Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies
Abstract
1. Introduction
2. Results
2.1. Effect of Strain, Wood Substrate and Cold Treatment on Successful Fruiting
2.2. Effect of Strain, Wood Substrate and Cold Treatment on Yield
2.3. Effect of Strain and Wood Substrate on Glucan Contents
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. Spawn Production
4.3. Substrate Preparation
4.4. Cold Treatment and Fruiting Body Yield
4.5. β-Glucan Content Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, K.; Villa, A.; Kuittinen, S.; Jänis, J.; Pappinen, A. An assessment of side-stream generation from Finnish forest industry. J. Mater. Cycles Waste Manag. 2018, 21, 265–280. [Google Scholar] [CrossRef]
- Verkasalo, E.; Leppälä, J.; Muhonen, T.; Korpinen, R.; Möttönen, V.; Kurppa, S. Novel industrial ecosystems and value chains to utilize side-streams of wood products industries–Finnish approach. Pro Ligno 2019, 15, 157–165. [Google Scholar]
- Korhonen, K.T.; Ihalainen, A.; Ahola, A.; Heikkinen, J.; Henttonen, H.M.; Hotanen, J.P.; Nevalainen, S.; Pitkänen, J.; Strandström, M.; Viiri, H. Suomen Metsät 2009–2013 Ja Niiden Kehitys 1921–2013; Luonnonvarakeskus: Helsinki, Finland, 2017; p. 88. ISBN 978-952-326-466-3. (In Finnish) [Google Scholar]
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Peksen, A.; Yakupoglu, G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World J. Microbiol. Biotechnol. 2008, 25, 611–618. [Google Scholar] [CrossRef]
- Gurung, O.; Budathoki, U.; Parajuli, G. Effect of Different Substrates on the Production of Ganoderma lucidum (Curt.:Fr.) Karst. Our Nat. 2013, 10, 191–198. [Google Scholar] [CrossRef]
- Hsieh, C.; Yang, F.C. Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresour. Technol. 2004, 91, 105–109. [Google Scholar] [CrossRef]
- Philippoussis, A.; Zervakis, G.I.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microbiol. Biotechnol. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Reverberi, M.; Di Mario, F.; Tomati, U. β-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [Google Scholar] [CrossRef]
- Croan, S.C. Conversion of conifer wastes into edible and medicinal mushrooms. For. Prod. J. 2004, 54, 68–76. [Google Scholar]
- Boh, B.; Berovič, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Li, S.; Dong, C.; Wen, H.; Liu, X. Development of Ling-zhi industry in China—Emanated from the artificial cultivation in the Institute of Microbiology, Chinese Academy of Sciences (IMCAS). Mycology 2016, 7, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Erkel, E.I. The effect of different substrate mediums on yield of Ganoderma lucidum (Fr.) Karst. J. Food Agric. Environ. 2009, 7, 841–844. [Google Scholar]
- Roy, S.; Jahan, M.A.A.; Das, K.K.; Munshi, S.K.; Noor, R. Artificial Cultivation of Ganoderma lucidum (Reishi Medicinal Mushroom) Using Different Sawdusts as Substrates. Am. J. Biosci. 2015, 3, 178. [Google Scholar] [CrossRef]
- Kuhar, F.; Postemsky, P.D.; Bianchinotti, M.V. Conditions Affecting Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Agaricomycetes) Basidiome Quality, Morphogenesis, and Biodegradation of Wood By-products in Argentina. Int. J. Med. Mushrooms 2018, 20, 495–506. [Google Scholar] [CrossRef]
- Azizi, M.; Tavana, M.; Farsi, M.; Oroojalian, F. Yield performance of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.) P. Karst. (higher Basidiomycetes), using different waste materials as substrates. Int. J. Med. Mushrooms 2012, 14, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral Agents From Fungi: Diversity, Mechanisms and Potential Applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, H.; Li, W.; Xie, M.-Y. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydrates Diet. Fibre 2013, 1, 10–20. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Papaspyridi, L.-M.; Zerva, A.; Topakas, E. Biocatalytic Synthesis of Fungal β-Glucans. Catalysts 2018, 8, 274. [Google Scholar] [CrossRef]
- Yang, F.-C.; Ke, Y.-F.; Kuo, S.-S. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzym. Microb. Technol. 2000, 27, 295–301. [Google Scholar] [CrossRef]
- Babitskaya, V.G.; Shcherba, V.V.; Puchkova, T.A.; Smirnov, D.A. Polysaccharides of Ganoderma lucidum: Factors affecting their production. Appl. Biochem. Microbiol. 2005, 41, 169–173. [Google Scholar] [CrossRef]
- Hsieh, C.; Tseng, M.-H.; Liu, C.-J. Production of polysaccharides from Ganoderma lucidum (CCRC 36041) under limitations of nutrients. Enzym. Microb. Technol. 2006, 38, 109–117. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Szypowski, J.; Łoś, R.; Siwulski, M.; Sobieralski, K.; Głowniak, K.; Malm, A. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc. Bot. Pol. 2012, 81, 17–21. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Wu, F.; Jia, X.; Yin, L.; Cheng, Y.; Miao, Y.; Zhang, X. The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation. Mology 2019, 24, 1834. [Google Scholar] [CrossRef]
- Wang, D.-M.; Wu, S.-H.; Su, C.-H.; Peng, J.-T.; Shih, Y.-H.; Chen, L.-C. Ganoderma multipileum, the correct name for ‘G. lucidum’ in tropical Asia. Bot. Stud. 2009, 50, 451–458. [Google Scholar]
- Cao, Y.; Wu, S.-H.; Dai, Y.-C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Pei, H.; Chen, Z.; Tan, X.; Hu, J.; Yang, B.; Sun, J. Intraspecific Variation and Phylogenetic Relationships Are Revealed by ITS1 Secondary Structure Analysis and Single-Nucleotide Polymorphism in Ganoderma lucidum. PLoS ONE 2017, 12, e0169042. [Google Scholar] [CrossRef]
- Karsten, P.A. Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Rev. Mycol. 1881, 3, 16–19. [Google Scholar]
- Curtis, W. Flora Londinensis, or, Plates and Descriptions of Such Plants as Grow Wild in the Environs of London; Printed by the author: London, UK, 1781; p. 530. [Google Scholar]
- Moncalvo, J.-M.; Wang, H.-F.; Hseu, R.-S. Gene phylogeny of the Ganoderma lucidum complex based on ribosomal DNA sequences. Comparison with traditional taxonomic characters. Mycol. Res. 1995, 99, 1489–1499. [Google Scholar] [CrossRef]
- Zhou, L.-W.; Cao, Y.; Wu, S.-H.; Vlasak, J.; Li, D.-W.; Li, M.-J.; Dai, Y.-C. Global diversity of the Ganoderma lucidum complex (Ganodermataceae, Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry 2015, 114, 7–15. [Google Scholar] [CrossRef]
- Niemelä, T.; Kotiranta, H. Polypore survey of Finland 4. Phaeolus, Fistulina, Ganoderma and Ischnoderma. Karstenia 1986, 26, 57–64. [Google Scholar] [CrossRef]
- Niemelä, T.; Kinnunen, J. Käävät, Puiden Sienet; Norrlinia; no. 13; Luonnontieteellinen Keskusmuseon Kasvimuseo: Helsinki, Finland, 2005; p. 319. (In Finnish) [Google Scholar]
- Cortina-Escribano, M.; Veteli, P.; Riikka, L.; Miina, J.; Vanhanen, H. Effect of wood residues on the growth of Ganoderma lucidum. Karstenia 2020, 58, 16–28. [Google Scholar] [CrossRef]
- Ibrahim, R.; Aziz, K.M.; Arshad, A.M.; Hasan, S.M. Enhancing mushroom production using physical treatments prior to fruiting body formation. Malays Appl. Biol. 2015, 44, 68–73. [Google Scholar]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Cho, J.-H.; Lee, J.-Y.; Lee, M.-J.; Oh, H.-N.; Kang, D.-H.; Jhune, C.-S. Comparative analysis of useful β-glucan and polyphenol in the fruiting bodies of Ganoderma spp. J. Mushrooms 2013, 11, 164–170. [Google Scholar] [CrossRef][Green Version]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J.; Goto, M. β-Glucan recovery from Ganoderma lucidum by means of pressurized hot water and supercritical CO2. Food Bioprod. Process. 2016, 98, 21–28. [Google Scholar] [CrossRef]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of Glucan Contents in the Fruiting Bodies and Mycelia of Lentinula edodes Cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef]
- Shalaby, S.; A Horwitz, B. Plant phenolic compounds and oxidative stress: Integrated signals in fungal–plant interactions. Curr. Genet. 2014, 61, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Witzell, J.; Martín, J.A. Phenolic metabolites in the resistance of northern forest trees to pathogens—past experiences and future prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Gao, Y.; Breuil, C.; Hiratsuka, Y. Biological degradation of resin acids in wood chips by wood-inhabiting fungi. Appl. Environ. Microbiol. 1995, 61, 222–225. [Google Scholar] [CrossRef]
- D07 Committee Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials. ASTM Int. 2016. [CrossRef]


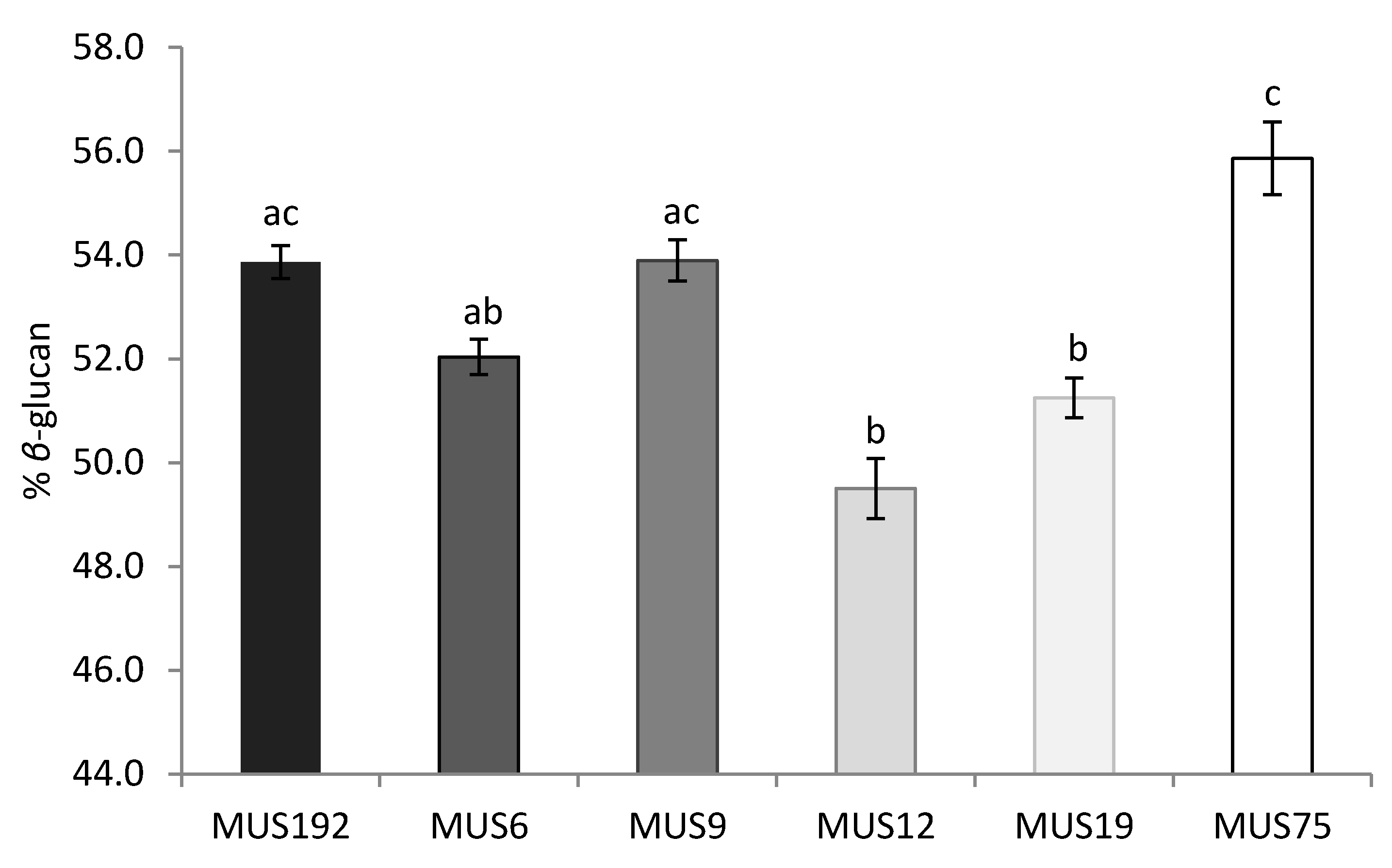
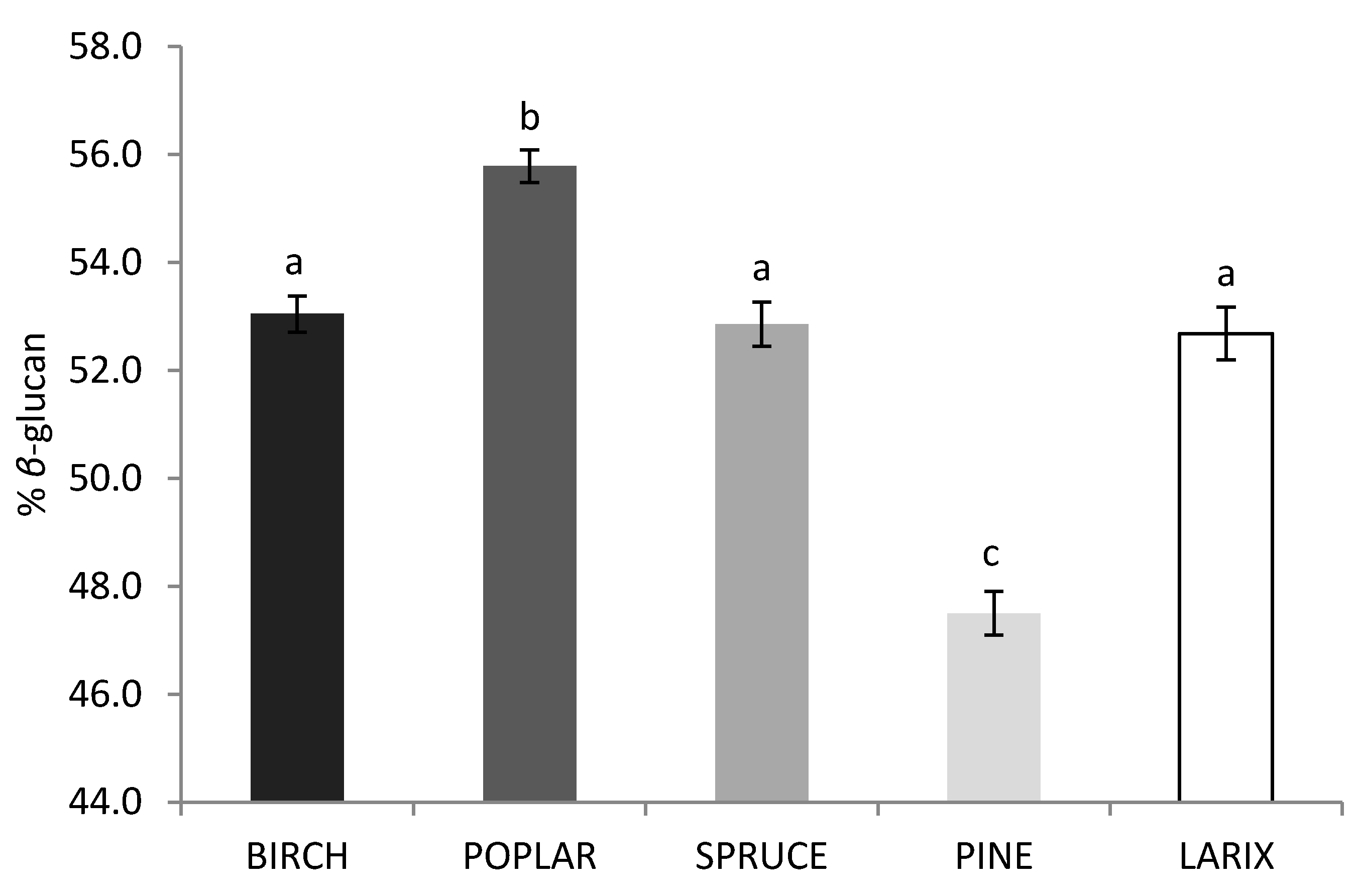
| Variables | Estimate | SE | Wald χ2 | p-Value |
|---|---|---|---|---|
| Constant | −1.30 | 0.57 | 5.20 | 0.023 |
| Strain (df = 5, ref. MU19) | 41.82 | <0.001 | ||
| MUS192 | −1.81 | 0.65 | 7.74 | 0.005 |
| MUS6 | −2.15 | 0.66 | 10.54 | 0.001 |
| MUS75 | −4.82 | 0.82 | 34.52 | <0.001 |
| MUS9 | −2.80 | 0.68 | 16.86 | <0.001 |
| MUS12 | −3.65 | 0.72 | 25.89 | <0.001 |
| Wood (df = 4, ref. P. abies) | 13.97 | 0.007 | ||
| Betula spp. | 0.30 | 0.55 | 0.30 | 0.583 |
| Larix sp. | −0.81 | 0.58 | 1.98 | 0.160 |
| P. sylvestris | −0.15 | 0.56 | 0.08 | 0.781 |
| P. tremula | 1.33 | 0.56 | 5.61 | 0.018 |
| Treatment +5 °C (df = 1, ref. −20 °C) | 4.14 | 0.54 | 58.78 | <0.001 |
| Source | Numerator df | Denominator df | F | p-Value |
|---|---|---|---|---|
| Intercept | 1 | 69 | 308.99 | <0.001 |
| Strain | 5 | 69 | 3.40 | 0.008 |
| Wood | 4 | 69 | 9.71 | <0.001 |
| Treatment | 1 | 69 | 0.48 | 0.492 |
| Strain * Wood | 14 | 69 | 1.12 | 0.361 |
| Wood * Treatment | 1 | 69 | 0.15 | 0.697 |
| Source | Num. Df | Den. Df | Total Glucan | α-Glucan | β-Glucan | |||
|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |||
| Intercept | 1 | 61 | 23,708.09 | <0.001 | 204.55 | <0.001 | 19,374.09 | <0.001 |
| Strain | 5 | 61 | 3.63 | 0.006 | 1.11 | 0.364 | 3.31 | 0.010 |
| Wood | 4 | 61 | 17.28 | <0.001 | 1.37 | 0.256 | 15.60 | <0.001 |
| Strain * Wood | 14 | 61 | 1.15 | 0.335 | 1.14 | 0.348 | 1.09 | 0.385 |
| Strain | Region | Host Species | Isolation Date | ITS GenBank ID |
|---|---|---|---|---|
| MUS192 | Uusimaa | P. abies | 07/2016 | MT334582 |
| MUS6 | Uusimaa | P. abies | 07/2016 | MT334583 |
| MUS75 | Uusimaa | P. abies | 07/2016 | MT334584 |
| MUS9 | Uusimaa | P. abies | 07/2016 | MT334585 |
| MUS12 | Satakunta | P. abies | 10/2016 | MT334586 |
| MUS19 | Uusimaa | B. pubescens | 03/2017 | MT334587 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortina-Escribano, M.; Pihlava, J.-M.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules 2020, 25, 4732. https://doi.org/10.3390/molecules25204732
Cortina-Escribano M, Pihlava J-M, Miina J, Veteli P, Linnakoski R, Vanhanen H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules. 2020; 25(20):4732. https://doi.org/10.3390/molecules25204732
Chicago/Turabian StyleCortina-Escribano, Marta, Juha-Matti Pihlava, Jari Miina, Pyry Veteli, Riikka Linnakoski, and Henri Vanhanen. 2020. "Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies" Molecules 25, no. 20: 4732. https://doi.org/10.3390/molecules25204732
APA StyleCortina-Escribano, M., Pihlava, J.-M., Miina, J., Veteli, P., Linnakoski, R., & Vanhanen, H. (2020). Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules, 25(20), 4732. https://doi.org/10.3390/molecules25204732







