Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma
Abstract
:1. Introduction
1.1. Putative Targets in Glioblastoma
1.1.1. Genetic Alterations
1.1.2. Autophagy
1.1.3. Immunomodulation
1.1.4. Dysregulated Metabolism
2. Chemopreventive Activities of Flavonoids
2.1. Bioavailability of Flavonoids
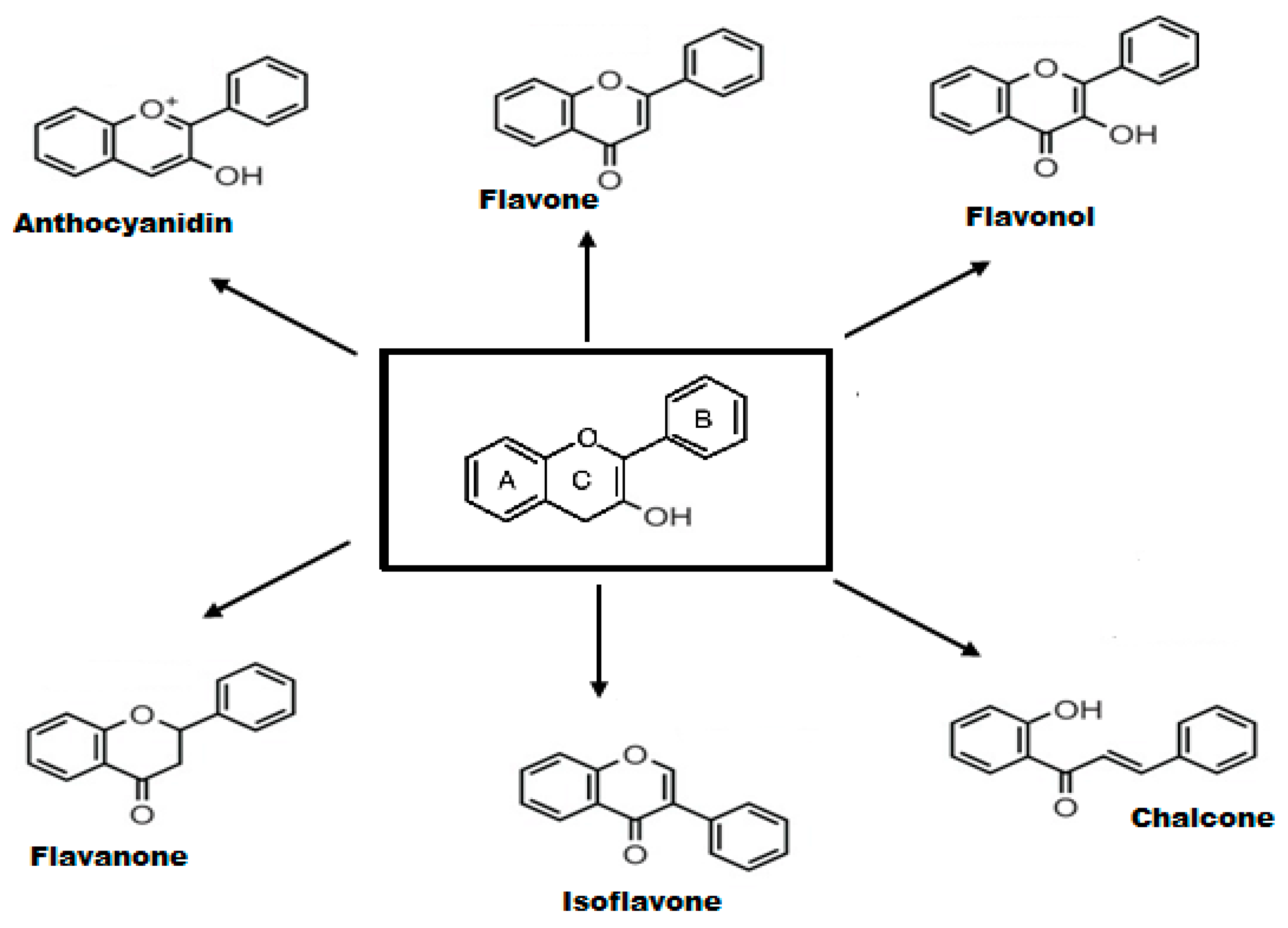
2.2. Structure-Activity Relationship of Flavonoids
2.3. Chrysin
Modified Chrysin
2.4. Quercetin
Modified Quercetin
2.5. Genistein
Modified Genistein
2.6. Epigallocatechin Gallate
Modified EGCG
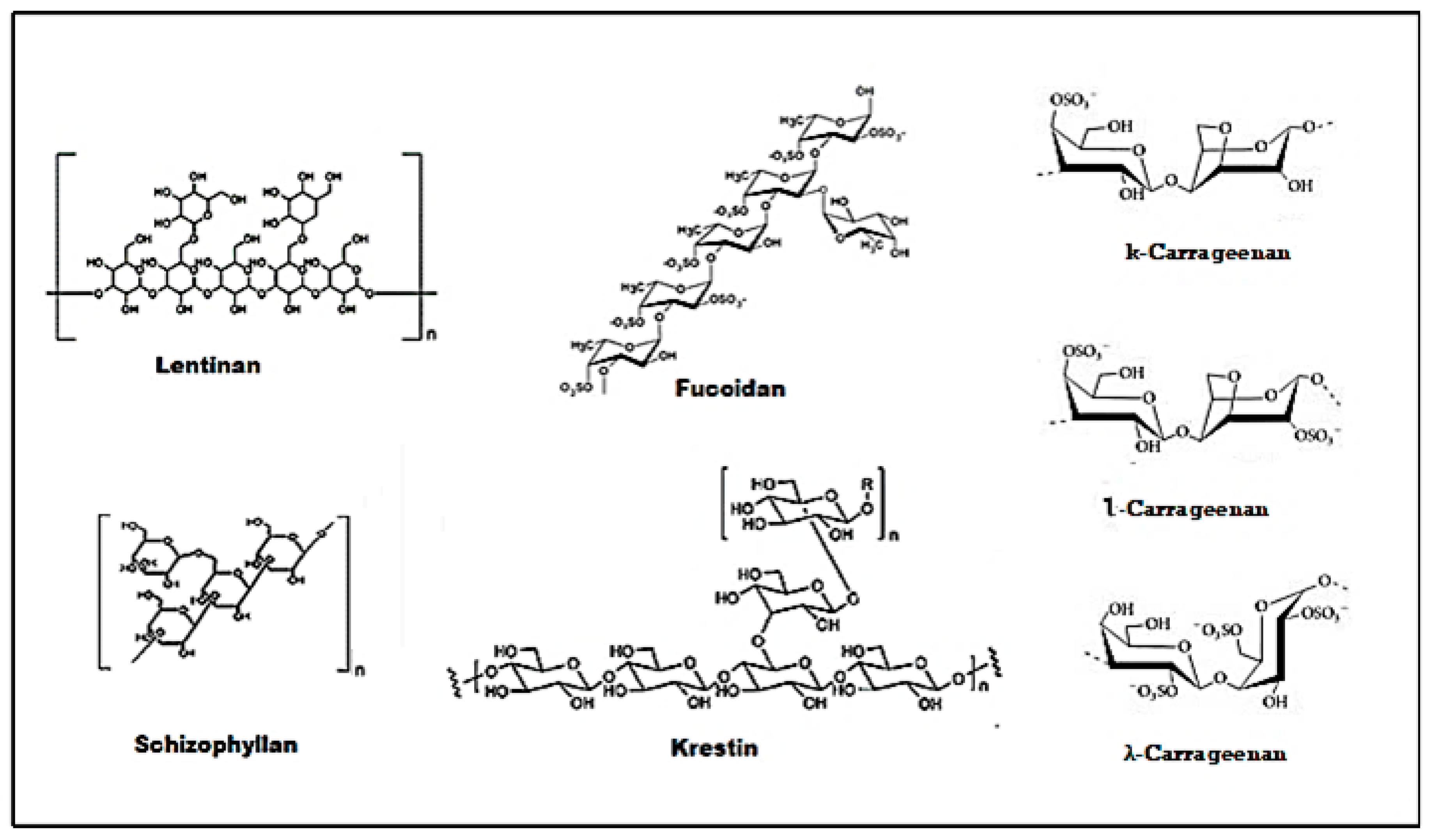
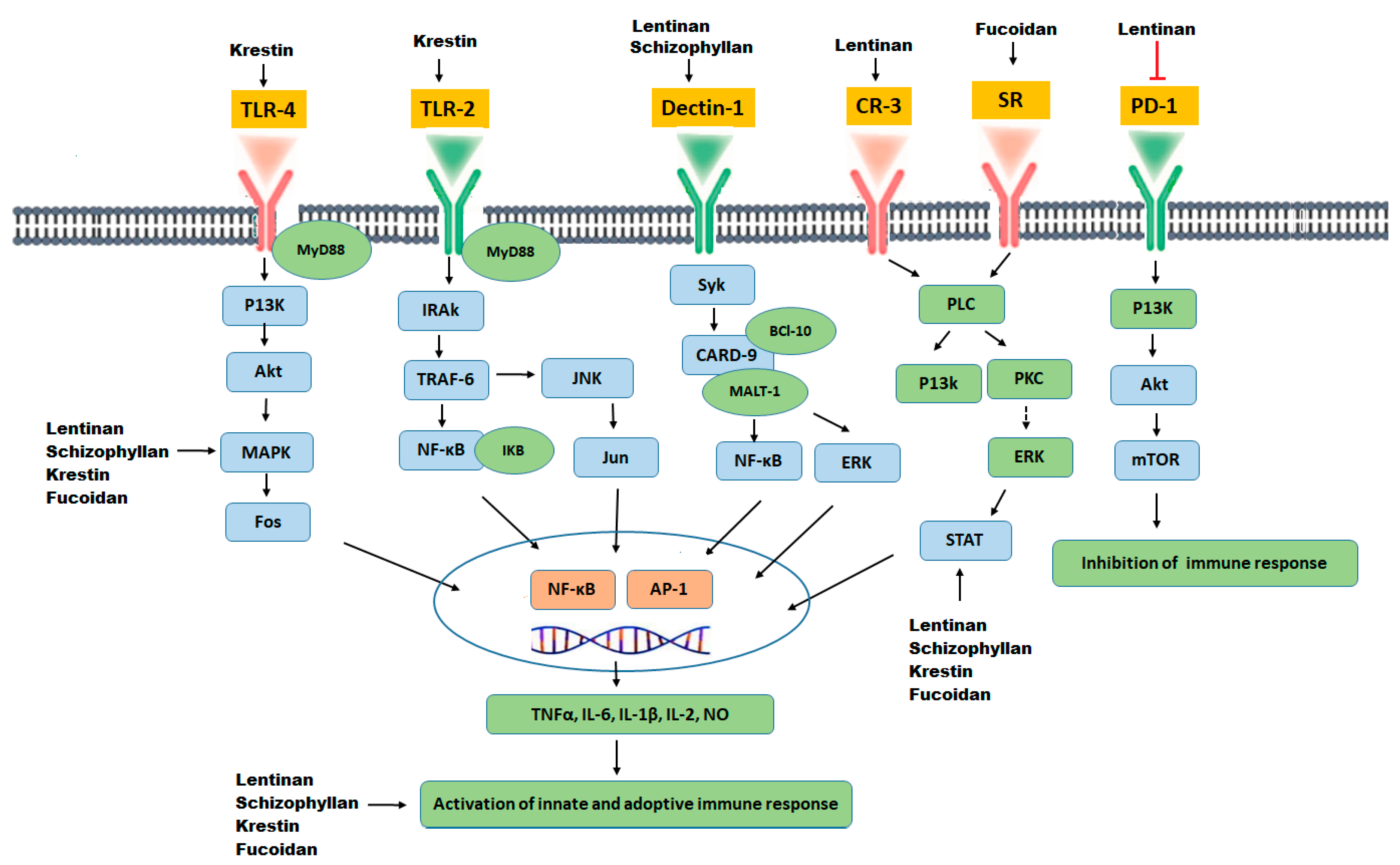
3. Chemopreventive Activities of Polysaccharides
3.1. Structure-Activity Relationship of Polysaccharides
3.2. Lentinan
Modified Lentinan
3.3. Schizophyllan
Modified Schizophyllan
3.4. Krestin
Modified Krestin
3.5. Fucoidan
Modified Fucoidan
3.6. Carrageenan
4. Conclusions
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| TMZ | Temozolomide |
| MGMT | O6 methyl guanine DNA methyltransferase |
| MDM2 | Mouse double minute 2 homolog |
| ARF | Alternative reading frame |
| PTEN | Phosphatase and tensin homolog |
| OXPHOS | Oxidative phosphorylation |
| IDH | Isocitrate dehydrogenase |
| CDK | Cyclin-dependent kinases |
| MAPK | Mitogen activated kinases |
| NF-κB | Nuclear factor kappa B |
| SGLT-1 | Sodium-glucose transport protein 1 |
| OATPs | Organic anion-transporting polypeptides |
| MMP | Matrix metalloproteinase |
| EGCG | Epigallocatechin gallate |
| PLGA | Poly (d, l-lactic-co-glycolic acid) |
| PEG | Polyethylene glycol |
| APE1 | Apyrimidinic (AP) endonuclease 1 |
| GSLCs | Glioma stem like cells |
| PDGFR | Platelet-derived growth factor receptor |
| EGFR | Epidermal growth factor receptor |
| IGF-1R | Insulin-like growth factor type 1 receptor |
| TIMP1 | TIMP metallopeptidase inhibitor 1 |
References
- Kane, J.R. The role of brain vasculature in glioblastoma. Mol. Neurobiol. 2019, 56, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-H.; Chang, T.-Y.; Lin, W.-C.; Wei, K.-C.; Shin, J.-W. GADD45A plays a protective role against temozolomide treatment in glioblastoma cells. Sci. Rep. 2017, 7, 8814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laug, D.; Glasgow, S.M.; Deneen, B. A glial blueprint for gliomagenesis. Nat. Rev. Neurosci. 2018, 19, 393–403. [Google Scholar] [CrossRef]
- Desai, V.; Bhushan, A. Natural bioactive compounds: Alternative approach to the treatment of glioblastoma multiforme. BioMed Res. Int. 2017, 2017, 9363040. [Google Scholar] [CrossRef] [Green Version]
- Koul, D. PTEN signaling pathways in glioblastoma. Cancer Biol. Ther. 2008, 7, 1321–1325. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, S2. [Google Scholar] [CrossRef] [Green Version]
- Anton, K.; Baehring, J.M.; Mayer, T. Glioblastoma multiforme: Overview of current treatment and future perspectives. Hematol. Oncol. Clin. 2012, 26, 825–853. [Google Scholar] [CrossRef]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Vidak, M.; Rozman, D.; Komel, R. Effects of flavonoids from food and dietary supplements on glial and glioblastoma multiforme cells. Molecules 2015, 20, 19406–19432. [Google Scholar] [CrossRef]
- Haque, A.; Banik, N.L.; Ray, S.K. Molecular alterations in glioblastoma: Potential targets for immunotherapy. In Progress in Molecular Biology and Translational Science; Elsevier: Charleston, SC, USA, 2011; Volume 98, pp. 187–234. [Google Scholar]
- Wu, C.-X.; Lin, G.-S.; Lin, Z.-X.; Zhang, J.-D.; Liu, S.-Y.; Zhou, C.-F. Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J. Surg. Oncol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-Oncology 2009, 11, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Singhal, N.; Selva-Nayagam, S.; Brown, M.P. Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment-case study and review of literature. J. Neuro-Oncol. 2007, 85, 229–230. [Google Scholar] [CrossRef]
- Cheema, T.A.; Wakimoto, H.; Fecci, P.E.; Ning, J.; Kuroda, T.; Jeyaretna, D.S.; Martuza, R.L.; Rabkin, S.D. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl. Acad. Sci. USA 2013, 110, 12006–12011. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Kausar, S.; Cui, H. Therapeutic potential of natural products in glioblastoma treatment: Targeting key glioblastoma signaling pathways and epigenetic alterations. Clin. Transl. Oncol. 2020, 22, 963–977. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Du, L.; Li, F. Effect and mechanism of total flavonoids extracted from cotinus coggygria against glioblastoma cancer in vitro and in vivo. BioMed Res. Int. 2015, 2015, 856349. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517. [Google Scholar] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Vega-Stromberg, T. Chemotherapy-induced secondary malignancies. J. Infus. Nurs. 2003, 26, 353–361. [Google Scholar] [CrossRef]
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Mao, H.; LeBrun, D.G.; Yang, J.; Zhu, V.F.; Li, M. Deregulated signaling pathways in glioblastoma multiforme: Molecular mechanisms and therapeutic targets. Cancer Investig. 2012, 30, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K. The p53 pathway in glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.R.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct. Target. Ther. 2017, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, S.; Siu, T.L.; Huang, S. Glioblastoma multiforme formation and EMT: Role of FoxM1 transcription factor. Curr. Pharm. Des. 2015, 21, 1268–1271. [Google Scholar] [CrossRef] [Green Version]
- Sami, A.; Karsy, M. Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: Novel therapeutic agents and advances in understanding. Tumor Biol. 2013, 34, 1991–2002. [Google Scholar] [CrossRef]
- Tuncel, G.; Kalkan, R. Receptor tyrosine kinase-Ras-PI 3 kinase-Akt signaling network in glioblastoma multiforme. Med. Oncol. 2018, 35, 122. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Niu, L.; Bai, Y.; Le, W. Glioblastoma: Targeting the autophagy in tumorigenesis. Brain Res. Bull. 2019, 153, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, Z.; Dai, S.; Qian, L.; Sun, L.; Gong, Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 23. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.A.; Das, B.C.; Ray, S.K. Targeing autophagy for combating chemoresistance and radioresistance in glioblastomat. Apoptosis 2018, 23, 563–575. [Google Scholar] [CrossRef]
- Avril, T.; Vauleon, E.; Tanguy-Royer, S.; Mosser, J.; Quillien, V. Mechanisms of immunomodulation in human glioblastoma. Immunotherapy 2011, 3, 42–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the immune system in glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Decollogne, S.; Dilda, P.J.; Hau, E.; Chung, S.A.; Luk, P.P.; Hogg, P.J.; McDonald, K.L. Dual-targeting of aberrant glucose metabolism in glioblastoma. J. Exp. Clin. Cancer Res. 2015, 34, 14. [Google Scholar] [CrossRef] [Green Version]
- Griguer, C.E.; Oliva, C.R. Bioenergetics pathways and therapeutic resistance in gliomas: Emerging role of mitochondria. Curr. Pharm. Des. 2011, 17, 2421–2427. [Google Scholar] [CrossRef]
- Jelluma, N.; Yang, X.; Stokoe, D.; Evan, G.I.; Dansen, T.B.; Haas-Kogan, D.A. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol. Cancer Res. 2006, 4, 319–330. [Google Scholar] [CrossRef] [Green Version]
- McBrayer, S.K.; Mayers, J.R.; DiNatale, G.J.; Shi, D.D.; Khanal, J.; Chakraborty, A.A.; Sarosiek, K.A.; Briggs, K.J.; Robbins, A.K.; Sewastianik, T. Transaminase inhibition by 2-hydroxyglutarate impairs glutamate biosynthesis and redox homeostasis in glioma. Cell 2018, 175, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, G.G.; Touil, Y.S.; Pham, M.H.; Dauzonne, D. Flavonoids in cancer prevention and therapy: Chemistry, pharmacology, mechanisms of action, and perspectives for cancer drug discovery. In Alternative and Complementary Therapies for Cancer; Springer: Paris, France, 2010; pp. 583–612. [Google Scholar]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Gupta, S.; Hussain, T.; Mukhtar, H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003, 410, 177–185. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Werdehausen, R.; Braun, S.; Essmann, F.; Schulze-Osthoff, K.; Walczak, H.; Lipfert, P.; Stevens, M.F. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiol. J. Am. Soc. Anesthesiol. 2007, 107, 136–143. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Begley, D.J. Efflux mechanisms in the central nervous system: A powerful influence on drug distribution within the brain. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Elsevier: London, UK, 2004; pp. 83–97. [Google Scholar]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Chen, M.; Wang, Y. Delivering flavonoids into solid tumors using nanotechnologies. Expert Opin. Drug Deliv. 2013, 10, 1411–1428. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier drug targeting: The future of brain drug development. Mol. Interv. 2003, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cordero, C.; López-Lázaro, M.; Gálvez, M.; Jesús Ayuso, M. Curcumin as a DNA topoisomerase II poison. J. Enzym. Inhib. Med. Chem. 2003, 18, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; Plygun, S.; Heydari, M. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Litim, N.; Di Paolo, T. Natural Phytoestrogens: A Class of Promising Neuroprotective Agents for Parkinson Disease. In Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Milton, ON Canada, 2018; pp. 9–61. [Google Scholar]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.; Krishna, D. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Mehdi, S.; Nafees, S.; Zafaryab, M.; Khan, M.; Rizvi, A. Chrysin: A promising anticancer agent its Current trends and future Perspectives. Eur. Exp. Biol. 2018, 8, 16. [Google Scholar] [CrossRef]
- Sun, L.-R.; Zhou, W.; Zhang, H.-M.; Guo, Q.-S.; Yang, W.; Li, B.-J.; Sun, Z.-H.; Gao, S.-H.; Cui, R.-J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.; Badruddeen; Akhtar, J.; Uddin MS, S.; Khan, M.I.; Khalid, M.; Ahmad, M. A Naturally Occurring Flavone (Chrysin): Chemistry, Occurrence, Pharmacokinetic, Toxicity, Molecular Targets and Medicinal Properties. J. Biol. Act. Prod. Nat. 2018, 8, 208–227. [Google Scholar] [CrossRef]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.; Pitanga, B.P.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.d.F.D.; El-Bachá, R.S.; Tardy, M. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem. Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.B.; Rahumatullah, A.; Yogarajah, T.; Ahmad, M.; Yin, K.B. Potential effects of chrysin on MDA-MB-231 cells. Int. J. Mol. Sci. 2010, 11, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, H.; Pi, J.; Jiang, J.-H.; Liu, L.; Bai, H.-H.; Yang, P.-H.; Cai, J.-Y. Anti-tumor activity evaluation of novel chrysin-organogermanium (IV) complex in MCF-7 cells. Bioorg. Med. Chem. Lett. 2013, 23, 5544–5551. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.-S.; Ho, Y.-S.; Lin, J.-K. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated protein kinase. Biochem. Pharmacol. 2005, 69, 1815–1827. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des. Dev. Ther. 2018, 12, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, L.C.; Antunes, M.S.; Borges Filho, C.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gülden, M.; Appel, D.; Syska, M.; Uecker, S.; Wages, F.; Seibert, H. Chrysin and silibinin sensitize human glioblastoma cells for arsenic trioxide. Food Chem. Toxicol. 2017, 105, 486–497. [Google Scholar] [CrossRef]
- Markiewicz-zukowska, R.; Borawska, M.H.; Fiedorowicz, A.; Naliwajko, S.K.; Sawicka, D.; Car, H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complementary Altern. Med. 2013, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-L.; Chen, C.-M.; Chang, Y.-Z.; Liu, G.-Y.; Hung, H.-C.; Hsieh, T.-Y.; Lin, C.-L. Pine (Pinus morrisonicola Hayata) Needle Extracts Sensitize GBM8901 Human Glioblastoma Cells to Temozolomide by Downregulating Autophagy and O 6-Methylguanine-DNA Methyltransferase Expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef]
- Borawska, M.H.; Naliwajko, S.K.; Moskwa, J.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Soroczyńska, J. Anti-proliferative and anti-migration effects of Polish propolis combined with Hypericum perforatum L. on glioblastoma multiforme cell line U87MG. BMC Complementary Altern. Med. 2016, 16, 367. [Google Scholar] [CrossRef] [Green Version]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Aiyelabegan, H.T.; Negahdari, B.; Rajeian, B.; Zarghami, N. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed. Pharmacother. 2016, 84, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, S.; Pu, Y.; Lai, Y.; He, B.; Gu, Z. Nanoparticles generated by PEG-Chrysin conjugates for efficient anticancer drug delivery. Eur. J. Pharm. Biopharm. 2014, 87, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Sabzichi, M.; Mohammadian, J.; Bazzaz, R.; Pirouzpanah, M.B.; Shaaker, M.; Hamishehkar, H.; Chavoshi, H.; Salehi, R.; Samadi, N. Chrysin loaded nanostructured lipid carriers (NLCs) triggers apoptosis in MCF-7 cancer cells by inhibiting the Nrf2 pathway. Process Biochem. 2017, 60, 84–91. [Google Scholar] [CrossRef]
- Anari, E.; Akbarzadeh, A.; Zarghami, N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1410–1416. [Google Scholar] [CrossRef]
- Mohammadian, F.; Pilehvar-Soltanahmadi, Y.; Mofarrah, M.; Dastani-Habashi, M.; Zarghami, N. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1972–1978. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly (lactic acid)/poly (lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 1–34. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Gogoi, R. Chrysin and its emerging role in cancer drug resistance. Chem. Biol. Interact. 2015, 236, 7–8. [Google Scholar] [CrossRef]
- Jung, J. Emerging utilization of chrysin using nanoscale modification. J. Nanomater. 2016, 2016, 2894089. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, G.M.; Jabir, M.S.; Hameed, A.H. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 708–720. [Google Scholar] [CrossRef]
- Nosrati, H.; Abbasi, R.; Charmi, J.; Rakhshbahar, A.; Aliakbarzadeh, F.; Danafar, H.; Davaran, S. Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells. Int. J. Biol. Macromol. 2018, 117, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, V.; Sumathi, T. Enhanced blood-brain barrier transmigration using the novel chrysin embedded solid lipid nanoformulation: A salient approach on physico-chemical characterization, pharmacokinetics and biodistribution studies. Int. J. Pharm. Clin. Res. 2016, 8, 1574–1582. [Google Scholar]
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, D.-P.; Li, R.-J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Khaw, A.K.; Yong, J.W.Y.; Kalthur, G.; Hande, M.P. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Genes Chromosomes Cancer 2012, 51, 961–974. [Google Scholar] [CrossRef]
- Le, C.T.; Leenders, W.P.; Molenaar, R.J.; van Noorden, C.J. Effects of the green tea polyphenol epigallocatechin-3-gallate on glioma: A critical evaluation of the literature. Nutr. Cancer 2018, 70, 317–333. [Google Scholar] [CrossRef]
- Verma, A.K.; Pratap, R. The biological potential of flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Nigam, V.; Sodhi, J. Some medicinal plants with antioxidant activity—A review. Int. J. Pharm. Biol. Sci. 2014, 4, 173–178. [Google Scholar]
- Chen, J.L.-Y.; Sperry, J.; Ip, N.Y.; Brimble, M.A. Natural products targeting telomere maintenance. MedChemComm 2011, 2, 229–245. [Google Scholar] [CrossRef]
- Lee, K.W.; Kang, N.J.; Heo, Y.-S.; Rogozin, E.A.; Pugliese, A.; Hwang, M.K.; Bowden, G.T.; Bode, A.M.; Lee, H.J.; Dong, Z. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008, 68, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Choi, C.H.; Park, J.Y.; Kang, S.K.; Kim, Y.K. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem. Res. 2008, 33, 971–979. [Google Scholar] [CrossRef]
- Pan, H.-C.; Jiang, Q.; Yu, Y.; Mei, J.-P.; Cui, Y.-K.; Zhao, W.-J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Rami, A.; von Deimling, A. Quercetin promotes degradation of survivin and thereby enhances death-receptor–mediated apoptosis in glioma cells. Neuro-Oncology 2009, 11, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yang, G.-Y.; Zhang, X.-H.; Zeng, N.; Ye, F. Experimental studies on existence form in plasma and brain targeting of quercetin nano-liposomes. Chin. J. Hosp. Pharm. 2011, 18, 610075. [Google Scholar]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.M.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes β-catenin cellular localization. Anti-Cancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, P.; Ferry, D.; Anderson, D.; Hussain, S.; Young, A.; Cook, J.; Hodgkin, E.; Seymour, L.; Kerr, D. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Calias, P.; Galanopoulos, T.; Maxwell, M.; Khayat, A.; Graves, D.; Antoniades, H.N.; d’Alarcao, M. Synthesis of inositol 2-phosphate-quercetin conjugates. Carbohydr. Res. 1996, 292, 83–90. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Rubio, N.; Wang, J.T.-W.; Al-Jamal, W.T.; Bourgognon, M.; Kafa, H.; Naeem, M.; Klippstein, R.; Abbate, V.; Leroux, F. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 2014, 8, 1384–1401. [Google Scholar] [CrossRef]
- Kikuta, S. The Cytotoxic Effect of Genistein, a Soybean Isoflavone, against Cultured Tribolium Cells. Insects 2020, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complementary Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.U.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G.; Heath, E.; Vaishampayan, U.; Cher, M.L.; Andic, F.; Rossi, P.J.; Kucuk, O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr. Cancer 2010, 62, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Singh-Gupta, V.; Joiner, M.C.; Runyan, L.; Yunker, C.K.; Sarkar, F.H.; Miller, S.; Gadgeel, S.M.; Konski, A.A.; Hillman, G.G. Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, M.M.K.; Mahmoud, S.S.; El-Sayed, S.H.; Rizk, E.M.A.; Raafat, A.; Negm, M.S.I. Impact of treatment with a protein tyrosine kinase inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 2018, 185, 115–123. [Google Scholar] [CrossRef]
- Puli, S.; Jain, A.; Lai, J.C.; Bhushan, A. Effect of combination treatment of rapamycin and isoflavones on mTOR pathway in human glioblastoma (U87) cells. Neurochem. Res. 2010, 35, 986–993. [Google Scholar] [CrossRef]
- Khoshyomn, S.; Nathan, D.; Manske, G.C.; Osler, T.M.; Penar, P.L. Synergistic effect of genistein and BCNU on growth inhibition and cytotoxicity of glioblastoma cells. J. Neuro-Oncol. 2002, 57, 193–200. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Viswanathan, S. Anticancer therapeutic potential of soy isoflavone, genistein. In Complementary and Alternative Approaches to Biomedicine; Springer: Santa Monica, CA, USA, 2004; pp. 121–165. [Google Scholar]
- Schmidt, F.; Knobbe, C.B.; Frank, B.; Wolburg, H.; Weller, M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol. Rep. 2008, 19, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-K. The therapeutic potential of flavonoids. Expert Opin. Investig. Drugs 2000, 9, 2103–2119. [Google Scholar] [CrossRef]
- Myers, D.E.; Sicheneder, A.; Clementson, D.; Dvorak, N.; Venkatachalam, T.; Sev, A.R.; Chandan-Langlie, M.; Uckun, F.M. Large scale manufacturing of B43 (anti-CD19)-genistein for clinical trials in leukemia and lymphoma. Leuk. Lymphoma 1998, 29, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Gaeti, M.P.N.; De Ávila, P.H.M.; de Sousa Vieira, M.; dos Santos Rodrigues, B.; de Ávila Marcelino, R.I.; Dos Santos, L.C.R.; Valadares, M.C.; Lima, E.M. Multicompartimental nanoparticles for co-encapsulation and multimodal drug delivery to tumor cells and neovasculature. Pharm. Res. 2014, 31, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Chuan, L.; Zhang, J.; Yu-Jiao, Z.; Shu-Fang, N.; Jun, C.; Qian, W.; Shao-Ping, N.; Ze-Yuan, D.; Ming-Yong, X.; Shu, W. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar]
- Azambuja, C.R.L.; dos Santos, L.G.; Rodrigues, M.R.; Rodrigues, R.F.M.; da Silveira, E.F.; Azambuja, J.H.; Flores, A.F.; Horn, A.P.; Dora, C.L.; Muccillo-Baisch, A.L. Physico-chemical characterization of asolectin–genistein liposomal system: An approach to analyze its in vitro antioxidant potential and effect in glioma cells viability. Chem. Phys. Lipids 2015, 193, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, V.; Walters, J.; Brownlow, B.; Elbayoumi, T. Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. J. Drug Target. 2013, 21, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja Borges, C.R.L.; Silva, N.O.; Rodrigues, M.R.; Marinho, M.A.G.; de Oliveira, F.S.; Cassiana, M.; Horn, A.P.; Parize, A.L.; Flores, D.C.; Clementin, R.M. Dimiristoylphosphatidylcholine/genistein molecular interactions: A physico-chemical approach to anti-glioma drug delivery systems. Chem. Phys. Lipids 2019, 225, 104828. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Zhang, Y.; Wang, S.-X.; Ma, J.-W.; Li, H.-Y.; Ye, J.-C.; Xie, S.-M.; Du, B.; Zhong, X.-Y. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J. Neuro-Oncol. 2015, 121, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wang, W.; Golden, E.B.; Thomas, S.; Sivakumar, W.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011, 302, 100–108. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, V.; Tewari, R.; Koul, N.; Joseph, C.; Sen, E. Epigallocatechin-3-gallate exhibits anti-tumor effect by perturbing redox homeostasis, modulating the release of pro-inflammatory mediators and decreasing the invasiveness of glioblastoma cells. Mol. Med. Rep. 2008, 1, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shervington, A.; Pawar, V.; Menon, S.; Thakkar, D.; Patel, R. The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol. Biol. Rep. 2009, 36, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.-W.; Chen, W.; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Xu, Y.-M.; Wu, Y.; Yu, K.-K.; Zhang, C.; Ji, Y.-H.; Ding, G.; Chen, F.-X. Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci. Bull. 2014, 30, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, N.; Annabi, B.; Bouzeghrane, M.; Temme, A.; Bahary, J.-P.; Moumdjian, R.; Béliveau, R. The Survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (−)-epigallocatechin-3-gallate. Brain Res. 2006, 1071, 1–9. [Google Scholar] [CrossRef]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839. [Google Scholar] [CrossRef] [Green Version]
- Seimiya, H.; Oh-hara, T.; Suzuki, T.; Naasani, I.; Shimazaki, T.; Tsuchiya, K.; Tsuruo, T. Telomere Shortening and Growth Inhibition of Human Cancer Cells by Novel Synthetic Telomerase Inhibitors MST-312, MST-295, and MST-199 1 Supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan. 1. Mol. Cancer Ther. 2002, 1, 657–665. [Google Scholar] [PubMed]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Sanna, V.; Pintus, G.; Roggio, A.M.; Punzoni, S.; Posadino, A.M.; Arca, A.; Marceddu, S.; Bandiera, P.; Uzzau, S.; Sechi, M. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J. Med. Chem. 2011, 54, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Lin, J.-N.; Hsieh, J.-T.; Chou, S.-C.; Lai, C.-H.; Yun, E.-J.; Lo, U.-G.; Pong, R.-C.; Lin, J.-H.; Lin, Y.-H. Nanoparticle targeting CD44-positive cancer cells for site-specific drug delivery in prostate cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 30722–30734. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Fadul, C.E.; Fisher, J.L.; Gui, J.; Hampton, T.H.; Côté, A.L.; Ernstoff, M.S. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro-Oncology 2011, 13, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Cassileth, B.R. Complementary therapies: The American experience. Supportive Care Cancer 2000, 8, 16–23. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal mushrooms and cancer therapy: Translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006, 49, 159–170. [Google Scholar] [CrossRef]
- Ayeka, P.A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: A review. Evid. Based Complementary Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhou, X.; Chen, H. Structure-activity relationship of plant polysaccharides. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2017, 42, 4104–4109. [Google Scholar]
- De Silva, D.D.; Rapior, S.; Fons, F.; Bahkali, A.H.; Hyde, K.D. Medicinal mushrooms in supportive cancer therapies: An approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012, 55, 1–35. [Google Scholar] [CrossRef]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of molecular structure on antitumor activities of (1→ 3)-β-d-glucans from different Lentinus edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Seviour, R. Medicinal importance of fungal β-(1→ 3), (1→ 6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Bae, I.Y.; Kim, H.W.; Yoo, H.J.; Kim, E.S.; Lee, S.; Park, D.Y.; Lee, H.G. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Res. Int. 2013, 51, 195–200. [Google Scholar] [CrossRef]
- Li, G.H.; Shen, Y.M.; Zhang, K.Q. Nematicidal activity and chemical component of Poria cocos. J. Microbiol. 2005, 43, 17–20. [Google Scholar] [PubMed]
- Cheng, J.-J.; Chang, C.-C.; Chao, C.-H.; Lu, M.-K. Characterization of fungal sulfated polysaccharides and their synergistic anticancer effects with doxorubicin. Carbohydr. Polym. 2012, 90, 134–139. [Google Scholar] [CrossRef]
- Zen, K.; Liu, Y.; Cairo, D.; Parkos, C.A. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J. Immunol. 2002, 169, 5270–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, Z.; Zeng, Y.; Zhang, M.; Cui, Y. Chinese FDA Approved Fungal Glycan-Based Drugs: An Overview of Structures, Mechanisms and Clinical Related Studies. Transl. Med. 2014, 90, H20003510. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, S.; Tabata, T.; Hazama, S.; Iizuka, N.; Yamamoto, K.; Hirayama, M.; Tangoku, A.; Oka, M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000, 20, 4707–4711. [Google Scholar]
- Wang, X.-E.; Wang, Y.-H.; Zhou, Q.; Peng, M.; Zhang, J.; Chen, M.; Ma, L.-J.; Xie, G.-M. Immunomodulatory effect of lentinan on aberrant T subsets and cytokines profile in non-small cell lung cancer patients. Pathol. Oncol. Res. 2020, 26, 499–505. [Google Scholar] [CrossRef]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Kuai, J.; Fan, P.; Wang, X.; Zhou, P.; Yang, D.; Zheng, X.; Liu, X.; Wu, Q. Lentinan inhibits tumor angiogenesis via interferon γ and in a T cell independent manner. J. Exp. Clin. Cancer Res. 2018, 37, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, D.; Seki, K.; Ishibashi, Y.; Egawa, Y.; Koga, M.; Sasaki, T.; Hirano, K.; Mikami, K.; Futami, K.; Maekawa, T. The effect of lentinan combination therapy for unresectable advanced gastric cancer. Anticancer Res. 2012, 32, 2365–2368. [Google Scholar]
- Wei, Y.-Z.; Zhou, B.; Fu, Q.; Song, S.-S.; Zheng, H.-L. Inhibitory effect of schizophyllan on rat glioma cells. Bangladesh J. Pharmacol. 2015, 10, 759–764. [Google Scholar]
- Ina, H.; Yoneda, M.; Kanda, M.; Kodera, Y.; Kabeya, M.; Yuasa, S. Lentinan, a shiitake mushroom beta-glucan, stimulates tumor-specific adaptive immunity through PD-L1 down-regulation in gastric cancer cells. Med. Chem. 2016, 6, 710–714. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Jin, L.; Zhang, X. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Wölfle, S.J.; Strebovsky, J.; Bartz, H.; Sähr, A.; Arnold, C.; Kaiser, C.; Dalpke, A.H.; Heeg, K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011, 41, 413–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wang, J.; Cheng, F.; Huang, X.; Cheng, Y.; Wang, K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016, 377, 117–125. [Google Scholar] [CrossRef]
- Xu, H.-L.; Dai, J.-H.; Hu, T.; Liao, Y.-F. Lentinan up-regulates microRNA-340 to promote apoptosis and autophagy of human osteosarcoma cells. Int. J. Clin. Exp. Pathol. 2018, 11, 3876. [Google Scholar] [PubMed]
- Li, X.; Zhang, M. In vitro inhibitory effects of lentinan on rat glioma cells. Biomed. Res. 2014, 25, 39–44. [Google Scholar]
- Hazama, S.; Watanabe, S.; Ohashi, M.; Yagi, M.; Suzuki, M.; Matsuda, K.; Yamamoto, T.; Suga, Y.; Suga, T.; Nakazawa, S. Efficacy of orally administered superfine dispersed lentinan (β-1, 3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res. 2009, 29, 2611–2617. [Google Scholar] [PubMed]
- Shen, J.; Ren, H.; Tomiyama-Miyaji, C.; Suga, Y.; Suga, T.; Kuwano, Y.; Iiai, T.; Hatakeyama, K.; Abo, T. Potentiation of intestinal immunity by micellary mushroom extracts. Biomed. Res. 2007, 28, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubala, L.; Ruzickova, J.; Nickova, K.; Sandula, J.; Ciz, M.; Lojek, A. The effect of (1→ 3)-β-d-glucans, carboxymethylglucan and schizophyllan on human leukocytes in vitro. Carbohydr. Res. 2003, 338, 2835–2840. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Banerjee, S.; Parasramka, M.; Paruthy, S. Polysaccharides in cancer prevention: From bench to bedside. Polysacch. Bioact Biotechnol. 2015, 8, 2179–2214. [Google Scholar] [CrossRef]
- Yoshiba, K.; Sato, T.; Osumi, T.; Ulset, A.-S.T.; Christensen, B.E. Conformation of carboxylated schizophyllan in aqueous solution. Carbohydr. Polym. 2015, 134, 1–5. [Google Scholar] [CrossRef]
- Oana, C.; Adriana, T.; Mircea, C.; Dragos, S.; Monica, H. Natural Macromolecules with Protective and Antitumor Activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2018, 18, 675–683. [Google Scholar] [CrossRef]
- Mansour, A.; Daba, A.; Baddour, N.; El-Saadani, M.; Aleem, E. Schizophyllan inhibits the development of mammary and hepatic carcinomas induced by 7, 12 dimethylbenz (α) anthracene and decreases cell proliferation: Comparison with tamoxifen. J. Cancer Res. Clin. Oncol. 2012, 138, 1579–1596. [Google Scholar] [CrossRef]
- Mousaviasl, S.; Saleh, T.; Shojaosadati, S.A.; Boddohi, S. Synthesis and characterization of schizophyllan nanogels via inverse emulsion using biobased materials. Int. J. Biol. Macromol. 2018, 120, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Fujisawa, T.; Haraguchi, S.; Numata, M.; Karinaga, R.; Kimura, T.; Okumura, S.; Sakurai, K.; Shinkai, S. Schizophyllan-folate conjugate as a new non-cytotoxic and cancer-targeted antisense carrier. Bioorg. Med. Chem. Lett. 2005, 15, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor: Physiological activity, uses, and production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Yang, Y.; Gad, E.; Disis, M.L. The role of TLR2 in the immunostimulatory effect of Polysaccharide krestin (PSK) (41.13). Am. Assoc. Immnol. 2009, 182, 4113. [Google Scholar]
- Fisher, M.; Yang, L.-X. Anticancer effects and mechanisms of polysaccharide-K (PSK): Implications of cancer immunotherapy. Anticancer Res. 2002, 22, 1737–1754. [Google Scholar] [PubMed]
- Yamashita, K.; Ougolkov, A.V.; Nakazato, H.; Ito, K.; Ohashi, Y.; Kitakata, H.; Yasumoto, K.; Omote, K.; Mai, M.; Takahashi, Y. Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic β-catenin activation. Dis. Colon Rectum 2007, 50, 1169–1181. [Google Scholar] [CrossRef]
- Hori, T.; Adachi, S.; Anno, Y.; Numata, H.; Hokama, Y.; Muraoka, K.; Matsutani, M.; Matsui, T. Immunochemotherapy of human gliomas transplanted into nude mice. No Shinkei Brain Nerve 1984, 36, 881–888. [Google Scholar]
- Wakisaka, S.; Maruoka, N.; Kaji, Y.; Moriyama, T.; Oyama, H.; Shimauchi, M.; Nonaka, A.; Goya, T.; Kinoshita, K.; Kodama, T. AUFRAP therapy: Combined modality treatment of malignant gliomas with intraarterial infusion of ACNU. Gan Kagaku Ryoho. Cancer Chemother. 1988, 15, 2405–2409. [Google Scholar]
- Matsuda, Y.; Suzuki, T.; Sato, E.; Sato, M.; KOIZUMI, S.; UNNO, K.; KATO, T.; NAKAI, K. Novel preparation of zein microspheres conjugated with PS-K available for cancer immunotherapy. Chem. Pharm. Bull. 1989, 37, 757–759. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Lai, I.; Kuo, H.-C.; Chuang, S.-E.; Lee, H.-L.; Whang-Peng, J.; Yao, C.-J.; Lai, G.-M. Epigenetic modification and differentiation induction of malignant glioma cells by Oligo-Fucoidan. Mar. Drugs 2019, 17, 525. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Kim, S.-K. Fucoidan, a sulfated polysaccharides from brown algae as therapeutic target for cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer: Berlin/Heidelberg, Germany, 2015; pp. 145–164. [Google Scholar]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Pomin, V.H. Marine carbohydrate-based compounds with medicinal properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [Green Version]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of antioxidant and anticancer potential of fucoidan from Sargassum polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Boo, H.-J.; Hong, J.-Y.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Kim, E.-J.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S.; Kwon, J.-M. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs 2013, 11, 2982–2999. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, H.Y.; Kwon, D.; Yoon, S. Detoxification and anticarcinogenic effects of Fucoidan in human hepatoblastoma and rat glioma cells. Fed. Am. Soc. Exp. Biol. 2007. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.-H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-α-and IFN-γ-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef]
- Do, H.; Choi, H.; Lee, S.; Joo, H.; Lee, H.; Sohn, E.; Lee, S.; Pyo, S.; Son, E. Fucoidan inhibits the production of NO induced by IFN-γ in C6 glioma cells; Regulation by p38, AP-1 and scavenger receptor B1. Fed. Am. Soc. Exp. Biol. 2007. [Google Scholar] [CrossRef]
- Chahal, M.; Xu, Y.; Lesniak, D.; Graham, K.; Famulski, K.; Christensen, J.G.; Aghi, M.; Jacques, A.; Murray, D.; Sabri, S. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro-Oncology 2010, 12, 822–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Song, Q.; Shao, Q.; Gao, W.; Mao, H.; Lou, H.; Qu, X.; Li, X. Comparison of the effects of marchantin C and fucoidan on sFlt-1 and angiogenesis in glioma microenvironment. J. Pharm. Pharmacol. 2012, 64, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lamszus, K.; Ulbricht, U.; Matschke, J.; Brockmann, M.A.; Fillbrandt, R.; Westphal, M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin. Cancer Res. 2003, 9, 1399–1405. [Google Scholar]
- Takano, S.; Kamiyama, H.; Tsuboi, K.; Matsumura, A. Angiogenesis and antiangiogenic therapy for malignant gliomas. Brain Tumor Pathol. 2004, 21, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.-Y.; Li, R.; Hsu, C.-H.; Lin, C.-W.; Chou, S.-C.; Tsai, M.-L.; Mi, F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic effects of fucoidan nanoparticles against osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-W.; Jung, W.; Choi, C.; Kim, S.-Y.; Son, A.; Kim, H.; Lee, N.; Park, H.C. Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis. Mar. Drugs 2018, 16, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.; Pereira, L. Antitumour potential of Gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.P.; Alves, C.; Pinteus, S.; Silva, J.; Valado, A.; Pedrosa, R.; Pereira, L. Antioxidant and antitumor potential of wild and IMTA-cultivated Osmundea pinnatifida. J. Oceanol. Limnol. 2019, 37, 825–835. [Google Scholar] [CrossRef]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.-W.; Li, Y.-L.; Wang, B.-L.; He, Z.-Y.; Liang, X.; Ye, T.-H.; Wei, Y.-Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 11062. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complementary Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, H.; Hawat, M.; Ekhtiar, A.; AlJapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.; Burns, W.W. Selective inhibition of cell proliferation and DNA synthesis by the polysulphated carbohydrate ι-carrageenan. Cancer Chemother. Pharmacol. 1995, 36, 325–334. [Google Scholar] [CrossRef]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef]
- Jazzara, M.; Ghannam, A.; Soukkarieh, C.; Murad, H. Anti-Proliferative Activity of λ-Carrageenan Through the Induction of Apoptosis in Human Breast Cancer Cells. Iran. J. Cancer Prev. 2016, 9, e3836. [Google Scholar] [CrossRef]
- Jazzara, M.; Ghannam, A.; Soukarieh, C.; Murad, H. Anti-proliferative effect of sulfated carrageenan extracted from Laurencia papillosa on T98G glioblastoma cancer cells. Arab J. Pharm. Sci. 2014, 286, 86–96. [Google Scholar]
- Ling, G.; Zhang, T.; Zhang, P.; Sun, J.; He, Z. Nanostructured lipid-carrageenan hybrid carriers (NLCCs) for controlled delivery of mitoxantrone hydrochloride to enhance anticancer activity bypassing the BCRP-mediated efflux. Drug Dev. Ind. Pharm. 2016, 42, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Gao, Y.; Yin, J.; Bai, M.; Wang, F. Green synthesis of gold nanoparticles using carrageenan oligosaccharide and their in vitro antitumor activity. Mar. Drugs 2018, 16, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, M.; Devi, V.; Doble, M. Biocompatible ι-carrageenan-γ-maghemite nanocomposite for biomedical applications–synthesis, characterization and in vitro anticancer efficacy. J. Nanobiotechnol. 2015, 13, 18. [Google Scholar] [CrossRef] [Green Version]

| Flavonoid Phytochemical | Structure | Mechanism of Action | References |
|---|---|---|---|
| Chrysin | 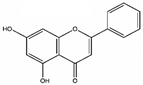 | Down-regulation of Wnt, NF-kB, and Akt. Reduction of Erk/Nrf2, modulation of MAPK/ERK, and P38 increase of SOD, CAT. Up-regulation of LC3-II and PARP | [88] |
| Genistein |  | Down-regulates NF-kB and Akt pathways, up-regulation of p53 and p21, inhibition of cyclin B, cyclin D1, TERT expression, activates Notch 1 Signaling pathway | [89,90] |
| Quercetin | 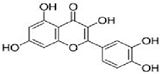 | Inhibition of MAPK/(ERK) kinase, (MEK) 1 and Raf1 kinase, STAT3, CDK1, MMP, Akt/P13k pathway. Stimulation of Bid, Bad, Bax, caspase-9, -3 release, Inhibition of Bcl-xL, Bcl-2, and cytochrome c | [69] |
| EGCG | 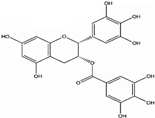 | Inhibition of PI3K/Akt pathway, modulation of MAPK, NFκB, Wnt/β-catenin, up-regulation of p53 and p21, G1,S, or G2/M arrest. | [91] |
| Polysaccharide Phytochemical | Molecular Target | References |
|---|---|---|
| Lentinan | Binding to CR-3 and Dectin-1 receptors, activation of macrophages, natural killer cells, T cells and B cell, Inhibition of T regulatory cells. Cell cycle arrest through the enhanced number of cells in the Go/G1 phase and reduced cells in the S phase. | [166] |
| Schizophyllan | A ligand of the Dectin-1 receptor. Potentiate natural killer cells (NK) and cytotoxic T cells. Inhibition of cell cycle at the Go/G1 and G2/M phase, p53 up-regulation, CDK1 inhibition. | [162] |
| Krestin | A specific TLR-2 agonist, up-regulation of NF-kB and Cytokines (TNF-α, IL-6), enhanced serum IgG, and IgM production. Activation of natural killer cells and lymphocytes activated killer cells. | [168] |
| Fucoidan | Binds specifically to scavenger receptors. Potentiates NK cells, dendritic cells (DC), and T cells. Down-regulated VEGF and elevated sFlt-1. Up-regulation of Myelin Basic Protein (MBP), Glial fibrillary acidic protein (GFAP), Oligodendrocyte transcription factor (OLIG2), and microtubule-associated protein-2 (MAP2). Up-regulation of NF-kB and AP-1 | [196] |
| Carrageenan | Induction of apoptosis through upregulation of caspase-8, caspase-9 and caspase-3. Causes cell cycle arrest at G1, G2 or S phase. | [197,198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiq, A.; Parhar, I. Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma. Molecules 2020, 25, 4895. https://doi.org/10.3390/molecules25214895
Atiq A, Parhar I. Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma. Molecules. 2020; 25(21):4895. https://doi.org/10.3390/molecules25214895
Chicago/Turabian StyleAtiq, Ayesha, and Ishwar Parhar. 2020. "Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma" Molecules 25, no. 21: 4895. https://doi.org/10.3390/molecules25214895
APA StyleAtiq, A., & Parhar, I. (2020). Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma. Molecules, 25(21), 4895. https://doi.org/10.3390/molecules25214895





