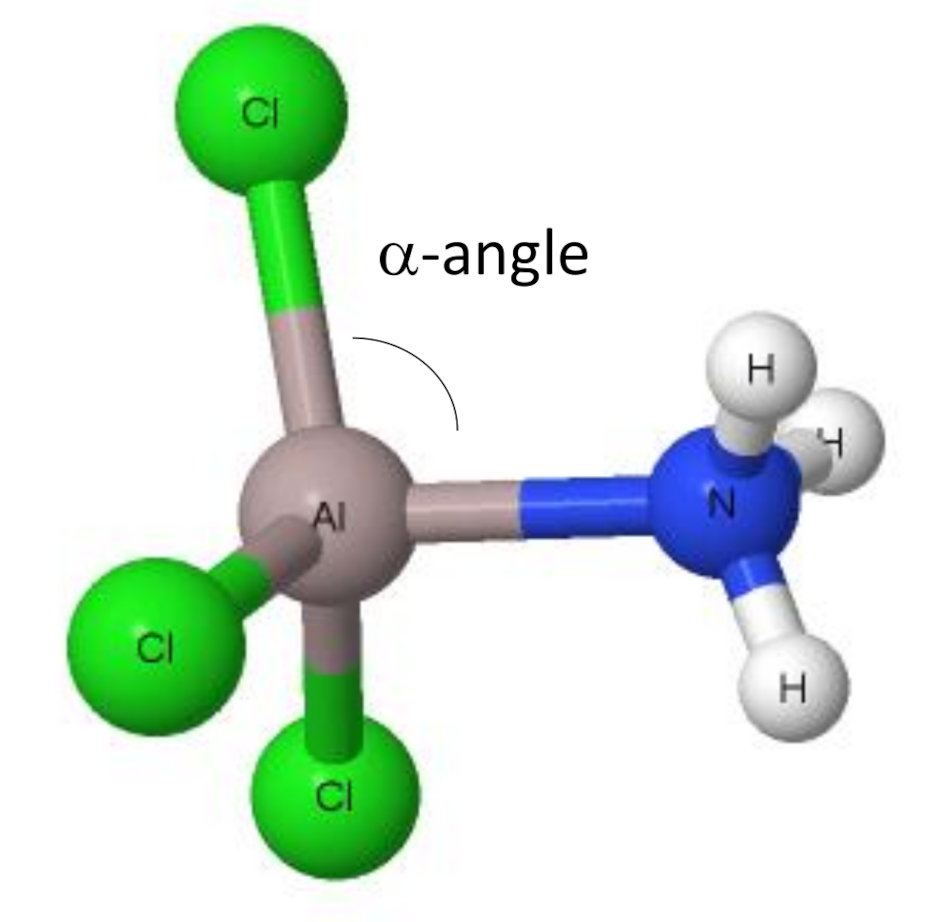
5. The Change of Trigonal Planar Triel Configuration into the Tetrahedral One—Triel Bonds
6. Tetrel Bonds and the SN2 Reaction
7. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Intermolecular Interactions in Crystals: Fundamentals of Crystal Engineering; Novoa, J.J. (Ed.) The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. Chem. Phys. Chem. 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; American Chemical Society Monograph Series; The Chemical Catalog Company, Inc.: New York, NY, USA, 1923; p. 146. [Google Scholar]
- Rauk, A. Orbital Interaction Theory of Organic Chemistry, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; p. XIII. [Google Scholar]
- Grabowski, S.J. Hydrogen Bond and Other Lewis acid–Lewis Base Interactions–Mechanisms of Formation. In Practical Aspects of Computational Chemistry IV; Leszczynski, J., Shukla, M.K., Eds.; Springer Science: New York, NY, USA, 2016; Chapter 9; pp. 245–278. [Google Scholar]
- Weinhold, F.; Landis, C. Valency and Bonding, a Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.G. Intermolecular Interactions: Physical Picture, Computational Methods and Model Potentials; John Wiley & Sons, Ltd.: Chichester, UK, 2006; p. 1. [Google Scholar]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds–mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Dunitz, J.D. Analysis of the Structure of Organic Molecules; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Bürgi, H.B. Stereochemistry and Reaction Paths as Determined from Crystal Structure Data—A Relationship between Structure and Energy. Angew. Chem. Int. Ed. 1975, 14, 460–473. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D. From Crystal Statics to chemical dynamics. Acc. Chem. Res. 1983, 16, 153–161. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D.; Shefter, E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973, 95, 5065–5067. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Krygowski, T.M. The proton transfer path for C=O…H-O systems modelled from crystal structure data. Chem. Phys. Lett. 1999, 305, 247–250. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Wong, R.; Allen, F.H.; Willett, P. The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Cryst. 2010, 43, 811–824. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.C. Single Crystal Neutron Diffraction from Molecular Materials; World Scientific Publishing Co. Pre. Ltd.: Singapore, 2000. [Google Scholar]
- Luger, P. Modern X-Ray Analysis on Single Crystals, 2nd ed.; Walter de Gruyter: Berlin, Germany, 2014. [Google Scholar]
- Brown, I.D. Bond valences—A simple structural model for inorganic chemistry. Chem. Soc. Rev. 1978, 7, 359–376. [Google Scholar] [CrossRef]
- Pauling, L. Atomic radii and interatomic distances in metals. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Covalent nature of the strong homonuclear hydrogen-bond–study of the OH…O system by crystal-structure correlation methods. J. Am. Chem. Soc. 1994, 116, 909–915. [Google Scholar] [CrossRef]
- Robertson, J.M.; Ubbelohde, A.R. Structure and thermal properties associated with some hydrogen bonds in crystals. I. The isotope effect. Proc. R. Soc. Lond. Ser. A 1939, 170, 222–240. [Google Scholar]
- Benedict, H.; Limbach, H.-H.; Wehlan, M.; Fehlhammer, W.-P.; Golubev, N.S.; Janoschek, R. Solid State 15N-NMR and Theoretical Studies on Primary and Secondary Geometric H/D Isotope Effects on Low-Barrier NHN-Hydrogen Bonds. J. Am. Chem. Soc. 1998, 120, 2939–2950. [Google Scholar] [CrossRef]
- Limbach, H.-H.; Tolstoy, P.M.; Pérez-Hernández, N.; Guo, J.; Shenderovich, I.G.; Denisov, G.S. OHO hydrogen bond geometries and NMR chemical shifts: From equilibrium structures to geometric H/D isotopic effects, with applications for water, protonated water, and compresses ice. Isr. J. Chem. 2009, 49, 199–216. [Google Scholar] [CrossRef]
- Benedict, H.; Shenderovich, I.G.; Malkina, O.L.; Malkin, V.G.; Denisov, G.S.; Golubev, N.S.; Limbach, H.-H. Nuclear Scalar Spin-Spin Couplings and Geometries of Hydrogen Bonds. J. Am. Chem. Soc. 2000, 122, 1979–1988. [Google Scholar] [CrossRef]
- Shenderovich, I.G.; Tolstoy, P.M.; Golubev, N.S.; Smirnov, S.N.; Denisov, G.S.; Limbach, H.-H. Low-Temperature NMR Studies of the Structure and Dynamics of a Novel Series of Acid−Base Complexes of HF with Collidine Exhibiting Scalar Couplings across Hydrogen Bonds. J. Am. Chem. Soc. 2003, 125, 11710–11720. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen bonds and other interactions as a response to protect doublet/octet electron structure. J. Mol. Model. 2018, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, E.S. Neutral Blue-Shifting and Blue-Shifted Hydrogen Bonds. In Hydrogen Bonding—New Insights; Grabowski, S.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Chapter 8; pp. 293–336. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules, a Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Matta, C.; Boyd, R.J. (Eds.) Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Jenkins, S.; Morrison, I. The chemical character of the intermolecular bonds of seven phases of ice as revealed by AB initio calculation of electron densities. Chem. Phys. Lett. 2000, 317, 97–102. [Google Scholar] [CrossRef]
- Arnold, W.D.; Oldfield, E. The Chemical Nature of Hydrogen Bonding in Proteins via NMR: J-Couplings, Chemical Shifts, and AIM Theory. J. Am. Chem. Soc. 2000, 122, 12835–12841. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J. Am. Chem. Soc. 2000, 122, 1154–11161. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Lipkowski, P. Characteristics of XH…π Interactions: Ab Initio and QTAIM Studies. J. Phys. Chem. A 2011, 115, 4765–4773. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on halogen bonding and other σ-hole interactions: Lex parsimoniae (Occam’s Razor). Comput. Theor. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7758. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Grabowski, S.J. Boron and other triel Lewis acid centers: From hypovalency to hypervalency. Chem. Phys. Chem. 2014, 15, 2985–2993. [Google Scholar] [CrossRef]
- Grabowski, S.J. π-hole bonds: Boron and aluminium Lewis acid centers. Chem. Phys. Chem. 2015, 16, 1470–1479. [Google Scholar] [CrossRef]
- Scheiner, S. Detailed Comparison of the Pnicogen Bond with Chalcogen, Halogen, and Hydrogen Bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen and halogen bonds are ruled by the same mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 7249–7259. [Google Scholar] [CrossRef] [PubMed]
- Turunen, L.; Erdélyi, M. Halogen Bonds and Halonium Ions. Chem. Soc. Rev. 2020, 49, 2688–2700. [Google Scholar] [CrossRef]
- Holl, M.G.; Pitts, C.R.; Lectka, T. Quest for a Symmetric [C−F−C]+ Fluoronium Ion in Solution: A Winding Path to Ultimate Success. Acc. Chem. Res. 2020, 53, 265–275. [Google Scholar] [CrossRef]
- Struble, M.D.; Scerba, M.T.; Siegler, M.; Lectka, T. Evidence for a Symmetrical Fluoronium Ion in Solution. Science 2013, 340, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Hennecke, U. Revealing the Positive Side of Fluorine. Science 2013, 340, 41–42. [Google Scholar] [CrossRef]
- Reiersølmoen, A.C.; Battaglia, S.; Øien-Ødegaard, S.; Gupta, A.K.; Fiksdahl, A.; Lindh, R.; Erdélyi, M. Symmetry of three-center, four-electron bonds. Chem. Sci. 2020, 11, 7979–7990. [Google Scholar] [CrossRef]
- Bedin, N.; Karim, A.; Reitti, M.; Carlsson, A.C.C.; Topić, F.; Cetina, M.; Pan, F.; Havel, V.; Al-Ameri, F.; Sindelar, V.; et al. Counterion influence on the N-I-N halogen bond. Chem. Sci. 2015, 6, 3746–3756. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Salinas, B.E.; Schlemper, E.O. Crystal structure of a salt of the pyridinium-pyridine ion by X-ray and neutron diffraction. J. Cryst. Mol. Struct. 1978, 8, 217–237. [Google Scholar] [CrossRef]
- Ward, J.S.; Fiorini, G.; Frontera, A.; Rissanen, K. Asymmetric [N-I-N]+ halonium complexes. Chem. Commun. 2020, 56, 8428–8431. [Google Scholar] [CrossRef]
- Zundel, G. Series of Ten Lectures On: Proton Polarizability of Hydrogen Bonds and Proton Transfer Processes, Their Role in Electrochemistry and Biology; Institut für Physikalische Chemie der Universität München: München, Germany, 1997. [Google Scholar]
- Kong, S.; Borissova, A.O.; Lesnichin, S.B.; Hartl, M.; Daemen, L.L.; Eckert, J.; Antipin, M.Y.; Shenderovich, I.G. Geometry and Spectral Properties of the Protonated Homodimer of Pyridine in the Liquid and Solid States. A Combined NMR, X-ray Diffraction and Inelastic Neutron Scattering Study. J. Phys. Chem. A 2011, 115, 8041–8048. [Google Scholar] [CrossRef]
- Gurinov, A.A.; Lesnichin, S.B.; Limbach, H.-H.; Shenderovich, I.G. How Short is the Strongest Hydrogen Bond in the Proton-Bound Homodimers of Pyridine Derivatives? J. Phys. Chem. A 2014, 118, 10804–10812. [Google Scholar] [CrossRef] [PubMed]
- Wenthold, P.G.; Squires, R.R. Bond Dissociation Energies of F2− and HF2−. A Gas-Phase Experimental and G2 Theoretical Study. J. Phys. Chem. 1995, 99, 2002–2005. [Google Scholar] [CrossRef]
- Grabowski, S.J. [FHF]−-The Strongest Hydrogen Bond under the Influence of External Interactions. Crystals 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, S.J.; Ugalde, J.M. High-level ab initio calculations on low barrier hydrogen bonds and proton bound homodimers. Chem. Phys. Lett. 2010, 493, 37–44. [Google Scholar] [CrossRef]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-Bond and Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef]
- Richardson, T.B.; de Gala, S.; Crabtree, R.H. Unconventional Hydrogen Bonds: Intermolecular B-H…H-N Interactions. J. Am. Chem. Soc. 1995, 117, 12875–12876. [Google Scholar] [CrossRef]
- Epstein, L.M.; Shubina, E.S. New types of hydrogen bonding in organometallic chemistry. Coord. Chem. Rev. 2002, 231, 165–181. [Google Scholar] [CrossRef]
- Bakhmutov, V.I. Dihydrogen Bonds, Principles, Experiments, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kubas, G.J. Metal Dihydrogen and σ-Bond Complexes–Structure, Theory, and Reactivity; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Rozas, I.; Alkorta, I.; Elguero, J. Field effects on dihydrogen bonded systems. Chem. Phys. Lett. 1997, 275, 423–428. [Google Scholar] [CrossRef]
- Custelcean, R.; Jackson, J.E. Topochemical Control of Covalent Bond Formation by Dihydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 12935–12941. [Google Scholar] [CrossRef]
- Custelcean, R.; Jackson, J.E. Topochemical Dihydrogen to Covalent Bonding Transformation in LiBH4·TEA: A Mechanistic Study. J. Am. Chem. Soc. 2000, 122, 5251–5257. [Google Scholar] [CrossRef]
- Marincean, S.; Jackson, J.E. Quest for IR-pumped reactions in dihydrogen-bonded complexes. J. Phys. Chem. A 2004, 108, 5521–5526. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Ruipérez, F. Dihydrogen bond interactions as a result of H2 cleavage at Cu, Ag and Au centres. Phys. Chem. Chem. Phys. 2016, 18, 12810–12818. [Google Scholar] [CrossRef]
- Grabowski, S.J. The Nature of Triel Bonds, a Case of B and Al Centres Bonded with Electron Rich Sites. Molecules 2020, 25, 2703. [Google Scholar] [CrossRef]
- Brinck, T.; Murray, J.S.; Politzer, P. A computational analysis of the bonding in boron trifluoride and boron trichloride and their complexes with ammonia. Inorg. Chem. 1993, 32, 2622–2625. [Google Scholar] [CrossRef]
- Bessac, F.; Frenking, G. Why Is BCl3 a Stronger Lewis Acid with Respect to String Bases than BF3? Inorg. Chem. 2003, 42, 7990–7994. [Google Scholar] [CrossRef]
- Jonas, V.; Frenking, G.; Reetz, M.T. Comparative Theoretical Study of Lewis Acid-Base Complexes of BH3, BF3, BCl3, AlCl3, and SO2. J. Am. Chem. Soc. 1994, 116, 8741–8753. [Google Scholar] [CrossRef]
- Van der Veken, B.J.; Sluyts, E.J. Reversed Lewis Acidity of Mixed Boron Halides: An Infrared Study of the Van der Waals Complexes of BFxCly with CH3F in Cryosolution. J. Am. Chem. Soc. 1997, 119, 11516–11522. [Google Scholar] [CrossRef]
- Phillips, J.A. Structural and energetic properties of nitrile–BX3 complexes: Substituent effects and their impact on condensed-phase sensitivity. Theor. Chem. Acc. 2017, 136, 16. [Google Scholar] [CrossRef]
- Giesen, D.J.; Phillips, J.A. Structure, Bonding, and Vibrational Frequencies of CH3CN-BF3: New Insight into Medium Effects and the Discrepancy between the Experimental and Theoretical Geometries. J. Phys. Chem. A 2003, 107, 4009–4018. [Google Scholar] [CrossRef]
- Phillips, J.A.; Cramer, C.J. B-N Distance Potential of CH3CN-BF3 Revisited: Resolving the Experiment-Theory Structure Discrepancy and Modeling the Effects of Low-Dielectric Environments. J. Phys. Chem. B 2007, 111, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Wrass, J.P.; Sadowsky, D.; Bloomgren, K.M.; Cramer, C.J.; Phillips, J.A. Quantum chemical and matrix-IR characterization of CH3CN-BCl3: A complex with two distinct minima along the B-N bond potential. Phys. Chem. Chem. Phys. 2014, 16, 16480–16491. [Google Scholar] [CrossRef] [PubMed]
- Helminiak, H.M.; Knauf, R.R.; Danforth, S.J.; Phillips, J.A. Structural and Energetic Properties of Acetonitrile-Group IV (A & B) Halide Complexes. J. Phys. Chem. A 2014, 118, 4266–4277. [Google Scholar]
- Grabowski, S.J. Two Faces of Triel Bonds in Boron Trihalide Complexes. J. Comput. Chem. 2018, 39, 472–480. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel bond and coordination of triel centres–Comparison with hydrogen bond interaction. Coord. Chem. Rev. 2020, 407, 213171. [Google Scholar] [CrossRef]
- Fau, S.; Frenking, G. Theoretical investigation of the weakly bonded donor—Acceptor complexes X3B—H2, X3B—C2H4, and X3B—C2H2 (X= H, F, Cl). Mol. Phys. 1999, 96, 519–527. [Google Scholar]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct. Chem. 2017, 28, 1163–1171. [Google Scholar] [CrossRef]
- Grabowski, S.J. Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions. Molecules 2020, 25, 3294. [Google Scholar] [CrossRef]
- Grabowski, S.J. Bifurcated Triel Bonds—Hydrides and Halides of 1,2-Bis(Dichloroboryl)Benzene and 1,8-Bis(Dichloroboryl)Naphthalene. Crystals 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonds with BF4− anion as a proton acceptor. Crystals 2020, 10, 460. [Google Scholar] [CrossRef]
- Grotewold, J.; Lisi, E.A.; Villa, A.E. Reactions of Co-ordinated Boron Compounds in the Gas Phase. Part I. Borine Carbonyl and Trimethylamine. J. Chem. Soc. A 1966, 1034–1037. [Google Scholar] [CrossRef]
- Grotewold, J.; Lisi, E.A.; Villa, A.E. Reactions of Co-ordinated Boron Compounds in the Gas Phase. Part II. Triethylamine as Scavanger of Borine. J. Chem. Soc. A 1966, 1038–1041. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Fleurat-Lessard, P.; Hristov, I.; Ziegler, T. Free Energy Profiles for the Identity SN2 Reactions Cl− + CH3Cl and NH3 + H3BNH3: A Constraint Ab Initio Molecular Dynamics Study. J. Phys. Chem. A 2004, 108, 9461–9468. [Google Scholar] [CrossRef]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-hole Strengths and Interactions of F3MX Molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef]
- Grabowski, S.J. Tetrel bond-σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions. Molecules 2018, 23, 1416. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, S.J. σ-Hole Bond versus Hydrogen Bond: From Tetravalent to Pentavalent, N., P and As Atoms. Chem. Eur. J. 2013, 19, 14600–14611. [Google Scholar] [CrossRef] [PubMed]
- Scherer, W.; McGrady, G.S. Agostic Interactions in d0 Metal Alkyl Complexes. Angew. Chem. Int. Ed. 2004, 43, 1782–1806. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S. A review of the π-complex theory. Bull. Soc. Chim. Fr. 1951, 18, C71–C79. [Google Scholar]
- Chatt, J.; Duncanson, L.A. Olefin Co-ordination Compounds. Part III. Infra-red Spectra and Structure: Attempted Preparation of Acetylene Complexes. J. Chem. Soc. 1953, 2939–2947. [Google Scholar] [CrossRef]
- Sundquist, W.I.; Bancroft, D.P.; Lippard, S.J. Synthesis, characterization, and biological activity of cis-diammineplatinum(II) complexes of the DNA intercalators 9-aminoacridine and chloroquine. J. Am. Chem. Soc. 1990, 112, 1590–1596. [Google Scholar] [CrossRef]
- Scherer, W.; Dunbar, A.C.; Barquera-Lozada, J.E.; Schmitz, D.; Eickerling, G.; Kratzert, D.; Stalke, D.; Lanza, A.; Macchi, P.; Casati, N.P.M.; et al. Anagostic Interactions under Pressure: Attractive or Repulsive? Angew. Chem. Int. Ed. 2015, 54, 2505–2509. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Neese, F.; Bistoni, G. Formation of Agostic Structures Driven by London Dispersion. Angew. Chem. Int. Ed. 2018, 57, 4760–4764. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Babashkina, M.G.; Robeyns, K.; Sagan, F.; Szczepanik, D.W.; Seredina, Y.V.; Garcia, Y.; Safin, D.A. Chameleon-like Nature of Anagostic Interactions and Its Impact on Metalloaromaticity in Square-Planar Nickel Complexes. Organometallics 2019, 38, 1973–1981. [Google Scholar] [CrossRef]








© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, S.J. Hydrogen Bond and Other Lewis Acid–Lewis Base Interactions as Preliminary Stages of Chemical Reactions. Molecules 2020, 25, 4668. https://doi.org/10.3390/molecules25204668
Grabowski SJ. Hydrogen Bond and Other Lewis Acid–Lewis Base Interactions as Preliminary Stages of Chemical Reactions. Molecules. 2020; 25(20):4668. https://doi.org/10.3390/molecules25204668
Chicago/Turabian StyleGrabowski, Sławomir J. 2020. "Hydrogen Bond and Other Lewis Acid–Lewis Base Interactions as Preliminary Stages of Chemical Reactions" Molecules 25, no. 20: 4668. https://doi.org/10.3390/molecules25204668
APA StyleGrabowski, S. J. (2020). Hydrogen Bond and Other Lewis Acid–Lewis Base Interactions as Preliminary Stages of Chemical Reactions. Molecules, 25(20), 4668. https://doi.org/10.3390/molecules25204668





